Abstract
The study of functionally relevant biological effects of serotonin transporter gene promoter region (5-HTTLPR) polymorphisms is especially important given the current controversy about the clinical relevance of these polymorphisms. Here we report an intrinsic immunobiological difference between individuals carrying two short (SS) versus long (LL) 5-HTTLPR alleles, that is observed in healthy subjects reporting low exposure to life stress. Given that 5-HTTLPR polymorphisms are thought to influence susceptibility to depression and are associated with robust neurobiological effects, that depression is associated with higher pro-inflammatory and lower anti-inflammatory cytokines, and that acute stressors increase circulating concentrations of pro-inflammatory cytokines, we hypothesized that compared to LL-individuals, SS-individuals may show a pro-inflammatory bias under resting conditions and/or during stress. 15-LL and 11-SS-individuals participated in the Trier Social Stress Test (TSST). Serum IL-6 and IL-10 were quantified at baseline and 30, 60, 90, and 120 minutes after beginning the 20-minute stress test. Compared to LL-individuals, SS-individuals showed a higher IL-6/IL-10 ratio at baseline and during stress. Importantly, this pro-inflammatory bias was observed despite both groups being healthy, reporting similar intensities of stress and negative emotionality during the TSST, and reporting similar low exposures to early and recent life stress. To our knowledge, this is the first report of a pro-inflammatory bias/phenotype in individuals carrying the SS genotype of 5-HTTLPR. Thus, healthy SS-individuals may be chronically exposed to a pro-inflammatory physiological burden under resting and stress conditions, which could increase their vulnerability to disorders like depression and other diseases that can be facilitated/exacerbated by a chronic proinflammatory state.
Keywords: depression, risk factors for depression, cytokine, IL-6, IL-10, immunomodulation, serotonin transporter promoter polymorphism, 5-HTTLPR, inflammation, psychosocial stress
Introduction
With a lifetime prevalence of over 15% (Schatzberg, 2005), major depressive disorder (MDD) is one of the most common, and most devastating, of psychiatric disorders. Stressful life events, especially those that involve loss or a threat to social standing, are clearly associated with MDD onset (Brown and Harris, 1978; Kendler et al., 1995). However, most individuals faced with such stressors recover without becoming depressed. Therefore, an important question that remains to be answered is: What makes some people more vulnerable than others? Twin studies show that one strong predictor of who will develop MDD after a stressful life event is the presence of a depressed identical twin (Kendler et al., 1995). These studies support the hypothesis that genetic factors modulate our vulnerability to life stressors, and recent work has identified genes that may confer vulnerability to depression after stressor exposure.
One such gene is the serotonin transporter, SLC6A4. A functional polymorphism in the promoter region of this gene (5-HTTLPR) results in a “short” allele variant (SS), with decreased mRNA for the presynaptic serotonin transporter, and a corresponding 50% decrease in serotonin reuptake (Lesch et al., 1996; Richell et al., 2005). Studies have shown that although SS individuals may be no more likely than others to be diagnosed with depression overall (Munafo et al., 2009), they may be at increased risk of developing depression after stressful life events (Caspi et al., 2003), or chronic illness (Otte et al., 2007). Attempts to replicate these findings have yielded both positive and negative results, and a recent, highly publicized meta-analysis concluded that the moderation may not exist at all (Risch et al., 2009). However, many attempts at replication of the original findings (Caspi et al., 2003) have been confounded by inconsistent assessment of early and recent life stressors (Monroe and Reid, 2008). Studies relying on structured interviews to assess the level of exposure to life stressors have consistently yielded positive findings, while most negative studies have used self-report questionnaires (Uher and McGuffin, 2007), which have been shown to have substantially less validity and reliability (Dohrenwend, 2006). A large body of research suggests that the 5-HTTLPR SS genotype may be a marker for stress-sensitivity more broadly: For example, SS individuals are more likely to attempt suicide, develop PTSD, or abuse substances after life stress (Roy et al., 2007; Kilpatrick et al., 2007; Brody et al., 2009). The association between serotonin transporter deficiency and stress-sensitivity also holds true in studies of both non-human primates and rodents. Infant macaques who carry the rhesus 5-HTTLPR S allele show higher emotional distress, more agitation, and an exaggerated HPA response to maternal separation (Champoux et al., 2002; Barr et al., 2004), while serotonin transporter deficient mice consistently show an anxious phenotype, with exaggerated neuroendocrine and behavioral startle responses to stressful stimuli (reviewed in Monroe et al., 2008).
Rather than focusing solely on specific negative health outcomes (such as depression) that might result from a long-term pattern of stress-sensitivity, recent research has sought to understand the physiologic mechanisms by which 5-HTTLPR associated health-vulnerability might be conferred. Studies have confirmed that the S-allele is associated with robust increases in amygdala reactivity (Hariri et al., 2005; Munafo et al., 2008), as well as with decreased cingulate-amygdala functional coupling (Pezawas et al., 2005). This evidence suggests that downstream effects of the S-allele, such as increased risk of depression after life stressors, might be preceded by stereotypic alterations in brain physiology. Here we explore psychological stress-related inflammation as another avenue by which the short allele might exert physiological effects that might, downstream, increase vulnerability to post-stressor depression.
Enhanced inflammation during acute or short-term stress responses is likely to have adaptive effects by increasing immuno-protection to defend the organism from the actions of the stressor (e.g. wound inflicted by a predator) (Dhabhar, 2009). Healthy individuals show elevated inflammatory cytokines during and following exposure to stress (Altemus et al., 2001; Steptoe et al., 2007). However, vulnerable individuals, such as those suffering from depression, may exhibit exaggerated inflammatory responses during stress (Pace et al., 2006). This is thought to induce excessive, and potentially detrimental pro-inflammatory physiological changes that contribute to the development of pathology. Several converging lines of evidence suggest that depression and systemic inflammation are linked (Pollak & Yirmiya, 2002; Dantzer et al., 2008; Miller et al., 2009; Raison et al., 2009). First, individuals with major depression have higher levels of circulating inflammatory markers, including pro-inflammatory cytokines, than non-depressed individuals (Tsao et al., 2006; Dantzer et al., 2008; Capuron et al., 2008; Cizza et al., 2008). These elevations are found in both serum and cerebrospinal fluid (CSF), have been associated with a history of treatment non-responsiveness (Maes et al., 1997), and are known to decrease following successful treatment with SSRI (Kenis and Maes, 2002; Dantzer et al., 2008). Second, non-depressed individuals given pro-inflammatory cytokines, both in experimental and therapeutic contexts, develop depressive symptoms (Reichenberg et al., 2001; Capuron et al., 2000; Miller, 2009; Miller et al., 2009; Raison et al., 2009). 20–50% of individuals who receive long-term interferon therapy develop clinical depression, and such depressive episodes are responsive to SSRI treatment (Capuron et al., 2002a). Third, new lines of evidence suggest that depression may be associated not only with increases in inflammatory cytokines such as IL-6, but also with impaired production of anti-inflammatory cytokines such as IL-10. In healthy individuals, there is a regulated balance between pro- and anti-inflammatory cytokines. For example, IL-6 mediates the early phase of the inflammatory process, and then induces the release of IL-10 that exerts immuno-regulatory effects and resolves inflammation (Daftarian et al., 1996; Ogawa, Duru, & Ameredes, 2008). It has been hypothesized that this regulatory loop is disrupted in individuals with depression, with one recent study showing both decreased IL-10 and an increased ratio of IL-6 to IL-10 in depressed individuals (Dhabhar et al., 2009), resulting in an overall bias towards inflammation. Despite these promising lines of evidence, no investigation has examined whether currently healthy individuals who may have a genetic predisposition towards depression after life stressors (such as SS homozygotes) show an abnormal inflammatory response to stress, with systemic inflammatory dysfunction preceding, or contributing to, overt psychiatric symptoms.
In the present study, we examined differences between healthy SS and LL individuals in the balance between pro- versus anti-inflammation under resting state and acute stress conditions. We chose the Trier Social Stress Test (TSST) (Kirschbaum et al., 1993) to induce acute stress because this test creates a controlled “real world” situation that is psychosocially threatening, inducing the sort of social threat noted by Kendler et al. (1995) to be particularly relevant to depression risk in genetically vulnerable subjects. We chose the ratio of circulating IL-6/IL-10 as an index of the balance between pro- and anti-inflammatory factors because it has recently been suggested that an important and relatively under-appreciated mechanism for increased susceptibility to depression and other diseases related to chronic inflammation may be a disruption of the immuno-regulatory balance between pro- and anti-inflammatory cytokines (Dhabhar et al., 2009). Such an immune balance can be shifted towards an inflammatory physiological milieu due to increased concentrations of pro-inflammatory cytokines, such as IL-6, decreased concentrations of anti-inflammatory/ immunomodulatory cytokines, such as IL-10, or a combination of the two. Importantly, there may be significant inter-individual differences in the absolute concentrations of pro- or anti-inflammatory factors, many of which exert their opposing effects on the same target cells and tissues. Therefore, examining the ratio of pro- to anti-inflammatory factors provides a useful measure of the net immunological effect that the circulating cytokines and/or other factors are likely to have on their targets, of the overall immune bias of an individual’s physiological milieu, and of immune dysregulation (Dhabhar et al., 2009). Moreover, the balance of pro- and anti-inflammatory cytokines at baseline and immediately after acute stress may be indicative of the body's ability to return to homeostasis after inflammation. Therefore, we measured circulating concentrations of IL-6, IL-10, and the IL-6/IL-10 ratio under relaxed resting state conditions and over time after the TSST, expecting that SS homozygotes would demonstrate an exaggerated inflammatory milieu under resting conditions, increased inflammatory reactivity during acute stress, or both.
Materials and Methods
Participants
Participants were 30 healthy, Caucasian women aged 18–25 (mean age: 21.7, SD 2.9) recruited via online advertisements in the San Francisco Bay Area. All potential participants were screened using a phone interview based on the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996). Eligible participants had no indication of any psychiatric disorder within the past year, no substance abuse within the past six months, no more than minimal depressive symptoms (Beck Depression Inventory–II (BDI, Beck et al., 1996) score < 14 (minimal range)), and no more than one past depressive episode. In addition, participants with a lifetime history of other major psychiatric illnesses were excluded. Participants endorsed no chronic or recent acute medical illnesses, took no prescription medications (other than hormonal contraceptives), were non-smokers, and were not obese (BMI 17.83 – 28.95; mean 22.4, SD 2.5).
We chose a population of healthy young individuals, homozygous for either the short or the long allele for the serotonin transporter, and excluded individuals with the less common "g" variant within the SNP rs75531 within 5-HTTLPR, given that the presence of this variant may be associated with lower transcriptional activity even in LL individuals (Hu et al., 2006). We included only subjects who were psychiatrically and medically healthy, to avoid confounds with psychiatric and medical illness. We included only female participants in order to maximize homogeneity of emotional responses (Kelly et al., 2008), and because women are twice as likely to develop major depressive disorder as men (APA, 1994). Only Caucasian individuals were studied in order to control for confounding effects of ethnic heterogeneity. Given the possible psychiatric and inflammatory effects of both recent and early life stressors, all participants completed questionnaires designed to assess both recent and early life stressors, and genotype groups were matched based on these measures.
Procedure
Following the phone interview, eligible participants came to the laboratory, provided informed consent, completed a variety of questionnaires and tasks, and provided a saliva sample for DNA analysis. Subjects homozygous for the short or long 5-HTTLPR genotype were invited to participate in the current study. Interested subjects underwent a second phone interview to assess for any new medical or psychiatric conditions and completed a short battery of online questionnaires. The Life Events Scale for Students (LESS, Linden, 1984) was used to assess recent life stress, and the Childhood Trauma Questionnaire (CTQ, Bernstein et al., 2003) was used to assess early life stress, in order to control for potential confounds of differences in exposure.
Thirty subjects elected to participate and were scheduled for a study session at the Stanford Hospital General Clinical Research Center (GCRC). All subjects were scheduled to arrive at 1 pm, and each baseline blood draw was performed at 2 pm, in order to control for circadian effects on cytokine levels. Most subjects were taking oral contraceptive medications; those who were not were scheduled to participate during the follicular phase of their menstrual cycle, given that prior studies have shown that women on OCPs and follicular phase women have similar free cortisol responses after TSST (Kirschbaum et al., 1999). Subjects were instructed to refrain from caffeinated beverages and heavy exercise on the day of the study session and to eat a light lunch, with no food starting an hour before study session start, to control for effects of blood glucose on variables of interest.
Upon arrival at the GCRC, each subject provided informed consent and completed baseline questionnaires to assess current emotional state, including the State Shame & Guilt Scale (SSGS, Marschall et al., 1994), the Russell Affect Grid (RAG, Russell et al., 1989), and the Subjective Units of Disturbance Scale (SUDS, Wolpe, 1969). A GCRC nurse obtained vitals and BMI and placed an indwelling IV catheter in the non-dominant arm. Subjects were then encouraged to rest comfortably while they viewed a relaxing nature video (Fothergill, 2006) for 30 minutes. The baseline blood sample was collected after this 30-minute rest period to control for any inflammatory response to venipuncture, as well as to help control for varying levels of stress and physiologic arousal in subjects upon arrival.
Stressor Protocol
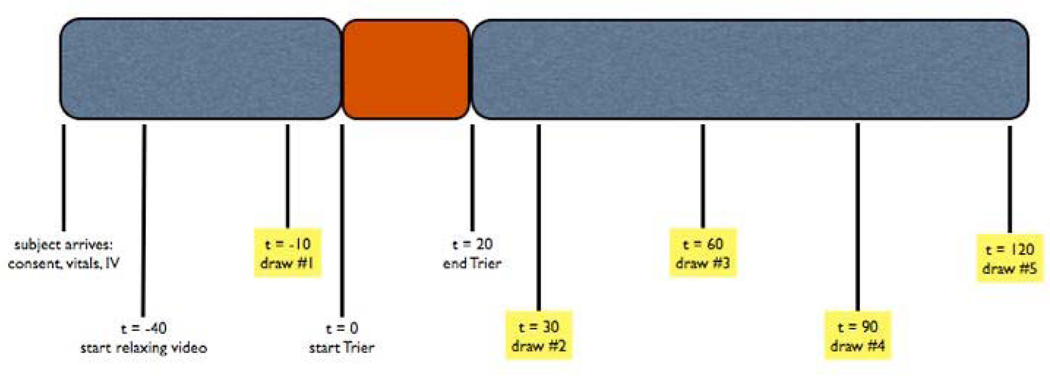
Ten minutes after the first blood draw, at 2 pm, participants began the TSST. For the first five minutes of the task, subjects read a printed job announcement and were instructed to imagine they were here to interview for the advertised job, which they would very much like to have. When subjects were finished reading, a panel of two researchers entered the room, one male and one female, both wearing lab coats. These panelists briefly explained the format of the experimental task, then left subjects alone for five more minutes to prepare a speech about their qualifications for the job. At ten minutes, the panelists returned, switched on a video camera aimed at the subject, visibly started a stop-watch, and instructed subjects to begin their speech. Panelists spoke their instructions in flat voices and provided as little non-verbal feedback as possible as subjects underwent the five-minute public speaking task, followed by five minutes of challenging mental arithmetic. After completing the TSST, subjects remained seated and completed another set of questionnaires. Subjects then continued to sit comfortably for 120 minutes and were invited to continue watching the nature video, watch TV, or do light reading. The baseline blood sample was drawn at t=−10 relative to TSST start; subsequent blood samples were drawn at 30, 60, 90, and 120 minutes after the start of the TSST (Fig. 1).
Figure 1.
Study Timeline.
Genotyping Procedure
DNA was amplified with primers 5’-TCCTCCGCTTTGGCGCCTCTTCC and 5’-TGGGGGTTGCAGGGGAGATCCTG as previously described (Wendland et al., 2006). This process generated two alleles: 469bp(S) or 512bp(L). PCR products were digested by 5 units of HpaII (New England Biolabs). The digestion mix was electrophoresed side by side with the undigested PCR product on a 3% Agarose gel. Digestion and PCR product sizes were: S(A)-469bp uncut and cut; S(G)- 469 uncut and 402+67bp cut; L(A)-512bp uncut and cut; L(G)-512 uncut and 402+110bp cut.
Cytokine Quantification
Blood samples were collected from an indwelling venous catheter for immunological analyses at all five timepoints. Whole blood was collected into 10ml SST tubes (Becton Dickinson, Franklin Lakes, NJ). Serum was allowed to clot for 30min at RT, then spun (1300 rpm for 15min), frozen, and stored at −75 C for subsequent cytokine quantification. A high sensitivity multiplexed sandwich immunoassay was used to quantify IL-6 and IL-10 concentrations (Mesoscale Discovery, Gaithersburg, MD). For IL-6, assay sensitivity is 0.6 pg/ml, and average intra- and inter- assay coefficients of variation are 6.2% and 5.6% respectively. For IL-10, assay sensitivity is 0.5 pg/ml, and average intra- and inter-assay coefficients of variation are 8.3% and 13.1% respectively. All measurements were performed by a trained technician without knowledge of the experimental conditions.
Statistical Analyses
Analyses were conducted using SPSS statistical software package version 17.0 (SPSS Inc., Chicago, IL). All tests of significance were two-tailed with an alpha level of p<0.05. Cohen’s d was used as a measure of effect size. Area under the curve analyses were conducted using methods described by Pruessner et al. (2003). We first analyzed the main effects of the task on the IL-6/IL-10 ratio, as well as its components (plasma IL-6 and plasma IL-10) by comparing the area under the curve (AUC) with respect to baseline (AUC-I) to zero. Next, we examined the effect of genotype on the IL-6/IL-10 ratio, IL-6, and IL-10. To assess baseline differences, we compared baseline cytokine values between groups. To assess for differences in reactivity to the stressful task, we compared AUC-I between groups. Finally, we calculated differences in AUC with respect to zero (AUC-G), a linear combination of the prior two analyses, to represent overall pro-inflammatory exposure during the study session. To improve homogeneity of variance, cytokine data were square-root transformed for all analyses. In all cases but one, this transformation improved homogeneity of variance enough to give a non-significant Levene’s test of equality of variances. In the one case where homogeneity of variance was not achieved, Welch’s t test was used. Of the 30 subjects enrolled in the study, 26 were included in the final analyses: three participants were excluded for demographic reasons and one because of cytokine outlier status. Subjects excluded for demographic reasons included one who was found to be a recent smoker and was using significant amounts of Nicorette gum; one was unable to complete the TSST; and one who was in the luteal phase of her menstrual cycle. One subject was noted to have an anomalous IL-6 value at a single timepoint and was excluded from all analyses.
Results
Participant Characteristics
Genotype groups did not differ significantly in terms of age, years of education, Beck Depression Inventory score, or scores on measures of early (CTQ) or recent (LESS) life stress. Additionally, the groups did not differ significantly in baseline heart rate or systolic or diastolic blood pressure (Table 1). Although obese subjects (BMI >30) were not eligible for the study, LL individuals had significantly lower BMI than SS individuals, t(22) = 2.22, p = 0.037, d=0.95, an effect which has previously been shown in the literature (Sookoian et al., 2007). BMI and oral contraceptive use were not included as covariates in analyses because neither contributed significantly to any observed effects.
Table 1.
Participant Characteristics. Values are mean +/− SD, or number of subjects (and % of sample).
| SS genotype (N = 11) | LL genotype (N = 15) | |
|---|---|---|
| Age (years) | 21.8 +/− 2.0 | 21.7 +/− 2.9 |
| Education (years) | 15.0 +/− 1.5 | 15.0 +/− 2.5 |
| BMI | 23.7 +/− 2.4 | 21.5 +/− 2.3 |
| HR | 75.1 +/− 19.5 | 66.7 +/− 10.0 |
| SBP | 113.1 +/− 12.7 | 110.0 +/− 10.6 |
| DBP | 67.0 +/− 9.8 | 64.8 +/− 7.3 |
| OCP user | 6 (55%) | 12 (80%) |
| non-OCP user, follicular | 5 (45%) | 3 (20%) |
| Beck Depression Inventory | 1.6 +/− 2.5 | 2.3 +/− 3.1 |
| Life Events Scale for Students | 3.5 +/− 2.6 | 2.8 +/− 2.3 |
| Childhood Trauma Questionnaire | 29.7 +/− 6.3 | 30.4 +/− 5.3 |
Effects of Genotype and Acute Stress On Mood
Across genotype groups, the TSST had substantial effects on participants’ emotional state. Participants reported higher scores on the shame subscale of the SSGS after the task compared to baseline, t(25) = 4.67, p < 0.001, d=1.87; they also reported lower scores on the pride subscale, t(24) = −3.09, p = 0.005, d=−1.26. Subjects’ SUDS scores increased from five minutes prior to the task to immediately after the task, t(25) = 4.09, p < 0.001, d=1.64. Subjects reported more negative emotional valence, t(25) = −8.09, p < 0.001, d=−3.24 and higher emotional arousal, t(25) = 6.17, p < 0.001, d=2.47 on the RAG immediately after the task, compared to five minutes before (Table 2). There were no differences by genotype group for any of these measures (all p > 0.3).
Table 2.
State emotion questionnaire responses at baseline and after TSST. Values are mean +/− SD.
| Baseline | Post-TSST | Significance | |
|---|---|---|---|
| SSGS - shame subscale | 5.6 +/− 1.1 | 9.4 +/− 3.9 | p <0.001 |
| SSGS - pride subscale | 18.7 +/− 5.4 | 15.2 +/− 4.0 | p = 0.005 |
| SUDS | 11.8 +/− 13.3 | 22.6 +/− 18.0 | p <0.001 |
| Russell Affect Grid - valence | 2.12 +/− 1.1 | −0.5 +/− 1.4 | p <0.001 |
| Russell Affect Grid - arousal | −1.4 +/− 1.9 | 1.6 +/− 1.4 | p <0.001 |
Effects of Genotype and Acute Stress On Serum Cytokines
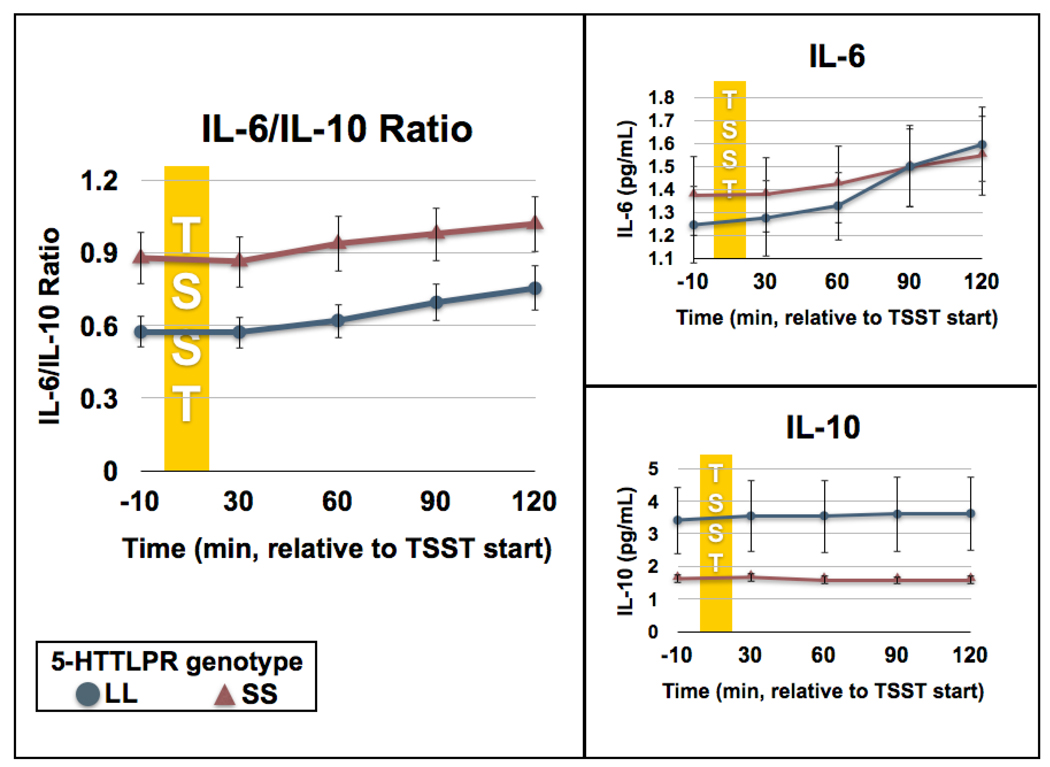
To test for main effects of the task (TSST) for each cytokine measurement, we compared AUC-I to zero using one-sample t-tests. The acute stressor affected IL-6 / IL-10 ratio, t(25)=13.62, p<0.001, d = 5.45, serum IL-6 conentrations, t(25)=19.79, p<0.001, d = 7.92, and serum IL-10 concentrations, t(25)=5.70, p<0.001, d = 2.28 (Fig. 2).
Figure 2. Acute stress induced changes in circulating cytokine concentrations by 5-HTTLPR genotype.
The left-hand panel displays IL-6/IL-10 ratio, at baseline and following administration of the Trier Social Stress Test (TSST), by genotype group. SS individuals had a higher baseline IL-6/IL-10 ratio compared to LLs (p = 0.036), resulting in greater inflammatory exposure overall (AUC-G, p = 0.032). The two groups had the same degree of reactivity (AUCI) to the stressful task (p = 0.690). The right-hand panel displays the components of the ratio (serum IL-6 and IL-10 concentrations) individually. Unlike the ratio, no significant between-group differences at baseline or in terms of reactivity were observed for either cytokine alone (all p > 0.1). Data are expressed as means ± SEM.
Individuals with the SS genotype showed a higher IL-6/IL-10 ratio than LL individuals at baseline, t(13.71) = 2.32, p = 0.036, d = 1.25. However, AUC-I was the same for both groups, t(24) = 0.40, p =0.690, d = 0.16, representing similar degrees of reactivity to the stressful task. AUC-G, a linear combination of the above two measures, was higher for SS individuals than LL individuals, t(24) = 2.28, p = 0.032, d = 0.93, representing higher overall inflammatory exposure during the study session (Fig. 2).
There was no statistically significant difference in IL-6 concentrations between genotype groups at baseline, t(24) = 0.52, p = 0.612, d = 0.21. Additionally, there were no significant differences between groups in AUC-I, t(24) = 0.66, p = 0.517, d = 0.27, or AUC-G, t(24) = 0.23, p = 0.820, d = 0.09. Although we observed lower circulating IL-10 concentrations in SS compared to LL individuals at baseline and following stress, these differences were not statistically significant, perhaps on account of the relatively small number of subjects in each group. Thus, there were no statistically significant differences in IL-10 concentrations between genotype groups at baseline, t(14.60) = 1.69, p = 0.113, d = 0.88, or between groups in AUC-I, t(14.15) = 1.49, p = 0.158, d = 0.79, or AUC-G, t(14.48) = 1.70, p = 0.110, d = 0.89. Interactions were assessed in all between-group analyses but none approached significance (all p > 0.2).
Discussion
5-HTTLPR genotype significantly affected the ratio of IL-6 to IL-10 under resting conditions and after acute psychosocial stress, with SS individuals exhibiting a significantly higher ratio across timepoints. This inflammatory bias was present despite both groups being medically healthy and reporting similarly low exposure to early and recent life stress, which may be independently associated with inflammation (Cohen et al., 1999; Danese et al., 2007; Danese et al., 2008). All participants reported strong subjective experiences of stress and negative emotionality immediately after the task, but these reports were also similar across genotype groups, suggesting that SS individuals do not exhibit a greater pro-inflammatory bias during the stress task simply because they perceive the task as more stressful. To our knowledge, this is the first report of a pro-inflammatory bias/phenotype in individuals carrying the SS genotype of 5-HTTLPR. Thus, even healthy SS-individuals may be chronically exposed to a pro-inflammatory burden under resting and stress conditions, which could increase their vulnerability to disorders like depression and other diseases that can be facilitated/exacerbated by a chronic pro-inflammatory state.
Given the current controversy over the relationships between 5-HTTLPR genotype, life stressors, and depression risk (Risch et al., 2009), the potential implications of the findings described here are significant, and call for further investigation. The pro-inflammatory bias shown by SS individuals, both at baseline and during psychosocial stress, parallels the increased inflammatory markers seen under resting conditions in individuals with depression (Dantzer et al., 2008; Capuron et al. 2008). Importantly, this bias is shown by SS individuals in the absence of psychiatric, immune, or other disease conditions which suggests that the pro-inflammatory bias may precede (and partially mediate) the development of pathology in conjunction with other factors. Individuals undergoing cytokine therapy often become clinically depressed (Miller, 2009; Miller et al., 2009; Raison et al., 2009) with individuals with weaker variants of 5-HTTLPR particularly at risk (Bull et al. 2008; Lotrich et al. 2009); a similar mechanism could lead to increased risk of depression in SS individuals after repeated mild to moderate stressors. Thus, the pro-inflammatory bias present under resting conditions that persists during stress, may cumulatively, after repeated stressful events, contribute to increased risk for development of depression in SS individuals.
Like other studies demonstrating acute stress-induced increases in pro-inflammatory cytokines (Altemus et al., 2001; Steptoe et al., 2007), the present study demonstrates an increase in circulating IL-6, and also demonstrates an increase in the IL-6/IL-10 ratio following exposure to the TSST. Moreover, our findings of a baseline pro-inflammatory bias in healthy SS individuals parallel similar findings in depressed populations: Pace et al. (2006) reported higher baseline IL-6 concentrations in depressed individuals compared to healthy controls, Dhabhar et al., (2009) reported lower IL-10 concentrations and higher IL-6/IL-10 ratio in depressed individuals relative to healthy controls, and Bob et al., (2009) reported that baseline IL-6 is correlated with both depressive symptoms and life stress in depressed individuals. Interestingly, Pace et al., (2006) also reported increased IL-6 reactivity to TSST in their depressed subjects, an effect that we did not observe in our young and healthy population of SS individuals. This difference may reflect the evolution of the stress-induced pro-inflammatory response over time, which might occur in parallel with the development of psychiatric symptoms after a critical level of exposure to stressors, or both. In an interesting and important study, Bull et al. (2008) have shown that individuals expressing a low IL-6-synthesizing polymorphism showed fewer depressive symptoms following interferon-alpha treatment. Their study also showed that individuals expressing the LL genotype of the 5-HTTLPR polymorphism, also showed fewer depressive symptoms following interferon-alpha administration, but with a significantly smaller effect. Their results suggest that interactions between circulating cytokine levels and 5-HTTLPR genotype may be important for the development of depressive symptoms, which is in keeping with the results presented here.
Given the findings described here, the biological mechanisms by which the SS genotype might result in a pro-inflammatory bias require further investigation. As discussed previously (Dhabhar et al., 2009), studies involving in vitro activation of immune cells in the presence or absence of serotonin, provide clues as to how the availability of serotonin (that may be reduced in SS individuals) may affect the balance between pro- and anti-inflammation. These studies suggest that serotonin-replete conditions tilt the immune balance in favor of anti-inflammatory cytokines, and that conversely, serotonin-deficient conditions, tilt the balance towards pro-inflammation (Menard, Turmel, & Bissonnette, 2007; Kubera, Kenis, Bosmans, Scharpé, & Maes, 2000).
Importantly, SS individuals may be at particular risk of developing serotonin deficiency-related pathology once a significant pro-inflammatory bias is established. This is because in addition to serotonin deficiency potentially increasing pro-inflammatory cytokine concentrations, pro-inflammatory cytokines can in turn precipitate serotonin deficiency by increasing the activity of indoleamine 2,3 dioxygenase (IDO) which diverts tryptophan metabolism towards the kynurenine pathway and decreases tryptophan availability for serotonin synthesis (Dantzer et al., 2008; Miller, 2009; Miller et al., 2009; Schiepers, Wichers, & Maes, 2005; Raison et al., 2009). Human and animal studies suggest that the interaction between serotonin metabolism and inflammatory cytokine activity, in particular via the IDO pathway, may play a key role in the development of depressive symptoms. In animal models, depression-like behavior induced by LPS, a common component of pathogenic bacterial cell walls, is mediated by IDO (O'Connor et al. 2009). Human studies also suggest that tryptophan depletion associated with inflammation may be responsible for the depressogenic effects of inflammatory cytokines. Cancer patients treated with interferon have depressive symptoms that are associated with the magnitude of tryptophan depletion they experience (Capuron et al 2002b), and two new studies have demonstrated that individuals with weaker 5-HTTLPR variants are more likely to experience depression after interferon-alpha therapy (Bull et al., 2008; Lotrich et al., 2009). Given that SS individuals already have a diminished capacity for serotonin uptake, the additional burden of depleted serotonin after even mild to moderate stressful events may act as a "second hit, " putting them at elevated risk for depression.
Both human and animal studies have suggested that, in addition to high levels of pro-inflammatory cytokines, low levels of anti-inflammatory cytokines are associated with depressive symptoms and depression-like behavior (Dhabhar et al., 2009; Song et al., 2009; Mesquita et al., 2008). Interestingly, the higher IL-6/IL-10 ratio shown by SS individuals under resting conditions and during acute stress suggests that even under healthy conditions, these individuals show increased levels of immune factors that are associated with the development of depression. These factors may reflect a higher-level disturbance in overall immune balance in SS individuals.
The approach taken in this study builds on the imaging genetics literature, which has identified a robust S allele-related effect of increased amygdala reactivity and decreased fronto-limbic functional coupling (Hariri et al., 2005). We identify a pro-inflammatory bias in SS individuals, highlighting the effects of the polymorphism on the immune system whose dysregulation can clearly lead to increased depression risk. This work highlights the need for examination of multiple, potentially interrelated biological systems in the search for proximal effects of genetic variants of interest.
This study has several limitations. First, given our small sample size, it will be important to replicate these findings in larger samples. However, the fact that we were able to detect between-group differences in IL-6/IL-10 ratio in our small sample speaks to the strength of these effects. Second, we chose a young and healthy sample in order to minimize confounding factors and to examine the inflammatory effects of a genetic risk factor that had not yet led to adverse medical or psychiatric outcomes. However, the homogeneity of our sample may limit the generalizability of our results. Future prospective studies should elucidate the association between a pro-inflammatory bias in healthy young individuals and their risk of developing depression later on. Third, due to the prolonged inflammatory cytokine response to acute stress, we were not able to examine genotype differences in the duration / resolution of this inflammatory stress response. Future studies that follow inflammatory markers for extended periods of time after acute stress may help broaden our understanding of genotype-related differences in cytokine response. Fourth, given that some studies have reported differences between SS and LL individuals in post-awakening (Chen et al., 2009) and stress-induced (Gotlib et al., 2008) salivary cortisol concentrations, while others have only found differences in post-awakening but not stress-induced cortisol (Wust et al., 2009), an investigation of circulating cortisol concentrations simultaneously with cytokines and other factors is likely to provide useful and important information. Such an investigation merits future consideration. Finally, any laboratory stressor can only approximate the effect of a real-life psychosocial stressor, and cannot predict the effects of multiple exposures to stressful events. We chose the TSST because the psychosocial stress it elicits is similar to the social rejection stress that has been especially associated with depression onset (Kendler et al., 1995). However, studies that measure the cumulative effects of multiple stressful events, both in and out of the laboratory setting, will increase our understanding of the cumulative impact of stressors on inflammation, and on subsequent psychiatric and medical health outcomes.
To our knowledge, these results are the first to show that even in the absence of a disease state, compared to LL individuals, SS individuals are exposed to higher levels of pro-inflammatory cytokines under resting conditions and during stress. Since life generally involves exposure to a series of stressors, this pro-inflammatory physiological bias is likely to result in a higher chronic inflammatory burden for SS individuals over time, which may increase their vulnerability to stress-related disorders like depression and other diseases that can be facilitated/exacerbated by a chronic pro-inflammatory state. Several studies have demonstrated that positive coping strategies such as values affirmation (Creswell et al., 2005), meditative practice (Pace et al., 2009) and social support (e.g., presence of a friend during TSST, Heinrichs et al., 2003) can decrease the neuroendocrine response to stressful tasks. These studies offer a hopeful perspective on the ability of individuals to cope more effectively with life stressors. They point to the need for further studies to assess whether individuals at increased risk for inflammation-induced depression due to genetic, cytokine treatment-related, or inflammatory disorder-associated causes, can use such coping strategies to reduce the negative impact of life stressors on their health. Clearly, future studies are needed to replicate the findings described here, investigate the biological mechanisms mediating the pro-inflammatory bias/phenotype shown by SS individuals, and explore the potential for using a three-pronged intervention involving behavioral, neurotransmitter-directed and immuno-modulatory approaches for the treatment of depression and other stress-related disorders.
Acknowledgements
This work was supported by National Institute of Mental Health R01 # MH58147 (JJG), the Carl & Elizabeth Naumann Fund Startup Grant (FSD), NARSAD Young Investigator Award 34676 (WR), and grant M01 RR-00070 from the National Center for Research Resources, National Institutes of Health. The authors would like to thank Lin Xiaoyan for performing genotype analyses, Katie Denny for her help in developing the TSST protocol, Cole Shiflett and Michael Mehler for their assistance as Trier panelists, and the nurses of the Stanford Hospital GCRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altemus M, Rao B, Dhabhar FS, Ding W, Granstein RD. Stress-induced changes in skin barrier function in healthy women. J. Invest. Dermatol. 2001;117:309–317. doi: 10.1046/j.1523-1747.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker ML, Schwandt M, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biol. Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bob P, Raboch J, Maes M, Susta M, Pavlat J, Jasova D, Vevera J, Uhrova J, Benakova H, Zima T. Depression, traumatic stress and interleukin-6. J. Affect. Disord. 2009 doi: 10.1016/j.jad.2009.03.017. doi:10.1016/j.jad.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SR, Philibert RA, Chen YF, Lei MK, Murry VM, Brown AC. Parenting moderates a genetic vulnerability factor in longitudinal increases in youths' substance use. J. Consult. Clin. Psychol. 2009;77:1–11. doi: 10.1037/a0012996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Social origins of depression: A study of psychiatric disorder in women. London: Tavistock; 1978. [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Molecular Psychiatry. 2008;13:1–10. doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. J. Clin. Oncol. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: Phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002a;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol. Psychiatry. 2002b;7:468–473. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Capuron L, Su S, Miller AH, Bremner JD, Goldberg J, Vogt GJ, Maisano C, Jones L, Murrah NV, Vaccarino V. Depressive symptoms and metabolic syndrome: is inflammation the underlying link? Biol. Psychiatry. 2008;64:896–900. doi: 10.1016/j.biopsych.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol. Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Chen MC, Joormann J, Hallmayer J, Gotlib IH. Serotonin transporter polymorphism predicts waking cortisol in young girls. Psychoneuroendocrinology. 2009;34:681–686. doi: 10.1016/j.psyneuen.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizza G, Marques AH, Eskandari F, Christie IC, Torvik S, Silverman MN, Phillips TM, Sternberg EM. Elevated Neuroimmune Biomarkers in Sweat Patches and Plasma of Premenopausal Women with Major Depressive Disorder in Remission: The POWER Study. Biol. Psychiatry. 2008;64:907–911. doi: 10.1016/j.biopsych.2008.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Skoner DP. Psychological stress, cytokine production, and severity of upper respiratory illness. Psychosom. Med. 1999;61:175–180. doi: 10.1097/00006842-199903000-00009. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Welch WT, Taylor SE, Sherman DK, Gruenewald TL, Mann T. Affirmation of personal values buffers neuroendocrine and psychological stress responses. Psychol. Sci. 2005;16:846–851. doi: 10.1111/j.1467-9280.2005.01624.x. [DOI] [PubMed] [Google Scholar]
- Daftarian PM, Kumar A, Kryworuchko M, Diaz-Mitoma F. IL-10 production is enhanced in human T cells by IL-12 and IL-6 and in monocytes by tumor necrosis factor-alpha. J. Immunol. 1996;157:12–20. [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Nat. Acad. Sci. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Archives of general psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: Implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI, Wolkowitz OM. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. Journal of Psychiatric Research. 2009;43:962–969. doi: 10.1016/j.jpsychires.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dohrenwend BP. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychol. Bull. 2006;132:477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW. User's guide for the structured clinical interview for DSM-IV Axis I disorders - Research Version (SCID-1, Version 2.0, February 1996 Final Version) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- Fothergill A., Director . "Jungles," Planet Earth [television series] United Kingdom: BBC; 2006. [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biological psychiatry. 2008;63:847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A Susceptibility Gene for Affective Disorders and the Response of the Human Amygdala. Archives of General Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol. Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hu X-Z, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. J. Behav. Ther. Exp. Psychiatry. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch. Gen. Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am. J. Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The 'Trier Social Stress Test' -- a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Scharpé S, Maes M. Effects of serotonin and serotonergic agonists and antagonists on the production of interferon-γ and interleukin-10. Neuropsychopharmacology. 2000;23:89–98. doi: 10.1016/S0893-133X(99)00150-5. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Linden W. Development and initial validation of a life event scale for students. Canadian Counsellor. 1984;18:106–110. [Google Scholar]
- Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biol. Psychiatry. 2009;65:344–348. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Bosmans E, De Jongh R, Kenis G, Vandoolaeghe E, Neels H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine. 1997;9:853–858. doi: 10.1006/cyto.1997.0238. [DOI] [PubMed] [Google Scholar]
- Marschall DE, Sanftner J, Tangney JP. The state shame and guilt scale. Fairfax, VA: George Mason University; 1994. [Google Scholar]
- Menard G, Turmel V, Bissonnette EY. Serotonin modulates the cytokine network in the lung: involvement of prostaglandin E2. Clin Exp Immunol. 2007;150:340–348. doi: 10.1111/j.1365-2249.2007.03492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita AR, Correla-Neves M, Roque S, Castro AG, Vieira P, Pedrosa J, Palha JA, Sousa N. IL-10 modulates depressive-like behavior. J. Psychiatr. Res. 2008;43:89–97. doi: 10.1016/j.jpsychires.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Miller AH. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and Its Discontents: The Role of Cytokines in the Pathophysiology of Major Depression. Biol. Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Reid MW. Gene-environment interactions in depression research: genetic polymorphisms and life-stress polyprocedures. Psychol. Sci. 2008;19:947–956. doi: 10.1111/j.1467-9280.2008.02181.x. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biol. Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene x environment interactions at the serotonin transporter locus. Biol. Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Duru EA, Ameredes BT. Role of IL-10 in the resolution of airway inflammation. Current Molecular Medicine. 2008;8:437–445. doi: 10.2174/156652408785160907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte C, McCaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the Heart and Soul Study. Am J Psychiatry. 2007;164:1379–1384. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am. J. Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Pace TW, Negi LT, Adame DD, Cole SP, Sivilli TI, Brown TD, Issa MJ, Raison CL. Effect of compassion meditation on neuroendocrine, innate immune and behavioral responses to psychosocial stress. Psychoneuroendocrinology. 2009;34:87–98. doi: 10.1016/j.psyneuen.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pollak Y, Yirmiya R. Cytokine-induced changes in mood and behaviour: implications for 'depression due to a general medical condition', immunotherapy and antidepressive treatment. Int J Neuropsychopharmacol. 2002;5:389–399. doi: 10.1017/S1461145702003152. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, Vogt GJ, Massung B, Miller AH. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biological Psychiatry. 2009;65:296–303. doi: 10.1016/j.biopsych.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang K-Y, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richell RA, Deakin JF, Anderson IM. Effect of acute tryptophan depletion on the response to controllable and uncontrollable noise stress. Biol. Psychiatry. 2005;57:295–300. doi: 10.1016/j.biopsych.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Russell JA, Weiss A, Mendelsohn GA. Affect grid: A single-item scale of pleasure and arousal. Journal of Personality and Social Psychology. 1989;57:493–502. [Google Scholar]
- Schatzberg A. Recent studies of the biology and treatment of depression. Focus. 2005;3:14–24. [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry. 2009;42:182–188. doi: 10.1055/s-0029-1202263. [DOI] [PubMed] [Google Scholar]
- Sookoian S, Gemma C, Garcia SI, Gianotti TF, Dieuzeide G, Roussos A, Tonietti M, Trifone L, Kanevsky D, Gonzalez CD, Pirola CJ. Short allele of serotonin transporter gene promoter is a risk factor for obesity in adolescents. Obesity. 2007;15:271–276. doi: 10.1038/oby.2007.519. [DOI] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Chen CC, Bai CH, Wu SR. Cytokines and serotonin transporter in patients with major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:899–905. doi: 10.1016/j.pnpbp.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain, Behavior, and Immunity. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of enivronmental adversity in the aetiology of mental illness: review and methological analysis. Mol. Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol. Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Wolpe J. The practice of behavior therapy. 2nd ed. New York: Pergamon Press; 1969. [Google Scholar]
- Wust S, Kumsta R, Treutlein J, Frank J, Entringer S, Schulze TG, Rietschel M. Sex-specific association between the 5-HTT gene-linked polymorphic region and basal cortisol secretion. Psychoneuroendocrinology. 2009;34:972–982. doi: 10.1016/j.psyneuen.2009.01.011. [DOI] [PubMed] [Google Scholar]