Abstract
Introduction
Differences among murine strains often lead to differential responses in models of human disease. The aim of the current study was to investigate whether differences exist among strains in models of hemostasis and thrombosis and whether these differences are reflected in differences in the tissue factor (TF) pathway.
Methods
We examined baseline hemostatic parameters and the response to FeCl3-induced arterial thrombosis and a tail vein bleeding model in C57BL/6J (C57), 129S1/SvImJ (129S), and Balb/cJ (BalbC) mice. Finally, we examined TF and tissue factor pathway inhibitor (TFPI) activities in blood and expression in vascular tissue to determine whether these factors covary with a thrombotic phenotype.
Results
No differences were observed in PT or aPTT among strains. 129S mice had lower platelet counts (p<0.001). BalbC had an increased rate of occlusion (mean occlusion time of 330±45 sec) in a FeCl3-induced model of thrombosis when compared to C57 (1182±349 sec) or 129S (1442±281 sec) (p<0.05). Similarly, BalbC demonstrated reduced blood loss in tail bleeding experiments when compared to C57 and 129S. Vascular expression of TF and TFPI content did not correlate with the thrombotic phenotype of BalbC. However, circulating TFPI activities were lower in BalbC compared to both C57 and 129S mice. When normalized to circulating TF activities, BalbC had lower circulating TFPI activity than C57 and 129S, and there was a significant correlation between tail bleeding and normalized TFPI activity (r= 0.67).
Conclusions
These data suggest that there are significant differences among strains in thrombosis and hemostasis and that circulating TFPI activity correlates with these differences.
Keywords: mouse, strain, hemostasis, thrombosis, tissue factor, tissue factor pathway inhibitor
Introduction
Hemostasis is critical for the prevention of excessive blood loss after vascular injury. Thrombus formation to halt blood loss and the subsequent removal of the hemostatic plug to resume normal blood flow are highly regulated processes. Deterioration of the hemostatic balance can lead to an increased susceptibility to vascular thrombosis leading to thrombotic occlusion of arteries supplying vital organs, such as the heart and brain, potentially resulting in myocardial infarction or stroke. Although many of the factors and mechanisms involved in the complex processes of hemostasis and thrombosis have been identified, more are likely to be discovered. In vivo models have been useful tools to elucidate many of the complex interactions that regulate the pathophysiology of thrombosis. With the advent of genetically modified mice, murine models of hemostasis and thrombosis have become increasingly useful tools to gain a more comprehensive understanding of the processes involved in maintaining vascular hemostasis. Deletion or overexpression of single or multiple gene targets is now possible allowing investigators to examine the resulting phenotype and gain insight into the role of specific gene product(s) in hemostasis and thrombosis.
The most common mouse models of thrombosis include ferric chloride (FeCl3) - induced arterial thrombosis, laser-induced vascular thrombosis, mechanical injury, and photochemical-induced arterial thrombosis (reviewed in [1]). Strain-specific differences have been reported in murine models of vascular thrombosis [1, 2]. These differences are presumably due to inherent differences in genetic makeup of these inbred strains of mice. Therefore, in addition to gene targeting approaches, these strain differences could be used to identify potential genes of interest and whose products may be involved in vascular thrombosis. This approach to identify gene products potentially involved in human disease has become an integral tool, particularly with the completion of the human genome project and the advent of microarray analysis. Genetic strain differences in platelet aggregation have also been reported in mice and rats [3, 4] as has strain differences in thrombus formation in rats [4]. In addition to thrombosis, murine strain differences have also been reported in models of vascular remodeling [5–7] and atherosclerosis [8, 9]. We therefore hypothesized that differences in hemostasis and thrombosis would exist in three commonly used inbred strains of mice, namely BalbC, C57, and 129S. Furthermore, as the tissue factor pathway is a central regulatory pathway in hemostasis and thrombosis, we hypothesized that differences between strains would be reflected in differences in this pathway.
Materials and methods
Animals and reagents
Age matched (8–12 weeks) male mice of three different strains: C57BL/6J (C57), 129S1/SvImJ (129S), and Balb/cJ (BalbC) were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were kept in an animal housing facility on a 12-h light-dark schedule with food and water available ad libitum. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic. All chemicals were purchased from Sigma-Aldrich unless otherwise noted.
Blood Analysis
Blood samples (100μl) were collected into tubes containing 10μl of 3.2% sodium citrate solution. Blood samples were analyzed within two hours of collection for complete blood count with differential (hemoglobin, red blood cell count, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin concentration, red cell distribution width, white blood cell count, lymphocytes, monocytes, granulocytes, platelet count, mean platelet volume, and platelet distribution width) and coagulation (prothrombin and activated partial thromboplastin times). For analysis, the VetScan HMT Hematology System (Union City, CA) and an SCA2000 Veterinary Coagulation Analyzer (San Diego, CA) were used according to the manufacturer’s instructions.
Murine model of arterial thrombosis
The FeCl3-induced model of vascular thrombosis has been previously described [10]. Briefly, mice were anesthetized by an intramuscular injection of approximately 0.02mL mouse cocktail (83.3 mg/mL ketamine, 16.7 mg/mL xylazine). After the left common carotid artery was exposed, a flow probe (model 0.5 VB, Transonic Systems) was placed on the artery. The probe was connected to a flowmeter (Transonic model T106), and data was collected via connection to a PC-driven PowerLab with ChartPro software (ADInstruments, Colorado Springs, CO). A strip of filter paper saturated with 10% FeCl3 solution was applied to the adventitial surface of the surgically exposed carotid artery for 3 min. Carotid artery blood flow was then followed by the T106 Small Animal Blood Flow Meter for 45 min or until complete occlusion (0 flow for at least 10 sec) occurred.
Murine model of hemostasis
Tail vein bleeding tests were performed on the three strains of mice as previous described [11]. Briefly, mice were anesthetized by an intramuscular injection of approximately 0.02mL mouse cocktail (83.3 mg/mL ketamine, 16.7 mg/mL xylazine). Several minutes later, the mice were immobilized in a mouse restraint device, and 1cm of the tip of the tail was removed with a sharp scalpel. The tail was immediately immersed into a 15-mL conical tube containing 12ml 0.9% NaCl at 37°C and was removed either at time of bleeding cessation or 60 sec, whichever came first. Samples were centrifuged at 520 × g for 5 min and supernatant was discarded. Subsequently, 5ml of red cell lysis buffer (10mM KHCO3, 150mM NH4Cl, and 1mM EDTA) was added to the cell pellets, and the lysis was allowed to proceed for 10 min at room temperature. The samples were centrifuged as described above, and the OD575 nm of the supernatants was used as a measure of hemoglobin present and therefore blood collected.
Quantification of Vascular TF and TFPI expression
Vascular TF and TFPI expression was measured semi-quantitatively using Western blot analysis. Aortas were removed, cleaned of adventitia, and placed in ice cold phosphate buffered saline. Aortas were then homogenized using an Omni tissue homogenizer (Omni International, Marrietta, GA, model TH-794) in lysis buffer consisting of 30mM CHAPS, 10μM E64, 1mM PMSF, and 10mM EDTA in PBS (Ph 7.4). The crude homogenates were then centrifuged at 10,000 × g, and supernatants (homogenates) were frozen and stored at −80°C until analyzed. Protein content of homogenates was measured using the DC protein assay (Bio-Rad Laboratories, Hercules, CA). Equal amounts of protein (12μg) were diluted 1:2 in Laemmli sample buffer (Bio-Rad) and placed in a boiling water bath for 5 min. Samples were then run on 10% Tris-HCl ready gels (Bio-Rad) using SDS-PAGE, proteins were transferred to PVDF membranes, and blocked overnight in TBST containing 0.05% Tween (TBST) and 5% milk. Membranes were incubated with primary antibodies to TF (1:400, overnight, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), TFPI (1:500, overnight, rabbit anti-mouse TFPI antiserum generated in house), or β-actin (1:2000, 1 hr, Abcam Inc., Cambidge, MA) and after washing were incubated for 45 min with horseradish peroxidase-conjugated secondary antibody (1:5000, 45 min). Western blots were developed using Amersham ECL Plus Western Blotting Detection Reagent (GE Healthcare, Piscataway, NJ) and quantified by densitometry. TF and TFPI were normalized to β-actin and are presented as relative quantification of TF and TFPI (relative to each individual sample β-actin).
Measurement of circulating TF and TFPI activities
Blood was collected into a tube containing 0.1M sodium citrate (one-tenth the volume of blood collected), centrifuged at 10,000 × g for 10 min at 4°C, and the resulting plasma was transferred to new tubes and frozen at −80°C until analysis. TFPI activities were measured in plasma diluted 1:30 with assay buffer using an ACTICHROME TFPI Activity Assay kit (American Diagnostica Inc.) per the manufacturer’s protocol. TF activities were measured in undiluted plasma using an AssaySense TF Chromogenic Activity Assay kit (Assaypro, St. Charles, MO) per the manufacturer’s protocol. These kits contain human reagents, and therefore, the data generated is best considered comparative and not quantitative.
Statistical analysis
Data are presented as mean±SEM. Statistical analysis was performed using one-way ANOVA, and all groups were compared using a multiple comparison test. For the Kaplan Meier plot (Figure 1A), a chi-squared analysis was performed. A Pearson correlation test was used to examine the correlation between blood loss in the tail bleeding assay and the ratio of circulating TFPI/TF. Differences were considered significant at P ≤ 0.05.
Fig. 1.
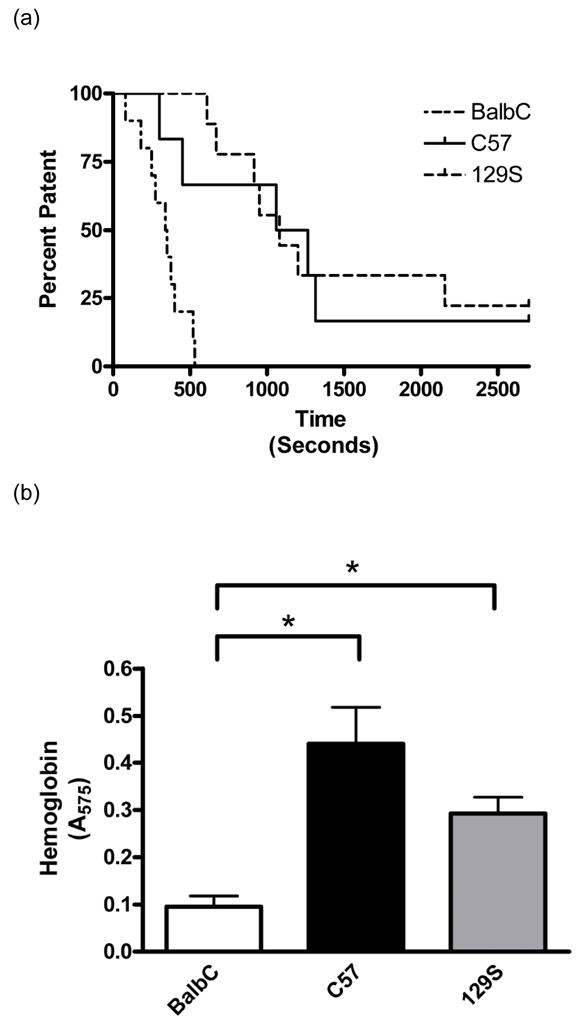
Arterial thrombosis and hemostasis. Patency of carotid arteries are shown in a Kaplan-Meier survival plot (A) demonstrating significant differences in FeCl3-induced occlusion of coronary arteries between the three strains of mice examined (P< 0.0001). A tail bleeding assay was used as a measure of hemostasis (B). A 1cm section of tail was excised from anesthetized mice, and the amount of blood collected in 60 sec (or until cessation of bleeding, whichever occurred first) was quantified using hemoglobin content (A575) as a measure of blood collected. Note that BalbC mice (n=10) had significantly lower blood loss than both the C57 (n=10) and 129S strains (n=10, * P < 0.05). Data are presented as means ± SEM.
Results
Baseline hemostatic parameters
To determine if there were any inherent differences in baseline hemostatic parameters among strains, we analyzed plasma samples for prothrombin and activated partial thromboplastin times. There were no significant differences in either of these two parameters among the three strains of mice. Prothrombin times were 21.7±0.6, 21.8±0.4, and 21.8±0.5 sec for BalbC, C57, and 129S, respectively. Activated partial thromboplastin times were 65.6±2, 65.8±3, and 69.5±2 sec for BalbC, C57, and 129S, respectively. Interestingly, 129S mice had significantly lower platelet counts (354±5 × 103/μl, n=10) when compared to either BalbC (728±18 × 103/μl, n=10) or C57 (741±23 × 103/μl, n=9) mice. This observation is similar to what has previously been established and reported in The Jackson Laboratory’s Mouse Phenome Database (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/home).
Strain and murine models of thrombosis and hemostasis
To assess strain differences in arterial thrombosis, we used a FeCl3-induced model of thrombosis. BalbC mice showed significantly reduced average time to occlusion (330±45 sec) when compared to either the C57 (1182±349 sec) or the 129S (1442±281 sec) strains of mice (P < 0.05, Figure 1A). These data suggest that there are inherent differences between strains that contribute to their propensity to thrombose in response to FeCl3.
To evaluate strain differences in hemostasis, we utilized a tail bleeding model. BalbC mice had significantly reduced blood loss when compared to either the C57 or 129S strains (Figure 1B). These data again are suggestive of inherent strain differences that account for their tendency to bleed and/or clot and further identify BalbC mice as those with the most thrombotic phenotype.
Vascular TF and TFPI expression
To determine whether the propensity of the BalbC mice to thrombose is related to differences in vascular expression of TF or TFPI, we analyzed aortic homogenates for TF and TFPI expression by Western blot analysis (Figure 2A). 129S mice had significantly lower TFPI protein expression compared to C57 mice (Figure 2B). C57 mice had significantly higher TF expression than either BalbC or 129S mice (Figure 2C). Thus, vascular TF and TFPI expression failed to suggest an obvious correlate for the thrombotic phenotype of BalbC mice.
Fig. 2.
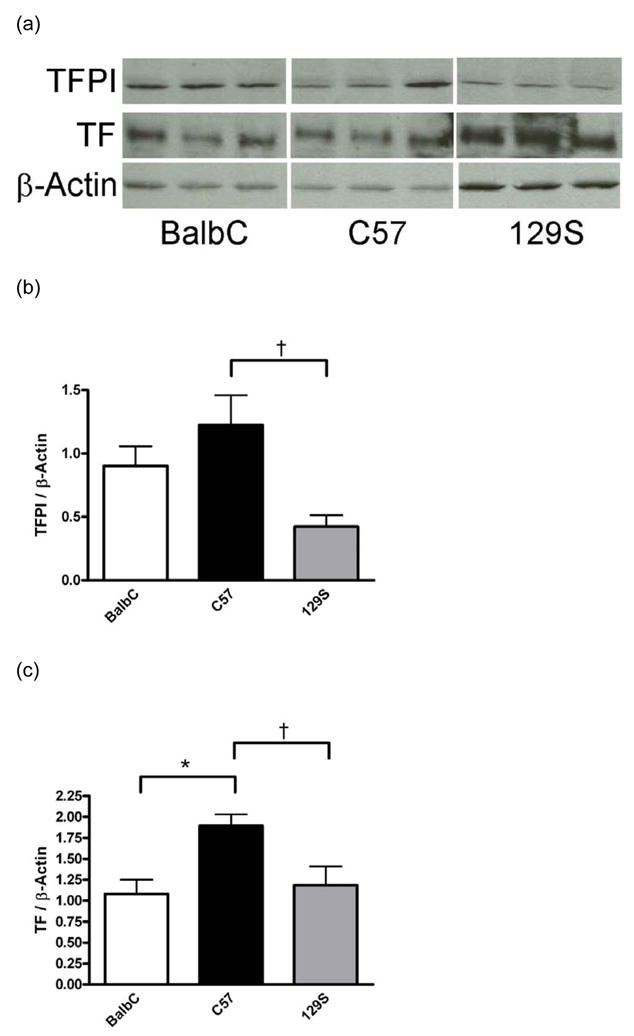
Western blot analysis of vascular TFPI and TF. Blots were probed with antibodies to TF, TFPI, and β-actin and analyzed by densitometry. Relative quantification was achieved by normalizing TFPI and TF protein expression to β-actin. Representative immunoblots for TF, TFPI, and β-actin (A). 129S mice had significantly lower TFPI protein expression than C57 mice (B, † P<0.05 compared to C57, n=5 mice/strain). C57 mice had significantly higher TF protein expression than either BalbC or 129S mice (C, * P< 0.01 when compared to BalbC, and † P<0.01 compared to 129S, n=5 mice/strain). Data are presented as means ± SEM.
Circulating TF and TFPI activities
Subsequently, we measured circulating TF and TFPI activities in plasma samples obtained from mice of the three strains to determine whether differences in these coagulation factors may contribute to strain differences in thrombosis and hemostasis. BalbC mice had significantly lower TFPI activity levels than either C57 or 129S mice (Figure 3A). BalbC mice had similar circulating levels of TF as the 129S and C57 mice while the C57 mice had significantly lower TF activities than the 129S mice (Figure 3B). When normalized to TF activity, TFPI activities in BalbC mice were significantly lower than those observed in either the C57 or 129S mice (Figure 3C).
Fig. 3.
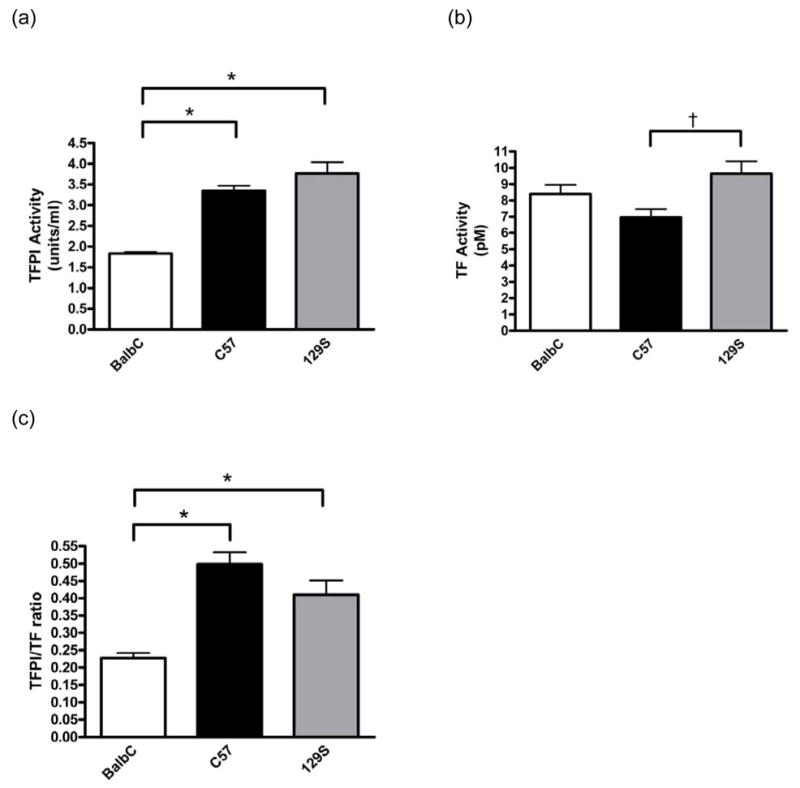
Analysis of circulating TF and TFPI activities. Plasma samples were analyzed for circulating TFPI (A) and TF (B) activity levels. BalbC mice had significantly reduced circulating TFPI activities compared to the C57 (n=10) or 129S mice (n=10, A, * P<0.001). C57 mice (n=10) had significantly lower circulating TF activities compared to the 129S strain (n=10, B, † P<0.05 when compared to 129S mice). No significant differences in TF activity was observed in BalbC mice (n=10) compared to the other two strains. The TFPI to TF ratios were also plotted (C), and BalbC mice had significantly lower ratios than the C57 or 129S strains (C, * P<0.001). Data are presented as means ± SEM.
As tail vein bleeding and circulating TFPI and TF activities were measured in the same mice, we correlated blood loss and normalized TFPI activities in individual mice. There was a tendency for each of the strains to segregate into groups, and there was a significant positive correlation observed between blood loss and the ratio of TFPI and TF (r = 0.6697, Figure 4).
Fig. 4.
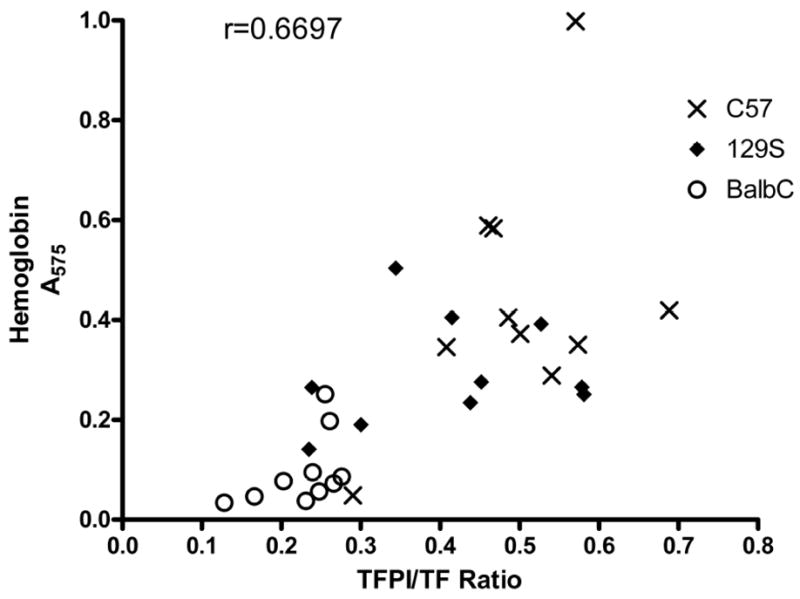
Hemostasis vs. TFPI/TF ratio. Blood collected during tail bleeding experiments was plotted against TFPI/TF ratios for individual mice. There was a significant positive correlation between blood collected and TFPI/TF ratios (r =0.6697, P<0.0001).
Discussion
In the current study, we evaluated three commonly utilized strains of mice for inherent differences in hemostasis and thrombosis. We also examined circulating activities and vascular expression of TFPI and TF hypothesizing that differences in these factors among strains may contribute to any differences observed in hemostasis and thrombosis. The results clearly demonstrate that there are differences among strains in hemostasis as evaluated in a tail bleeding model as well as differences in thrombosis utilizing a FeCl3-induced model of arterial thrombosis. These studies identified BalbC mice as the most thrombotic. We further identified that circulating TFPI levels (and not vascular levels) correlated with what we observed in the tail bleeding model of hemostasis, and we speculate that these differences may also contribute to differences we observed in the thrombosis model.
Differences in genetic makeup have long been known to be responsible for phenotypic differences among individual members of any given species. Inbred strains of mice have been available for nearly a century and have become valuable tools in biomedical research. These strains have been maintained to contain little genetic variation among members of the same strain. The genetic differences among strains may result in phenotypic differences which can be helpful to identify potential genes or gene products of interest that may be involved in the pathogenesis of various disease states.
In the present study, we found that there are strain differences in hemostasis as demonstrated in a tail bleeding model. We also observed strain differences in a FeCl3-induced model of arterial thrombosis. Strain differences in hemostasis and thrombosis have been reported previously. Hoover-Plow et al examined thrombosis and hemostasis in two strains of mice, namely C57 and A/J mice [2]. A/J mice were found to have significantly decreased occlusion times in a FeCl3-induced model of arterial thrombosis compared to C57 mice. Tail bleeding times were similar between the two strains but rebleeding times were increased in the A/J strain compared to C57. The authors of this study utilized a breeding scheme with chromosome substitution strains to identify mice that had a rebleeding phenotype similar to the A/J phenotype and found that the C57 background could modify this phenotype. The authors conclude that these genetic backgrounds and differences in response to thrombosis and hemostasis suggest the potential for identifying novel genetic determinants of thrombotic risk. Strain-specific differences in a photochemical injury model of thrombosis have also previously been reported [1]. In this review of murine models of vascular thrombosis, the authors reported occlusion times in 129S mice that were lower (19.1±2.9 min) when compared to BalbC (40.0±8.4 min) or C57 (42.2±4.2 min) mice. In the present study using a FeCl3-induced model of arterial thrombosis, we report significantly shorter occlusion times in BalbC (330±45 sec) mice when compared to C57 (1182±349 sec) or 129S (1080±281 sec) mice. We can only conclude that differences in the mechanism of injury are responsible for the variability in strain differences observed in the two models. The genetic differences that are responsible for this discrepancy between models may hold clues as to what mediators are involved in the two models.
We report here that there are strain differences in circulating TFPI and TF levels and that the ratio of TFPI to TF is positively correlated with blood loss in a tail bleeding model of hemostasis. As previous studies have demonstrated a role for the tissue factor pathway in murine models of hemostasis, thrombosis, atherosclerosis, angiogenesis, inflammation, and vascular remodeling [12–14], it is not surprising given the strain differences in circulating levels of TFPI and TF that there are strain differences in hemostasis and thrombosis. We have previously demonstrated that overexpression of TFPI leads to attenuation of vascular remodeling in a flow interruption model of vascular remodeling [14]. We have also previously reported that TFPI haploinsufficiency results in exacerbation of neointimal formation and proliferation in a murine model of vascular remodeling [15]. Transgenic mice overexpressing TFPI via a smooth muscle specific promoter have increased local vascular TFPI levels and show resistance to thrombotic occlusion in a FeCl3-induced model of arterial thrombosis [10]. We therefore speculate that differences in circulating TFPI and TF activity levels between strains may contribute to the differences observed in the FeCl3-induced model of arterial thrombosis reported in the present study.
The strength of the correlation between circulating TFPI levels and thrombotic risk in humans is somewhat controversial. Dahm and colleagues examined TFPI activity and free and total TFPI antigen levels in 474 patients and 474 controls from the Leiden Thrombophilia Study [16]. The authors found that low levels of TFPI correlate, albeit weakly, with risk for deep vein thrombosis (DVT). In another study, analysis of total TFPI levels in 122 DVT patients and 126 controls revealed an association between reduced TFPI levels and DVT [17]. In a study examining TFPI free antigen levels in 611 patients with a first venous thromboembolism (VTE), the relative risk of recurrence of VTE was significantly increased in patients with TFPI levels below or equal to the 2nd percentile [18]. The probability of recurrence was 48.6% among the patients with low TFPI levels and 16.8% in patients with higher levels. In yet another study, investigators studied 42 patients with peripheral artery disease (PAD) and 42 healthy controls [19]. They measured TF, free TFPI, and total TFPI in citrated plasma and found a significant elevation of TF and a significant decrease in total TFPI in the patients with PAD. No significant differences in free TFPI were found. The authors conclude that reduced total TFPI and increased TF may contribute to atherosclerosis and increased risk of thrombosis. These studies suggest that there is at least a mild correlation between circulating TFPI levels and thrombotic risk in humans. In contrast, patients with abetalipoproteinemia have very low levels of TFPI antigen and yet do not have an increased risk of thrombosis [20]. The authors of this study found that heparin administration in an individual with abetalipoproteinemia increased TFPI levels to that of normal individuals. The authors conclude that circulating TFPI levels may not be as important as the heparin releasable pool of TFPI in predicting thrombotic risk. In a review of the genetic susceptibility to thrombosis, Luyendyk and colleagues suggest that levels of TF may be more important than levels of TFPI in determining risk of thrombosis [21]. Little has been reported in the literature regarding circulating TFPI and/or TF levels and thrombotic risk in mice. Multiple studies investigating the role of TFPI in mice have used mice that not only have a reduction or increase in circulating TFPI but also decreased or increased vascular expression of TFPI as well [10, 13–15].
Strain differences have previously been reported in murine models of atherosclerosis [8, 9] and vascular remodeling [5–7], and differences in circulating TFPI and TF levels may contribute to these differences. While we suggest that differences in TFPI and TF levels contribute to the observed strain differences in thrombosis and hemostasis, there may be other differences between these strains that contribute to the observed differences. These could include differences in expression of other pro-coagulant or anti-coagulant proteins, differences in expression of proteins involved in the fibrinolytic system, or differences in platelet number and/or function. For example, Li et al examined integrinα2 expression between five strains of mice [22]. The authors observed significantly reduced integrinα2 expression in FVB mice compared to the other four strains which was associated with decreased platelet aggregation. The authors speculate that differences in platelet aggregation could contribute to differences in thrombotic risk.
In our analysis of vascular TFPI and TF protein levels, we predicted that the vascular expression of TFPI and TF would be reflected in the differences in time to occlusion observed in the FeCl3 model of thrombosis i.e. lower TFPI and higher TF in the BalbC mice compared to C57 or 129S. This was not the case as the BalbC mice had significantly higher TFPI/TF ratios than 129S mice and a ratio that was not significantly different from C57 mice (data not shown). It is important to note that in the present study, we examined TF and TFPI protein expression in the aorta as the use of mice precludes the measurement of microvascular TF and TFPI protein expression. Microvascular endothelial cells are purported to be a primary source of TFPI [23] with expression also present in endothelium and smooth muscle cells of large vessels such as aorta, carotid artery, and coronary artery [14, 24–26]. It is possible that had we measured TF and TFPI in the microvasculature that the TFPI/TF ratios may have correlated with the thrombotic phenotype. In the FeCl3 model of injury, thrombosis occurs due to chemical oxidation resulting in a predisposition of the injured area to platelet adherence and aggregation followed by the activation of coagulation, fibrin deposition, and eventual thrombotic occlusion. The fact that the 129S mice have significantly lower platelet counts than the other two strains could contribute to their resistance to thrombosis when compared to the BalbC mice in this model.
In conclusion, strain differences in murine models of hemostasis and thrombosis exist. We demonstrate here that circulating TFPI and TF levels correlate with strain differences in hemostasis and speculate that they also play a role in the strain differences observed in thrombosis, atherosclerosis, and vascular remodeling. Future studies could provide important insights into additional contributors to these differences and therefore lead to the discovery of as yet unknown mediators of these important processes.
Acknowledgments
Sources of Support
This study was supported by a grant from the National Institutes of Health (HL-65191).
Footnotes
Disclosure of Conflicts of Interests
The authors state that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis (Eitzman series) Arteriosclerosis, Thrombosis & Vascular Biology. 2007;27:2079–93. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- 2.Hoover-Plow J, Shchurin A, Hart E, Sha J, Hill AE, Singer JB, Nadeau JH. Genetic background determines response to hemostasis and thrombosis. BMC Blood Disord. 2006;6:6. doi: 10.1186/1471-2326-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sudo T, Ito H, Kimura Y. Genetic strain differences in platelet aggregation of laboratory mice. Thrombosis & Haemostasis. 2006;95:159–65. [PubMed] [Google Scholar]
- 4.Sudo T, Ito H, Hayashi H, Nagamura Y, Toga K, Yamada Y. Genetic strain differences in platelet aggregation and thrombus formation of laboratory rats. Thrombosis & Haemostasis. 2007;97:665–72. [PubMed] [Google Scholar]
- 5.Harmon KJ, Couper LL, Lindner V. Strain-dependent vascular remodeling phenotypes in inbred mice. American Journal of Pathology. 2000;156:1741–8. doi: 10.1016/S0002-9440(10)65045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berk BC, Korshunov VA. Genetic determinants of vascular remodelling. Canadian Journal of Cardiology. 2006;22 (Suppl B):6B–11B. doi: 10.1016/s0828-282x(06)70980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the “Glagov phenomenon” is genetically determined.[see comment] Circulation. 2004;110:220–6. doi: 10.1161/01.CIR.0000134958.88379.2E. [DOI] [PubMed] [Google Scholar]
- 8.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 9.Paigen B, Ishida BY, Verstuyft J, Winters RB, Albee D. Atherosclerosis susceptibility differences among progenitors of recombinant inbred strains of mice. Arteriosclerosis. 1990;10:316–23. doi: 10.1161/01.atv.10.2.316. [DOI] [PubMed] [Google Scholar]
- 10.Pan S, Kleppe LS, Witt TA, Mueske CS, Simari RD. The effect of vascular smooth muscle cell-targeted expression of tissue factor pathway inhibitor in a murine model of arterial thrombosis. Thrombosis & Haemostasis. 2004;92:495–502. doi: 10.1160/TH04-01-0006. [DOI] [PubMed] [Google Scholar]
- 11.Broze GJ, Jr, Yin ZF, Lasky N. A tail vein bleeding time model and delayed bleeding in hemophiliac mice. Thrombosis & Haemostasis. 2001;85:747–8. [PubMed] [Google Scholar]
- 12.Pawlinski R, Pedersen B, Erlich J, Mackman N. Role of tissue factor in haemostasis, thrombosis, angiogenesis and inflammation: lessons from low tissue factor mice. Thrombosis & Haemostasis. 2004;92:444–50. doi: 10.1160/TH04-05-0309. [DOI] [PubMed] [Google Scholar]
- 13.Westrick RJ, Bodary PF, Xu Z, Shen YC, Broze GJ, Eitzman DT. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044–6. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 14.Singh R, Pan S, Mueske CS, Witt T, Kleppe LS, Peterson TE, Slobodova A, Chang JY, Caplice NM, Simari RD. Role for tissue factor pathway in murine model of vascular remodeling.[see comment] Circulation Research. 2001;89:71–6. doi: 10.1161/hh1301.092508. [DOI] [PubMed] [Google Scholar]
- 15.Singh R, Pan S, Mueske CS, Witt TA, Kleppe LS, Peterson TE, Caplice NM, Simari RD. Tissue factor pathway inhibitor deficiency enhances neointimal proliferation and formation in a murine model of vascular remodelling. Thrombosis & Haemostasis. 2003;89:747–51. [PubMed] [Google Scholar]
- 16.Dahm A, Van Hylckama Vlieg A, Bendz B, Rosendaal F, Bertina RM, Sandset PM. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood. 2003;101:4387–92. doi: 10.1182/blood-2002-10-3188. [DOI] [PubMed] [Google Scholar]
- 17.Amini-Nekoo A, Futers TS, Moia M, Mannucci PM, Grant PJ, Ariens RA. Analysis of the tissue factor pathway inhibitor gene and antigen levels in relation to venous thrombosis. British Journal of Haematology. 2001;113:537–43. doi: 10.1046/j.1365-2141.2001.02752.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoke M, Kyrle PA, Minar E, Bialonzcyk C, Hirschl M, Schneider B, Kollars M, Weltermann A, Eichinger S. Tissue factor pathway inhibitor and the risk of recurrent venous thromboembolism. Thrombosis & Haemostasis. 2005;94:787–90. doi: 10.1160/TH05-06-0412. [DOI] [PubMed] [Google Scholar]
- 19.Blann AD, Amiral J, McCollum CN, Lip GY. Differences in free and total tissue factor pathway inhibitor, and tissue factor in peripheral artery disease compared to healthy controls. Atherosclerosis. 2000;152:29–34. doi: 10.1016/s0021-9150(99)00444-x. [DOI] [PubMed] [Google Scholar]
- 20.Novotny WF, Brown SG, Miletich JP, Rader DJ, Broze GJ., Jr Plasma antigen levels of the lipoprotein-associated coagulation inhibitor in patient samples. Blood. 1991;78:387–93. [PubMed] [Google Scholar]
- 21.Luyendyk JP, Tilley RE, Mackman N. Genetic susceptibility to thrombosis. Current Atherosclerosis Reports. 2006;8:193–7. doi: 10.1007/s11883-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 22.Li TT, Larrucea S, Souza S, Leal SM, Lopez JA, Rubin EM, Nieswandt B, Bray PF. Genetic variation responsible for mouse strain differences in integrin alpha 2 expression is associated with altered platelet responses to collagen. Blood. 2004;103:3396–402. doi: 10.1182/blood-2003-10-3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werling RW, Zacharski LR, Kisiel W, Bajaj SP, Memoli VA, Rousseau SM. Distribution of tissue factor pathway inhibitor in normal and malignant human tissues. Thrombosis & Haemostasis. 1993;69:366–9. [PubMed] [Google Scholar]
- 24.Caplice NM, Mueske CS, Kleppe LS, Peterson TE, Broze GJ, Jr, Simari RD. Expression of tissue factor pathway inhibitor in vascular smooth muscle cells and its regulation by growth factors. Circ Res. 1998;83:1264–70. doi: 10.1161/01.res.83.12.1264. [DOI] [PubMed] [Google Scholar]
- 25.Caplice NM, Mueske CS, Kleppe LS, Simari RD. Presence of tissue factor pathway inhibitor in human atherosclerotic plaques is associated with reduced tissue factor activity. Circulation. 1998;98:1051–7. doi: 10.1161/01.cir.98.11.1051. [DOI] [PubMed] [Google Scholar]
- 26.Crawley J, Lupu F, Westmuckett AD, Severs NJ, Kakkar VV, Lupu C. Expression, localization, and activity of tissue factor pathway inhibitor in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2000;20:1362–73. doi: 10.1161/01.atv.20.5.1362. [DOI] [PubMed] [Google Scholar]


