Abstract
Rationale
Mounting data suggest that immune cell abnormalities participate in the pathogenesis of pulmonary arterial hypertension (PAH).
Objective
To determine whether the T lymphocyte subset composition in the systemic circulation and peripheral lung is altered in PAH.
Methods
Flow cytometric analyses were performed to determine the phenotypic profile of peripheral blood lymphocytes in idiopathic PAH (IPAH) patients (n=18) and healthy controls (n=17). Immunocytochemical analyses of lymphocytes and T cell subsets were used to examine lung tissue from PAH patients (n=11) and controls (n=11).
Measurements and Main Results
IPAH patients have abnormal CD8+ T lymphocyte subsets, with a significant increase in CD45RA+ CCR7- peripheral cytotoxic effector-memory cells (p=0.02) and reduction of CD45RA+ CCR7+ naive CD8+ cells versus controls (p=0.001). Further, IPAH patients have a higher proportion of circulating regulatory T cells (Treg) and 4-fold increases in the number of CD3+ and CD8+ cells in the peripheral lung compared to controls (p<0.01).
Conclusions
Alterations in circulating T cell subsets, particularly CD8+ T lymphocytes and CD4+ Tregs, in patients with PAH suggest a dysfunctional immune system contributes to disease pathogenesis. A preponderance of CD3+ and CD8+ T lymphocytes in the peripheral lung of PAH patients supports this concept.
Keywords: T cells, immune system, lung lymphocytes, regulatory T cells
Introduction
Pulmonary arterial hypertension (PAH) is associated with multiple causes, but similar clinical and pathologic findings suggest common pathophysiologic processes (1). The occurrence of PAH in association with systemic inflammation and immune dysregulation has long been recognized but poorly understood. For example, PAH is associated with HIV (human immunodeficiency virus) infection, scleroderma, and other autoimmune diseases. The characteristic structural changes of idiopathic PAH (IPAH) are often accompanied by perivascular inflammatory cell infiltrates that include T and B lymphocytes, mast cells, macrophages, and other inflammatory cells around plexiform lesions (2). In addition, circulating anti-nuclear, anti-endothelial, and anti-fibroblast autoantibodies, elevated serum concentrations of proinflammatory cytokines, as well as local pulmonary inflammatory chemokine expression have been described in IPAH (3–7). These findings implicate immune cell dysfunction and a loss of self-tolerance in the pathogenesis of this disease (8).
Some investigators have suggested that a loss of immunoregulation could promote autoreactive lymphocytes and the expansion of other inflammatory cells (9–11). A recent study found diminished circulating CD8+ T cells in patients with IPAH compared to controls, as well as an elevated proportion of FoxP3+ cells within the CD4+ T cell population, presumably identifying an increase in circulating regulatory T cells (Tregs) with suppressor activity (12). These findings implicate immune dysregulation in humans as either a cause or consequence of disease pathogenesis.
The present study used flow cytometric analyses (FACS) of circulating lymphocytes from the blood of IPAH patients and controls as well as immunocytochemical techniques to study lung tissue from PAH patients and controls, to test the hypothesis that an abnormal lymphocyte composition contributes to the pathophysiology of PAH. Our results demonstrate alterations in the proportions of T lymphocyte subsets in the peripheral blood of these patients and infiltration of CD3+ and CD8+ cells in the lung, suggesting that a loss of self tolerance promotes disease expression.
Methods
Subjects
Peripheral blood samples were obtained from patients with IPAH recruited from the clinic of the Pulmonary Hypertension Center of Vanderbilt University Medical Center (n=18) (Table 1). All enrolled patients met diagnostic criteria for IPAH in accordance with accepted international standards, including a mean pulmonary arterial pressure of ≥ 25 mm Hg with a pulmonary capillary or left atrial pressure of ≤ 15 mm Hg, and exclusion of other causes of pulmonary hypertension (1). Six of the IPAH patients had been previously tested for a BMPR2 mutation according to the most advanced standards published to date, with no mutation detected (13). Healthy adult volunteers not using medications served as controls (n=17, mean age 48.3 years ± 16.1; 7 males and 10 females). Control subjects completed a medical questionnaire prior to the blood draw, and include only individuals without known co-morbid conditions such as autoimmune or cardiovascular disease.
Table 1.
Characteristics of IPAH Subjects Included in FACS Analysis
| Variable | IPAH Group |
|---|---|
| n = 18 | |
| Female (%) | 15 (83.3) |
| Age at Time of Blood Draw, yrs | 56.2 ± 8.3 |
| Age at Diagnosis, yrs | 50.7 ± 7.8 |
| Mean PAP at Diagnosis, mm Hg | 55.4 ± 15.0 |
| WHO Functional Class, n (%) | |
| I | 2 (11.1) |
| II | 7 (38.9) |
| III | 8 (44.4) |
| IV | 1 (5.6) |
| Current Prostanoid Therapy, n (%) | 14 (77.8) |
| Current Phosphodiesterase Inhibitor Therapy, n (%) | 8 (44.4) |
| Current Endothelin Receptor Antagonist Therapy, n (%) | 3 (16.7) |
Values are mean ± S.D.
Lung tissue samples were obtained from PAH patients (total n=11). Eight samples were obtained at autopsy and three were explanted lungs (n=3) (Table 2). All enrolled patients met diagnostic criteria for PAH in accordance with accepted international standards described below (1). Six patients were diagnosed with IPAH and 5 patients with heritable PAH. While subtle differences may exist, because the clinical presentation and pulmonary arterial changes from patients with IPAH and heritable PAH are known to be very similar, the cases were combined and are presented as the PAH group.(14) Control lung tissue (n=11, mean age 47.4 years ± 14.4; 6 males and 5 females) from subjects without systemic inflammatory or autoimmune diseases was obtained from the Vanderbilt University Medical Center Department of Pathology. This tissue consisted of either healthy areas of lung from patients with a lung biopsy performed for diagnostic or therapeutic purposes involving a focal lung process (5 subjects) or from autopsy cases (6 subjects) with no evidence of lung disease. All samples other than biopsy tissue were inflated with formalin by way of the bronchus; biopsy tissues were inflated with formalin by needle inflation.
Table 2.
Characteristics of PAH Patients Included in Tissue Analysis
| Variable | PAH Group |
|---|---|
| n = 11 | |
| Female (%) | 7 (63.6) |
| Age at Time of Tissue Acquisition | 32.2 ± 13.0 |
| Age at Diagnosis, yrs | 26.5 ± 14.1 |
| Mean PAP at Diagnosis, mm Hg | 67.7 ± 17.1 |
| Current Prostanoid Therapy, n (%) | 11 (100) |
| Current Phosphodiesterase Inhibitor Therapy, n (%) | 2 (0.2) |
| Current Endothelin Receptor Antagonist Therapy, n (%) | 3 (0.3) |
Values are mean ± S.D.
All aspects of the study were approved by the institutional review board at Vanderbilt University Medical Center, and written informed consent was obtained from all living subjects included in the study. Unique identifiers to conceal identity were assigned to the samples before their receipt in the laboratory.
Blood Samples and Lymphocyte Subsets Analysis
Venous blood samples were collected from each subject in heparin-treated tubes using a 21-gauge needle, and held at room temperature overnight prior to isolation of peripheral blood mononuclear cells (PBMC). PBMC were isolated by Ficoll-Hypaque (Sigma-Aldrich) density gradient centrifugation, and resuspended at a concentration of 107 cells/ml in freezing medium containing 90% FBS (Invitrogen Life Technologies) and 10% DMSO. The cells were aliquoted to cryogenic vials (Sarstedt) and stored at −80°C. Frozen specimens were transferred to a liquid nitrogen freezer and stored in the vapor phase. At the time of analysis, cryopreserved cells were thawed in a 37°C water bath, incubated with 20 µg/ml DNase (Roche), and washed twice. Viability was determined by trypan blue exclusion. Samples included for analysis had a viability of ≥ 80% (mean 88.8%, range 82 – 96%). Cryopreservation by this technique has been repeatedly used to successfully preserve mononuclear cells for future experiments, including intracellular staining and FACS analysis.(15, 16)
The following anti-human monoclonal antibodies (mAbs) were obtained from BD Immunocytometry Systems: anti-CD3 PE Cy5.5, anti-CD8 Pacific Orange, anti-CD4 Pacific Blue, anti-CD14 PE-Texas Red anti-CD19 PE-Texas Red, anti-CD25 APC-Cy7, anti-CCR7 PE-Cy7, anti-CD45RA PE-Cy5, anti-CD57 FITC, and anti-CD127 PE. The intracellular marker anti-FoxP3 APC was obtained from eBioscience (San Diego, CA, USA). An amine reactive live/dead viability dye (Invitrogen/Molecular Probes) was used as a dead cell exclusion marker, so that dead cells could be excluded from flow cytometric analysis (17).
Surface staining for phenotypic markers was performed with pre-titered, directly conjugated mAbs for 30 min at room temperature as described previously (18). In brief, approximately 2 × 106 PBMCs were resuspended in RPMI, washed twice with phosphate-buffered saline and incubated at room temperature in the dark for 30 minutes with appropriate mAbs. Samples were washed twice with cold FACS buffer (PBS supplemented with 1% bovine serum albumin and 0.1% sodium azide (NaN3)).
For intracellular staining with anti-FoxP3 monoclonal antibody, cells were prepared according to the manufacturer’s protocol (eBioscience, cat. 00-5523). Briefly, following a wash with cold FACS Buffer, the cells were incubated for 60 minutes at 4°C in the dark with freshly prepared fixation/permeabilization working solution and washed twice with permeabilization buffer. Permeabilization was followed by incubation with the fluorochrome conjugated anti-human FoxP3 antibody in permeabilization buffer for 4°C for 1 hour in the dark Following a wash with cold FACS Buffer, the cells were fixed in 0.5% paraformaldehyde diluted in PBS and stored in the dark at 4°C. Flow cytometric analysis was performed within 4 hours of preparation.
Multiparametric flow cytometry was performed using a 5-laser (355 nm, 405 nm, 488 nm, 532nm and 633 nm) BD LSRII cytometer (BD Immunocytometry Systems). Instrument compensation was performed with antibody capture beads (BD Pharmingen) stained singly with individual antibodies used in the test samples. For each sample, 50,000 CD3+CD8+ events were collected. Data analysis was performed using Flow Jo version 8.7.1 (Tree Star, Inc.).
Lung Tissue Analysis
Immunocytochemical analyses were performed on random formalin-fixed, paraffin embedded 5 µm thick sections of lung tissue from 11 patients with PAH and 11 control subjects. Serial tissue sections were reacted with CD3, CD8, CD19, CD23 and S100 (Dako North America, Inc., Carpinteria, CA), and CD4 (Novocastra, Leica, Bannockburn, IL) antibodies. Visualization was by biotin-streptavidin/horseradish peroxidase, counterstained with hematoxylin. Hematoxylin and eosin staining of each case also was performed. Morphometric assessment of the CD3+, CD4+ and CD8+ cell numbers was determined by counting the number of immunoreactive cells in the alveolar wall and its capillaries, and vessels with lumens less than 100 µm in 10 fields (taken at a magnification of ×20) and relating this number to the total number of nuclei in these same fields for each case (number of immunoreactive cells/total number of cells × 100). Alveolar macrophages and alveolar epithelial cells were not included in these counts.
Statistical Analysis
All cell counts are expressed as the mean ± standard deviation, or median values with inter-quartile ranges. To account for any non-normal data distributions, and for consistency of analysis, statistical significance was evaluated using nonparametric statistical techniques with two-sided testing, with a p value < 0.05 considered statistically significant. Specifically, comparisons of the percent of CD3+, CD4+, and CD8+ lymphocyte populations between the patient and control groups in the peripheral lung were made using the nonparametric Mann-Whitney U test (Wilcoxon rank-sum test). This test also was used to compare all lymphocyte markers in the blood analyzed by FACS between patient and control groups. Statistical analysis was performed using the statistical package SPSS for Windows (Version 16.0, SPSS Inc., Chicago, IL).
Results
Blood Lymphocytes and T Lymphocyte Subsets
We determined circulating T lymphocyte subsets in patients with IPAH compared to healthy control subjects. Anti-CD14 PE-Texas Red antibody was used to filter out macrophages and monocytes, which along with dead cells were excluded from analysis. Anti-CD19 PE-Texas Red identified B lymphocytes, which were not different between groups (IPAH = 12.7% ± 8.0, control = 8.2% ± 6.0, as a percent of total lymphocytes; p = 0.068). Analysis of CD3+ T cells revealed no significant differences between IPAH and control groups in terms of the percentage of CD4+ (IPAH = 61.5% ± 13.5, control = 59.1% ± 15.4 as a percent of total CD3+ T cells), CD8+ (IPAH = 26.7% ± 10.3, control = 29.7% ± 10.6 as a percent of total CD3+ T cells), and CD57+ (IPAH = 36.4% ± 20.5, control = 24.7% ± 16.8 as a percent of total CD8+ T cells) T cells. The CD4+ / CD8+ T cell ratio also did not significantly differ between the two groups (IPAH = 2.73 ± 1.4, control = 2.33 ± 1.3).
CD8+ T cells expressing the antigen protein tyrosine phosphatase’s RA form (CD45RA) but not the chemokine (C-C motif) receptor 7 (CCR7) were significantly more abundant in the IPAH group (IPAH = 17.0% ± 6.0, control = 12.1% ± 10.2 as a percent of total CD3+CD8+ T cells; p = 0.019) (Figure 1). These effector memory (CD45RA+CCR7-) T lymphocytes are believed to have stronger cytolytic activity and travel from blood to nonlymphoid tissues for a direct encounter with the tissue-based antigen to which they are primed (19, 20). In contrast, CD45RA+CCR7+ cells were significantly lower in the IPAH group (IPAH = 24.8% ± 10.7, control = 41.3% ± 14.7 as a percent of total CD3+CD8+ T cells; p = 0.001). CD45RA+CCR7+ cells are primarily naive T cells, unable to immediately release inflammatory cytokines, which migrate from the blood directly to secondary lymphoid tissues (19–21). There were no statistically significant differences in the two populations in terms of CD8+ T cells that were CD45RA-CCR7- (IPAH = 25.4% ± 8.4, control = 12.19.3% ± 9.8 as a percent of total CD3+CD8+ T cells; p = 0.097) and CD45RA-CCR7+ (IPAH = 30.2% ± 12.3, control = 27.4% ± 12.6 as a percent of total CD3+CD8+ T cells; p = 0.418).
Figure 1. Circulating blood CD8+ T lymphocyte subsets are abnormal in IPAH.
Circulating blood lymphocytes were analyzed by flow cytometry in IPAH and control subjects. Patients with IPAH have a significantly higher percentage of CD45RA+CCR7- CD8+ T lymphocytes (left box) and a significantly lower percentage of CD45RA+CCR7+ CD8+ T lymphocytes. (A) Box plots demonstrate the median value (line), and inter-quartile range for each group (grey boxplots are IPAH group and open boxplots are Control group; note that values in the text are given as mean ± S.D.). (B) Representative flow cytometric dot plots from an IPAH patient (left panel) and a healthy control (right panel). CD8+ T cells were gated based on CCR7 and CD45RA expression. The numbers in the corners indicate the percentages of cells within that particular quadrant.
Regulatory T cells were characterized based upon the expression of cell surface markers (CD3, CD4, CD25, and CD127) and intracellular expression of FoxP3. Co-expression of CD25 (the α-chain of the IL-2 receptor) and intracellular FoxP3 has classically been used to characterize Tregs (CD25+FoxP3+) (22). The use of CD127 (the α-chain of the IL-7 receptor) expression may provide a more sensitive and specific characterization of the circulating Treg population in peripheral blood (Treg-127low) (23).
Significant differences in the proportion of circulating Tregs were found in the IPAH versus control group (IPAH = 4.8% ± 2.6, controls=2.4% ± 1.5; p=0.004; Figure 2). Inclusion of CD127 during the analysis of Tregs further supported the significant differences between the groups, with a significantly higher proportion of the Tregs 127low cells in the IPAH group (IPAH=3.8% ± 2.3%, control=1.8% ± 1.1; p=0.003; Figure 2A). Consistent with this finding, the total population of CD4+ T cells with low expression of CD127 was significantly higher in the IPAH versus control group (IPAH = 35.4% ± 10.7, control = 22.8% ± 7.8; p < 0.001). Consistent with published reports that CD127 expression is inversely correlated with FoxP3 expression, among Treg cells, the proportion of Treg-CD127low cells was the same in each group (IPAH=76.5% ± 10.6, control= 75.4% ± 15.9).
Figure 2. Elevated circulating blood CD4+ regulatory T lymphocytes (Tregs) in IPAH.
(A) Circulating blood lymphocytes were analyzed by flow cytometry in IPAH and control subjects. Patients with IPAH have a significantly higher percentage of CD25+FoxP3+ Tregs and CD25+FoxP3+CD127low Tregs. Box plots demonstrate the median value (line), and inter-quartile range for each group (grey boxplots are IPAH group and open boxplots are Control group; note that values in the text are given as mean ± S.D.). (B) Representative dot plots from an IPAH patient and healthy control (upper panels). CD4+ T cells were gated based on CD25 and FoxP3 expression. The numbers indicate the percentages of CD25+FoxP3+ cells within the gated region. Histogram (lower panels) to illustrate low CD127 expression on Tregs (grey) compared to total CD4+ T cell population (black).
Lymphocytes in Peripheral Lung
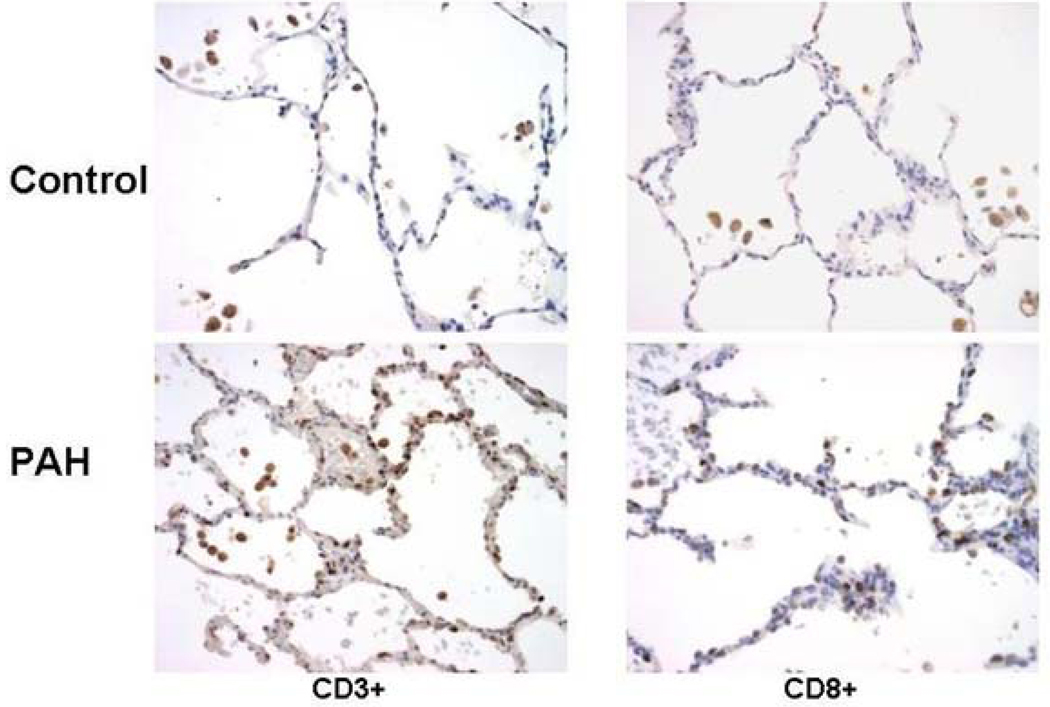
Initial microscopic analysis of the lung sections revealed increased numbers of CD3+ and CD8+ cells in the peripheral lung of the PAH samples compared to controls. Counts of immunoreactive cells related to total number of nuclei revealed an 4.5-fold increase in CD3+ cells in the PAH samples compared to controls (PAH = 4.93% ± 2.60, control = 0.76% ± 0.70 as a percent of total nuclei, p<0.01) (Figure 3). CD8+ cells were numerous and showed a 4.4-fold increase above controls (PAH = 2.09% ± 1.20, control = 0.69% ± 0.40, p<0.01) (Figure 3); CD4+ T cells were rare in the peripheral lung (PAH =1.5% ± 1.89, control = 0.55% ± 0.54). Like the CD4+ cells, CD19+ B cells and CD56+ natural killer cells were rarely seen in peripheral lung. Areas and numbers of bronchial associated lymphoid tissues were increased in the PAH cases although the distribution of lymphocyte subsets in these regions was normal. Increased numbers of lymphocytes also were sometimes apparent around remodeled arteries but no one subset was consistently responsible for this increase.
Figure 3. Elevated CD3+ and CD8+ lymphocytes in the peripheral lung of patients with PAH compared to controls.
(A) Counts of lymphocyte subsets related to total number of nuclei per field in the peripheral lung of PAH patients (grey blocks) and controls (open blocks). Box plots demonstrate the median value (line), and inter-quartile range for each group; * p<0.01 compared to controls. (B) Peripheral lung from a patient with PAH and a control showing increased numbers of CD3+ and CD8+ lymphocytes in the PAH patient. Line in lower right micrograph = 100 µm.
Discussion
This study demonstrates significant alterations in lymphocyte subsets in blood and lung tissue of patients with PAH compared to healthy controls. In the blood, CD19+ B cells were not different, and analysis of CD3+ T cells revealed no differences in the percentages of CD4+, CD8+ and CD57+ T cells between the two groups. However, characterization of circulating blood CD8+ T lymphocytes revealed a significant increase in CD45RA+CCR7- T lymphocytes (effector memory cells with enhanced cytolytic activity to peripheral antigens) and a significant decrease in CD45RA+CCR7+ T lymphocytes (naïve cells deficient in immediate cytokine production properties which home to secondary lymphoid organs) in IPAH samples compared to controls. Our results also demonstrate a significant increase in circulating Tregs in patients with IPAH as compared to controls. Peripheral lung tissue shows significantly elevated numbers of CD3+ and CD8+ lymphocytes in patients with PAH compared to controls.
In humans, the association of PAH with autoimmune diseases and diseases of immune dysfunction such as HIV and POEMS Syndrome (Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal immunoglobulin, Skin changes), suggests a role for inflammatory dysregulation and autoimmunity in PAH (9, 24–26). The current study identified subsets of circulating CD8+ T lymphocytes that are abnormal in IPAH. Effector memory T lymphocytes, which posses an immunophenotype of differentiated memory cells with potent cytolytic activity that home to inflamed peripheral tissues, are elevated in IPAH patients compared to controls. In contrast, quiescent CD8+ T lymphocytes are depressed in these patients as compared to controls (20, 27). Thus, the CD8+ T lymphocyte subset balance appears shifted in favor of cytotoxic effector-memory cells primed to peripheral antigens.
The current study confirms and more precisely characterizes the immunophenotype of Tregs described as elevated in PAH by Ulrich and colleagues, suggesting altered immune control by CD4+ T lymphocytes (12). Elevated Tregs are seen in other inflammatory diseases with and without pulmonary manifestations, at levels of comparison to healthy controls similar to the findings in this study (28). For example, in severe pulmonary sarcoidosis, Tregs accumulate in the peripheral blood, pulmonary granulomas, and bronchoalveolar lavage fluid. Furthermore, Tregs are expanded in the blood and lungs of patients with active pulmonary tuberculosis, at percentages of comparison (2.5-fold elevation) within the CD4+ T cell populations between healthy controls and tuberculosis patients virtually identical to the findings in this study comparing healthy controls to IPAH patients (29). In sarcoidosis, pulmonary tuberculosis, and IPAH, this may reflect a global Treg amplification aimed at controlling (unsuccessfully) local lung inflammation and a loss of self tolerance (30). While the identification of cells with surface and intracellular markers consistent with a particular immunophenotype contributes to the overall understanding of the contribution of immune cells to IPAH, we recognize that further studies are needed to characterize the true function of these cells. It is not clear whether these cells cause disease, or are biologic markers reflecting an ongoing disease process.(31–34).
Inflammatory cells, in particular lymphocytes, have been noted previously in the lungs of IPAH patients. For example, Tuder and colleagues noted perivascular inflammatory cell infiltrates consisting of T and B lymphocytes and macrophages exclusively around plexiform lesions (2). Bonnet et al reported CD3+ T lymphocytes in the walls of resistance arteries in IPAH patients; the majority of the cells showed NFATc2 (a nuclear Ca2+/calcineurin-sensitive transcription factor found in T lymphocytes) activation (35). Further, dendritic cells have been identified in the walls of muscular pulmonary arteries in patients with IPAH and an experimental model of monocrotaline-induced PAH (36). RANTES (regulated upon activation, normal T-cell expressed and secreted (CC chemokine ligand 5 [CCL5]), a critical regulator of activated T cell homing and migration, is elevated in the vascular lesions of PAH patients (37, 38). Animal studies also implicate the lymphocyte in the pathogenesis of PAH. Athymic rats lacking T cells have been shown to develop pulmonary vascular changes, right ventricular hypertrophy and an increase in pulmonary artery pressure (11). Prolonged and intermittent exposure to aerosolized OVA antigen in mice has been linked to the development of PAH, a response that is suppressed in CD4+ T lymphocyte depleted mice (39). Thus, there is ample evidence for a link between lymphocytes and the pathophysiology of PAH. Importantly, the increases in peripheral lung CD3+ and CD4+ T cells found in this study are consistent with elevations in lung lymphocytes reported for other lung diseases with an inflammatory etiology, such as posttransplant rejection for lung recipients (40).
The present study demonstrates an increase in CD3+ and CD8+ lymphocytes in the peripheral lung of patients with PAH compared to controls. As far as we are aware this is the first assessment of lymphocyte subsets in the peripheral lung of PAH patients. Our finding suggests that the localization of lymphocytes in the lungs of PAH patients is not limited to the regions of the remodeled arteries and plexiform lesions. Ulrich and colleagues reported a reduction in total CD8+ T lymphocytes in the blood of IPAH patients and although we were unable to confirm this, it is tempting to speculate that the increase in peripheral lung CD8+ cells in our study reflects their reduction in circulating blood (12). Cytotoxic T lymphocytes have been linked to various organ-specific autoimmune diseases such as Behcet’s disease, ankylosing spondylitis, type 1 diabetes mellitus and allogeneic pancreatic islet cell graft failure (23, 41–43). In the lung, respiratory infections result in cytotoxic CD8+ T lymphocyte migration from the vascular space into the lung tissue to promote a local inflammatory response, responding to RANTES activity (37, 44). Furthers studies are needed to prove whether PAH is also driven by an organ-specific autoimmune attack.
One hundred percent and 78 percent of patient samples for the tissue and blood analyses, respectively, were receiving some form of prostanoid therapy at the time of study. While this study was not powered for subset analyses, data from the subset of subjects receiving therapy were indistinguishable from data of those subjects not receiving prostanoid therapy in terms of lung tissue analyses (data not shown). Furthermore, there was a significant elevation of CD3+, CD4+ and CD8+ cells in the peripheral lung of patients with PAH not receiving prostanoid therapy compared to controls, although the magnitude of the increase is not as marked (a significant two-fold increase was found; data not shown). Thus, while prostanoid therapy may exacerbate the lymphocyte infiltration, it does not appear to account entirely for this change. Since our circulating lymphocyte studies did not include any known cases of heritable PAH, future studies will investigate subjects with this disease, as it is conceivable that changes in lymphocyte subsets could be a biologic marker for early disease in asymptomatic subjects at known genetic risk to develop disease. In addition, because our tissue and circulating blood investigations were not performed on the same subjects, we cannot definitively conclude that the findings in the lung tissue and circulating blood will be concordant in each individual. However, as a rare disease, there is a paucity of available lung tissue for study; also, lung biopsy is not recommended in living patients, making a comprehensive comparison of circulating blood and lung tissue within the same patient extremely difficult. Finally, future studies should work to characterize the circulating lymphocytes, so as to further understand the abnormal lymphocyte milieu, as well as explore any functional alterations in these lymphocytes attributable to current PAH medications.
In summary, we evaluated circulating blood lymphocytes and peripheral lung tissue to determine whether alterations in lymphocyte subsets were associated with the pathophysiology of PAH. Patients with IPAH have an imbalance of circulating CD8+ T cell subsets, with a preponderance of cytotoxic, differentiated, peripheral effector-memory cells known to migrate into peripheral tissues and fewer CD8+ T cells less prone to home to peripheral tissues. We also found a higher proportion of circulating CD4+ Tregs in patients with IPAH, suggesting a systemic response attempting to suppress peripheral pro-inflammatory activity. Further, patients with IPAH and heritable PAH exhibit an abnormally elevated number of CD3+ and CD8+ cells in the peripheral lung. These findings provide novel information about alterations in the lymphocyte milieu, advancing the concept that exuberant inflammation, due to abnormal immune function and a loss of self-tolerance, contributes to the pathophysiology of PAH.
Acknowledgements
We are grateful to the Vanderbilt University Medical Center (VUMC) Flow Cytometry Shared Resource for our flow cytometry measurements. The VUMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Supported by grants from the Cardiovascular Medical Research and Education Fund, National Institutes of Health (P01 HL072058 and NIH K12 RR1 7697), and Vanderbilt University Medical Center (GCRC RR000095).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST NOTIFICATION PAGE: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004 Jun 16;43(12 Suppl S):5S–12S. doi: 10.1016/j.jacc.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 2.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol. 1994 Feb;144(2):275–285. [PMC free article] [PubMed] [Google Scholar]
- 3.Steen VD, Lucas M, Fertig N, Medsger TA., Jr. Pulmonary arterial hypertension and severe pulmonary fibrosis in systemic sclerosis patients with a nucleolar antibody. J Rheumatol. 2007 Nov;34(11):2230–2235. [PubMed] [Google Scholar]
- 4.Humbert M, Monti G, Brenot F, Sitbon O, Portier A, Grangeot-Keros L, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med. 1995 May;151(5):1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 5.Otterdal K, Andreassen AK, Yndestad A, Oie E, Sandberg WJ, Dahl CP, et al. Raised LIGHT levels in pulmonary arterial hypertension: potential role in thrombus formation. Am J Respir Crit Care Med. 2008 Jan 15;177(2):202–207. doi: 10.1164/rccm.200703-506OC. [DOI] [PubMed] [Google Scholar]
- 6.Tamby MC, Chanseaud Y, Humbert M, Fermanian J, Guilpain P, Garcia-de-la-Pena-Lefebvre P, et al. Anti-endothelial cell antibodies in idiopathic and systemic sclerosis associated pulmonary arterial hypertension. Thorax. 2005 Sep;60(9):765–772. doi: 10.1136/thx.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamby MC, Humbert M, Guilpain P, Servettaz A, Dupin N, Christner JJ, et al. Antibodies to fibroblasts in idiopathic and scleroderma-associated pulmonary hypertension. Eur Respir J. 2006 Oct;28(4):799–807. doi: 10.1183/09031936.06.00152705. [DOI] [PubMed] [Google Scholar]
- 8.Barst RJ, Loyd JE. Genetics and immunogenetic aspects of primary pulmonary hypertension. Chest. 1998 Sep;114(3 Suppl):231S–236S. doi: 10.1378/chest.114.3_supplement.231s. [DOI] [PubMed] [Google Scholar]
- 9.Nicolls MR, Taraseviciene-Stewart L, Rai PR, Badesch DB, Voelkel NF. Autoimmunity and pulmonary hypertension: a perspective. Eur Respir J. 2005 Dec;26(6):1110–1118. doi: 10.1183/09031936.05.00045705. [DOI] [PubMed] [Google Scholar]
- 10.Mouthon L, Guillevin L, Humbert M. Pulmonary arterial hypertension: an autoimmune disease? Eur Respir J. 2005 Dec;26(6):986–988. doi: 10.1183/09031936.05.00112105. [DOI] [PubMed] [Google Scholar]
- 11.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, Scerbavicius R, Burns N, Cool C, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med. 2007 Jun 15;175(12):1280–1289. doi: 10.1164/rccm.200608-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich S, Nicolls MR, Taraseviciene L, Speich R, Voelkel N. Increased regulatory and decreased CD8+ cytotoxic T cells in the blood of patients with idiopathic pulmonary arterial hypertension. Respiration. 2008;75(3):272–280. doi: 10.1159/000111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogan JD, Pauciulo MW, Batchman AP, Prince MA, Robbins IM, Hedges LK, et al. High Frequency of BMPR2 Exonic Deletions/Duplications in Familial Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2006 May 25; doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sztrymf B, Yaici A, Girerd B, Humbert M. Genes and pulmonary arterial hypertension. Respiration. 2007;74(2):123–132. doi: 10.1159/000098818. [DOI] [PubMed] [Google Scholar]
- 15.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996 Apr;36(4):303–308. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 16.Axelsson S, Faresjo M, Hedman M, Ludvigsson J, Casas R. Cryopreserved peripheral blood mononuclear cells are suitable for the assessment of immunological markers in type 1 diabetic children. Cryobiology. 2008 Dec;57(3):201–208. doi: 10.1016/j.cryobiol.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, et al. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J Immunol Methods. 2006 Jun 30;313(1–2):199–208. doi: 10.1016/j.jim.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Rock MT, Yoder SM, Wright PF, Talbot TR, Edwards KM, Crowe JE., Jr. Differential regulation of granzyme and perforin in effector and memory T cells following smallpox immunization. J Immunol. 2005 Mar 15;174(6):3757–3764. doi: 10.4049/jimmunol.174.6.3757. [DOI] [PubMed] [Google Scholar]
- 19.Ebert LM, Schaerli P, Moser B. Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues. Mol Immunol. 2005 May;42(7):799–809. doi: 10.1016/j.molimm.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 20.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007 Apr 1;178(7):4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 21.Lewis M, Tarlton JF, Cose S. Memory versus naive T-cell migration. Immunol Cell Biol. 2008 Mar–Apr;86(3):226–231. doi: 10.1038/sj.icb.7100132. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006 Aug;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 23.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008 Jun;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 24.Lesprit P, Godeau B, Authier FJ, Soubrier M, Zuber M, Larroche C, et al. Pulmonary hypertension in POEMS syndrome: a new feature mediated by cytokines. Am J Respir Crit Care Med. 1998 Mar 157;(3 Pt 1):907–911. doi: 10.1164/ajrccm.157.3.9707095. [DOI] [PubMed] [Google Scholar]
- 25.Jouve P, Humbert M, Chauveheid MP, Jais X, Papo T. POEMS syndrome-related pulmonary hypertension is steroid-responsive. Respir Med. 2007 Feb;101(2):353–355. doi: 10.1016/j.rmed.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 26.Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003 Aug;22(2):358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 27.Fearon DT, Manders P, Wagner SD. Arrested differentiation, the self-renewing memory lymphocyte, and vaccination. Science. 2001 Jul 13;293(5528):248–250. doi: 10.1126/science.1062589. [DOI] [PubMed] [Google Scholar]
- 28.Perros F, Cohen-Kaminsky S, Humbert M. Understanding the role of CD4+CD25(high) (so-called regulatory) T cells in idiopathic pulmonary arterial hypertension. Respiration. 2008;75(3):253–256. doi: 10.1159/000114655. [DOI] [PubMed] [Google Scholar]
- 29.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006 Apr 1;173(7):803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 30.Miyara M, Amoura Z, Parizot C, Badoual C, Dorgham K, Trad S, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med. 2006 Feb 20;203(2):359–370. doi: 10.1084/jem.20050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luttmann W, Herzog V, Matthys H, Thierauch KH, Virchow JC, Kroegel C. Modulation of cytokine release from mononuclear cells by prostacyclin, IL-4 and IL-13. Cytokine. 1999 Feb;11(2):127–133. doi: 10.1006/cyto.1998.0410. [DOI] [PubMed] [Google Scholar]
- 32.Luttmann W, Herzog V, Virchow JC, Jr., Matthys H, Thierauch KH, Kroegel C. Prostacyclin modulates granulocyte/macrophage colony-stimulating factor release by human blood mononuclear cells. Pulm Pharmacol. 1996 Feb;9(1):43–48. doi: 10.1006/pulp.1996.0005. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Blackwell TS, Goleniewska K, O'Neal JF, Fitzgerald GA, Lucitt M, et al. Prostaglandin I2 analogs inhibit Th1 and Th2 effector cytokine production by CD4 T cells. J Leukoc Biol. 2007 Mar;81(3):809–817. doi: 10.1189/jlb.0606375. [DOI] [PubMed] [Google Scholar]
- 34.Zhou W, Hashimoto K, Goleniewska K, O'Neal JF, Ji S, Blackwell TS, et al. Prostaglandin I2 analogs inhibit proinflammatory cytokine production and T cell stimulatory function of dendritic cells. J Immunol. 2007 Jan 15;178(2):702–710. doi: 10.4049/jimmunol.178.2.702. [DOI] [PubMed] [Google Scholar]
- 35.Bonnet S, Rochefort G, Sutendra G, Archer SL, Haromy A, Webster L, et al. The nuclear factor of activated T cells in pulmonary arterial hypertension can be therapeutically targeted. Proc Natl Acad Sci U S A. 2007 Jul 3;104(27):11418–11423. doi: 10.1073/pnas.0610467104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perros F, Dorfmuller P, Souza R, Durand-Gasselin I, Mussot S, Mazmanian M, et al. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur Respir J. 2007 Mar;29(3):462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 37.Galkina E, Thatte J, Dabak V, Williams MB, Ley K, Braciale TJ. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J Clin Invest. 2005 Dec;115(12):3473–3483. doi: 10.1172/JCI24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dorfmuller P, Zarka V, Durand-Gasselin I, Monti G, Balabanian K, Garcia G, et al. Chemokine RANTES in severe pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002 Feb 15;165(4):534–539. doi: 10.1164/ajrccm.165.4.2012112. [DOI] [PubMed] [Google Scholar]
- 39.Daley E, Emson C, Guignabert C, de Waal Malefyt R, Louten J, Kurup VP, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008 Feb 18;205(2):361–372. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tavora F, Drachenberg C, Iacono A, Burke AP. Quantitation of T lymphocytes in posttransplant transbronchial biopsies. Hum Pathol. 2009 Apr;40(4):505–515. doi: 10.1016/j.humpath.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Blanco P, Pitard V, Viallard JF, Taupin JL, Pellegrin JL, Moreau JF. Increase in activated CD8+ T lymphocytes expressing perforin and granzyme B correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Rheum. 2005 Jan;52(1):201–211. doi: 10.1002/art.20745. [DOI] [PubMed] [Google Scholar]
- 42.Blanco P, Viallard JF, Pellegrin JL, Moreau JF. Cytotoxic T lymphocytes and autoimmunity. Curr Opin Rheumatol. 2005 Nov;17(6):731–734. doi: 10.1097/01.bor.0000179942.27777.f8. [DOI] [PubMed] [Google Scholar]
- 43.Campbell PD, Estella E, Dudek NL, Jhala G, Thomas HE, Kay TW, et al. Cytotoxic T-lymphocyte-mediated killing of human pancreatic islet cells in vitro. Hum Immunol. 2008 Jul 16; doi: 10.1016/j.humimm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Ely KH, Cauley LS, Roberts AD, Brennan JW, Cookenham T, Woodland DL. Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol. 2003 Feb 1;170(3):1423–1429. doi: 10.4049/jimmunol.170.3.1423. [DOI] [PubMed] [Google Scholar]