Abstract
Prenatal and postnatal methylmercury (MeHg) exposure has been shown to increase neuronal excitability and seizure susceptibility. To determine if early postnatal MeHg exposure causes a similar effect, we examined changes in field potentials in layer II/III neurons in cortical slices of rat following in vivo MeHg treatment. Rats received 0 (0.9% NaCl), 0.75 or 1.5 mg/kg/day MeHg subcutaneously for 15 or 30 days beginning on postnatal day 5, after which cortical slices were prepared for field potential recordings. In slices from rats treated with vehicle, single pulse stimulation of layer IV of cortical slices induced a typical field excitatory postsynaptic potential (fEPSP) with a single spike. This type of fEPSPs was also seen in slices from rats with 15 day treatment with 0.75 or 1.5 mg/kg/day MeHg. However, 30 day treatment with either MeHg dose resulted in fEPSPs with multiple spikes (epileptiform activity) in 40% of animals examined. This epileptiform activity remained observable in 50 – 60% animals in which MeHg exposure had been terminated for 30 days. However, slices from control animals still showed fEPSPs with single spike. Thus, these data suggest that postnatal MeHg exposure in vivo altered neuronal excitability and induced a long-lasting hyperexcitability in cortical neurons.
Keywords: Methylmercury, extracellular microelectrode recording, synaptic transmission, epileptiform activity, rat cortical slice
Introduction
Methylmercury (MeHg), a ubiquitous environmental neurotoxicant, is particularly toxic to the developing brain (Amin-Zaki et al., 1974; Choi, 1989). Prenatal exposure to low levels of MeHg from maternal fish diet has been shown to be associated with deficits in neurological and neuropsychological development in children (Grandjean et al., 1997, 1998, 1999; Lebel et al., 1998; Cordier et al., 2002), whereas children with prenatal exposure to higher levels of MeHg in the cases of Minamata disease in Japan during 1950s to 1960s (Harada, 1968, 1979, 1995) and acute MeHg poisoning in Iraq in 1970s (Amin-Zaki et al., 1976, 1979; Marsh et al., 1987) and other sporadic cases (Synder, 1971; Davis, 1994) often suffered severe neurological disorders such as ataxia, visual disturbance, mental retardation and death. Notably, hyperactivity and seizures are often seen in children who were born to women who had acute or chronic MeHg exposure due to consumption of MeHg-contaminated fish or food (Harada, 1968, 1979; Marsh et al., 1987; Davis, 1994). Similarly, the offspring of experimental rats that were exposed to low levels of MeHg prenatally and postnatally showed an increase in neuronal excitability and sensitivity to seizure induction (Szász et al., 1999; Világi et al., 2000). Moreover, prenatal exposure of pregnant mice to a single dose of 12 mg/kg MeHg on day 10 of gestation or three dose of 4 mg/kg/day on day 10, 11 and 12 of gestation by subcutaneous (s.c.) injection significantly increased the susceptibility of postnatal offspring to flurothy-induced convulsion (Su and Okita, 1976) and the audiogenic seizures (Menashi et al., 1982). Thus, prenatal plus postnatal MeHg exposure appears to cause abnormal neuronal excitability and facilitate seizure induction. However, information on the effect of early postnatal exposure in vivo to MeHg on neuronal excitability in the developing brains remains limited.
During the early postnatal development, extensive modifications of the neuronal circuits and associated synaptic function take place in the brains of humans and animals. These modifications are neuronal activity-dependent and require highly specific formation and elimination of certain synapses between neurons located in the same or different cortical layers (for review, see Katz and Shatz, 1996; Hohnke and Sur, 1999; Sur et al., 1999; Penn, 2001; Grubb, 2004; Sur and Rubenstein, 2005; Majewska and Sur, 2006; Marsh et al., 2008). Thus, the early postnatal life is also a critical period for brain development and relatively vulnerable to diseases or environmental insults (for review, see Rodier, 1995; Selevan et al., 2000; Rice and Barone, 2000; Hass, 2006). Since MeHg affects the entire process of the brain development including proliferation, migration and differentiation of neurons and synaptogensis (for review, see Rice and Barone, 2000), it is conceivable that early postnatal exposure to MeHg causes changes in neuronal excitability. Moreover, it has been previously shown that MeHg preferentially affected GABAergic neurons or GABAergic inhibitory synaptic responses to glutamatergic excitatory responses (Shaw et al., 1975; O’Kusky, 1985; Yuan and Atchison, 1997; Fountain and Rowan, 2000). Therefore, it will be not surprising if early postnatal MeHg exposure also causes increased neuronal excitability. In the present study, we specifically examined changes in synaptic responses of layer II/III neurons in cerebral cortical slices of rat following in vivo MeHg exposure using field potential recording techniques. Our results show that early postnatal MeHg exposure in vivo induced a time-dependent, long-lasting increase in neuronal excitability in the cortical slices of rat.
Materials and Methods
Chemicals and Solutions
Methylmercury chloride (MeHg) was obtained from ICN Biomedical Inc. (Costa Mesa, CA). It was dissolved in physiological saline to the final concentration of 4 mg/ml as a stock solution. Solutions for s.c. injection were diluted from the stock solution with 0.9% NaCl based on the principle of equal volume injection per kg of animal body weight. 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and amino-5-phosphonopentanoic acid (APV), (-)-bicuculline methiodide were purchased from Sigma Chemical Co. (St Louis, MO).
Brain “slicing” solution contains (in mM): 125 NaCl; 2.5 KCl; 4 MgCl2; 1.25 KH2PO4; 26 NaHCO3; 1 CaCl2 and 20 D-glucose, pH 7.35–7.4 when saturated with 95% O2 −5% CO2.
Artificial cerebrospinal fluid (ACSF) contains (in mM): 125 NaCl; 2.5 KCl; 1 MgCl2; 1.25 KH2PO4; 26 NaHCO3; 2 CaCl2 and 25 D-glucose; pH 7.35–7.4 when saturated with 95% O2 −5% CO2.
Animals and Treatment
All animal procedures complied with the National Institutes of Health of the USA guidelines on animal care and use (http://grants.nih.gov/grants/olaw/olaw.htm and http://grants.nih.gov/grants/olaw/references/phspol.htm#USGovPrinciples) and were approved by Michigan State University Institutional Animal Use and Care Committee (http://www.iacuc.msu.edu). Male Sprague-Dawley rat pups along with mother rats were purchased from Charley River Laboratories (Wilmington, MA). Animals were provided with water and food ad libitum with 12:12 light/dark cycle. 90 rat pups from 16 litters were randomly assigned to three groups: control (0.9% NaCl), low dose of MeHg (0.75mg/kg/day) and high dose of MeHg (1.5mg/kg/day). The MeHg dosages are chosen based on previous literature (Kakita et al., 2000; Shigematsu et al., 2000; Sakamoto et al., 2004) in which the selected doses for the present study did not cause any significant pathological alteration in the cerebral cortex. MeHg exposure began at postnatal day 5 (PND5). Because rat pups at this age are not capable of drinking water themselves, we used the model described by O’Kusky (1985) to administer MeHg to rat pups. Physiological saline or MeHg were injected s.c. for 15 (PND20) or 30 consecutive days (PND35), respectively. During exposure, the animal body weight, general health and behaviors were monitored daily. After completion of 15 or 30 day injections, 7 – 11 rats from each group were sacrificed for brain slice preparations. The remaining rats of each group were kept for an additional 30 days without any further treatment. These rats were euthanized at 60 days and brain slices were prepared using identical procedures to those for rats at 15 or 30 days. These subgroups were designed specifically to examine if any effect of MeHg on synaptic transmission is reversible after an extra 30 day of tissue “Clearance” of MeHg. The total mercury contents in brain tissues were examined and reported previously by Dasari and Yuan (2009).
Preparation of cortical slices
At PND20, 35 or 65, after completion of designed durations for injections or “clearance”, the rat brains were quickly isolated after rats were first exposed to CO2 to induce narcosis and then decapitated. Coronal cortical slices (350 – 400 µm) were prepared from the brains of rats in ice-cold, oxygenated “slicing” solution saturated with 95% O2 /5% CO2. The cortical slices were prepared from the neocortex using procedures described previously (Dasari and Yuan, 2009). In brief, a brain tissue block containing the neocortex portion was glued onto the tissue pedestal of an NVMSL1 motorized advanced Slicer (World Precision Instruments, Inc. Sarasota, FL) with cyanocrylate glue. After cutting off the first 500 – 600 µm from the occipital pole, the tissue block was transected transversely to produce 5 – 8 coronal slices. Slices were then transferred to a home-made holding chamber containing ACSF (see Chemicals and Solutions) aerated continuously with 95% O2 /5% CO2, incubated at room temperature of 22–25 0C for at least 60 min before electrophysiological recording.
Extracellular recordings of field potentials
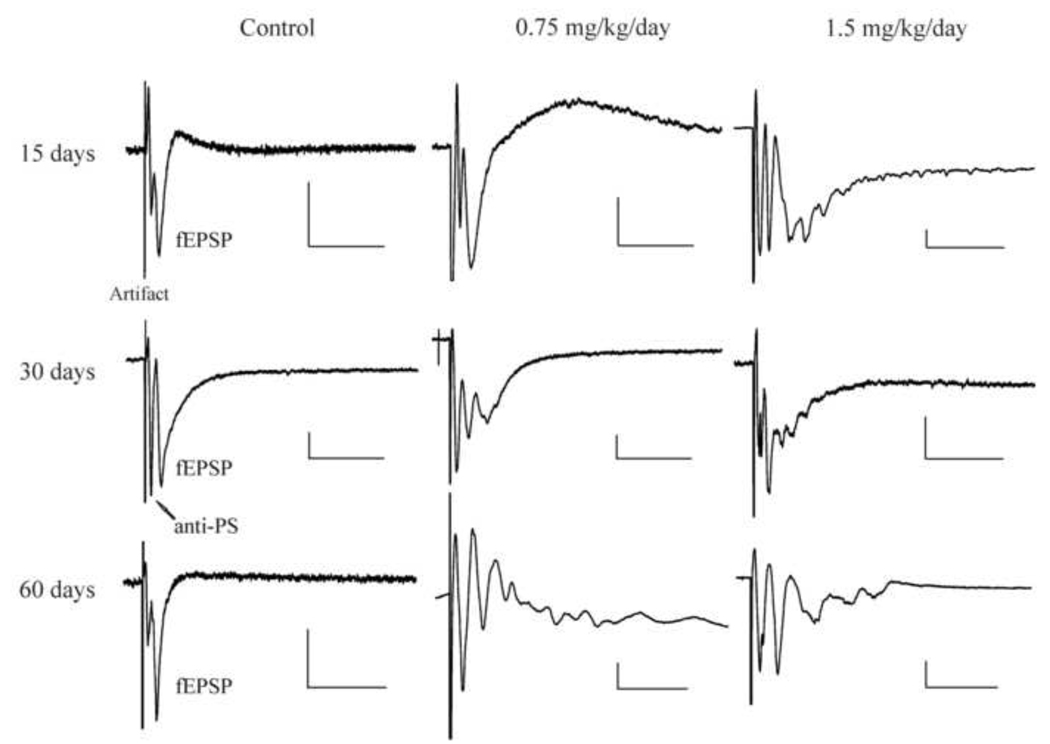
One slice was transferred to a recording chamber and mechanically fixed using a “U” shape anchor made of a platinum wire frame with nylon mesh. The slice was superfused (2 – 4 ml/min) continuously with ACSF by gravity. The laminar structure of the cerebral cortex in slices was visually identified under low power (4 x) magnification of an upright microscope. The field potentials were recorded from layer II/III neurons using a glass electrode with resistance of 2 – 7 MΩ when filled with ACSF and evoked by a broken-tip glass stimulating electrode in layer IV. Isolated stimuli were generated from a Grass S88 stimulator (Grass, Inc, Quincy, MA) at a frequency of 0.2 Hz, 0.1 ms duration and intensity that was adjusted to cause about 50% of the maximal response. The stimulating electrode had an impedance of 1 – 4 MΩ when filled with ACSF. The field potentials of layer II/III neurons in cortical slice evoked by a single pulse stimulation of layer IV neurons usually consists of two components as shown in Figure 1 (Left panel): The first downward potential is an antidromically-activated population spike mediated possibly by antidromic activation of axons of layer II/III neurons, whereas the second downward potential (fEPSP) is a presynaptic stimulation-evoked, glutamatergic excitatory postsynaptic response. Detailed characterization of these responses has been previously described in Dasari and Yuan (2009). Normally, for a healthy slice, only one fEPSP should be evoked by a single stimulus even at stimulation intensity that induces the maximal response. If more than one fEPSP in a given slice appear in response to a single submaximal stimulation, it indicates that neuronal excitability in the slice is enhanced (hyperexcitability). This multiple spike activity in field potential recording is also often termed as burst-like or epileptiform activity (Hablitz, 1984; Hochmann et al., 1999; Margineanu et al., 2004).
Figure 1.
In vivo MeHg exposure induced epileptiform responses in cortical slices of rat. Left, field potentials (fEPSPs) in layer II/III evoked by stimulating layer IV of cortical slice prepared from three representative rats treated by subcutaneous injection (s.c.) of 0.9% NaCl for consecutive 15, 30 or 30 days of injection plus an additional 30 observation day without any further treatment (60 days). Middle and Right, similar recordings as shown in Left panel made in cortical slices prepared from rats treated by s.c. injection of 0.75 (Middle) or 1.5 mg/kg/day MeHg (Right) for consecutive 15, 30 or 30 days of injection plus an additional 30 observation day without any further treatment (60 days). Note that each representative trace is the average of 15 – 20 subsequent traces recorded in a given experiment under the same conditions. Because appearances of the multiple spikes in each trace were not synchronized exactly at the same time points, multiple spikes in the averaged trace were often smaller than those appearing in the individual traces prior to averaging. Abbreviation: Artifact, stimulation artifact; anti-PS, antidromic population spike evoked by stimulation of axons of layer II/III neurons. The size of antidromic responses vary from slice to slice; fEPSP, synaptically evoked glutamatergic excitatory postsynaptic responses. Note that multiple spikes following fEPSP are showing in slices prepared from animals that were treated with 0.75 or 1.5 mg/kg/day for 30 days or 30 days plus an addition 30 observation days. Each trace is a representative of 7 – 12 individual experiments. Calibration bars: vertical, 0.2 mV; horizontal, 40 ms.
The neuronal synaptic response properties can also be characterized by means of the input-output relation or curve. To examine the effects of MeHg exposure on the input-output relation of neurons, fEPSPs were evoked at a series of stimulus intensities varying from sub-threshold to maximum stimulations. Due to the nature of field potential recording, it is nearly impossible to make direct comparison of amplitudes of field potentials from different individual experiments. That is because amplitudes and shapes of field potentials vary greatly in different slices even from the same animal, in different locations of the recording electrode in the same slice, or even in the same region of the same slice but with different depths of the electrode tips in tissue and relative location of the recording and stimulating electrodes. The variation is even greater among slices from different animals. Thus, for comparison, the amplitudes of fEPSPs evoked by different stimulus intensities in a given experiment were normalized to the amplitude that is ~10% of the maximum response obtained in the same experiment. The primary reason for using ~10% of the maximum amplitude rather than the threshold or maximum amplitude for normalization is that either threshold or maximum amplitude is relatively insensitive to changes in stimulation intensity and thus could potentially introduce more variations. Correspondingly, all stimuli with different intensities were normalized to the stimulus that generated the 10% of the maximum response (amplitude). To construct the stimulus-response (input-output) curve, the normalized amplitudes of fEPSPs were plotted as a function of the normalized stimulus intensity.
Data acquisition was as described previously in Yuan and Atchison (1993). Field potential signals from recording electrodes were amplified using a Model 3000 AC/DC differential amplifier (A-M Systems, Inc., Carlsborg, WA) and filtered at 5 kHz with a 4-pole low-pass Bessel filter and digitized at 10 – 20 kHz for later off-line analysis using pClamp 9.0 program (Axon Instruments, Union City, CA). All experiments were carried out at room temperature of 22 – 25 °C.
Statistical analysis
Data were analyzed statistically using Fisher’s exact test or one-way analysis of variance (ANOVA) for multiple MeHg dose- or stimulus-dependent measures. Dunnett’s procedure was used for post hoc comparison (Tukey-Kramer multiple comparison test). Values were considered statistically significant at P ≤ 0.05. Statistical analyses were done using SigmaPlot 11.0 (Systat Software Inc., San Jose, CA). Each experiment was replicated in a minimum of 7 – 10 animals; the actual number of replicates for each experiment is listed in the corresponding figure legend or table. Although multiple slices from a given animal or multiple cells from a given slice might be examined, the data were averaged and the sample size # (n) was counted as one.
Results
We have shown previously that there was no significant change in the rate of body weight gain of rats among three groups during MeHg treatments under the present experimental conditions, (Dasari and Yuan, 2009). There were also no overt abnormal physical development and neurobehavioral changes during MeHg exposure and “clearance”. However, field potential recordings in acutely isolated cortical slices revealed an abnormal change in postsynaptic potentials following MeHg exposure. In slices prepared from control rats, injection of 0.9% NaCl for different durations did not cause any significant change in glutamtate receptor-mediated fEPSPs (Figure 1, left panel). Exposure of animals to 0.75 mg/kg/day MeHg for 15 days also did not cause any change in fEPSPs (Figure 1, middle panel). The majority slices from rats treated with 1.5 mg/kg/day MeHg for 15 days showed no burst-like or epileptiform activity, although slices from one animal exposed to 1.5 mg/kg/day for 15 days showed multiple spike responses (Figure 1, right panel). However, after exposure of animals to either dose of MeHg for 30 days, multiple spikes were observed in about 40% of the animals examined (P < 0.05). Interestingly, the epileptiform activity became observable in 50% and 60%, respectively for 0.75 or 1.5 mg/kg/day MeHg dose, of rats examined even after MeHg exposure had been terminated for 30 days (Figure 1, 60 days). The differences between control and MeHg (0.75 or 1.5 mg/kg/day) treated animals are statistically significant (P < 0.01). These data are summarized in Table 1. Thus, these results suggest that early postnatal MeHg exposure induced a time-dependent and long-lasting alteration of neuronal membrane excitability in the cerebral cortex.
Table 1.
Comparison of rates of cortical slices showing epileptiform activity following postnatal exposure of rats to 0.9% NaCl, 0.75 or 1.5 mg/kg/day MeHg for 15, 30 days or 30 days plus extra 30 days of observation.
| Treatment (Day) |
Control (0.9% NaCl) |
MeHg (0.75 mg/kg/day) |
MeHg (1.5 mg/kg/day) |
|---|---|---|---|
| 15 | 0% (0/10)# | 0% (0/10) | 14.3% (1/7) |
| 30 | 0% (0/10) | 45.4% (5/11) | 44.4% (4/9) |
| 60 | 0% (0/10) | 50.0% (6/12)** | 60.0% (6/10)** |
The number of rats from all animals examined showed epileptiform activity.
Differences between control and MeHg treated groups are statistically significant (* p < 0.05, ** p < 0.01).
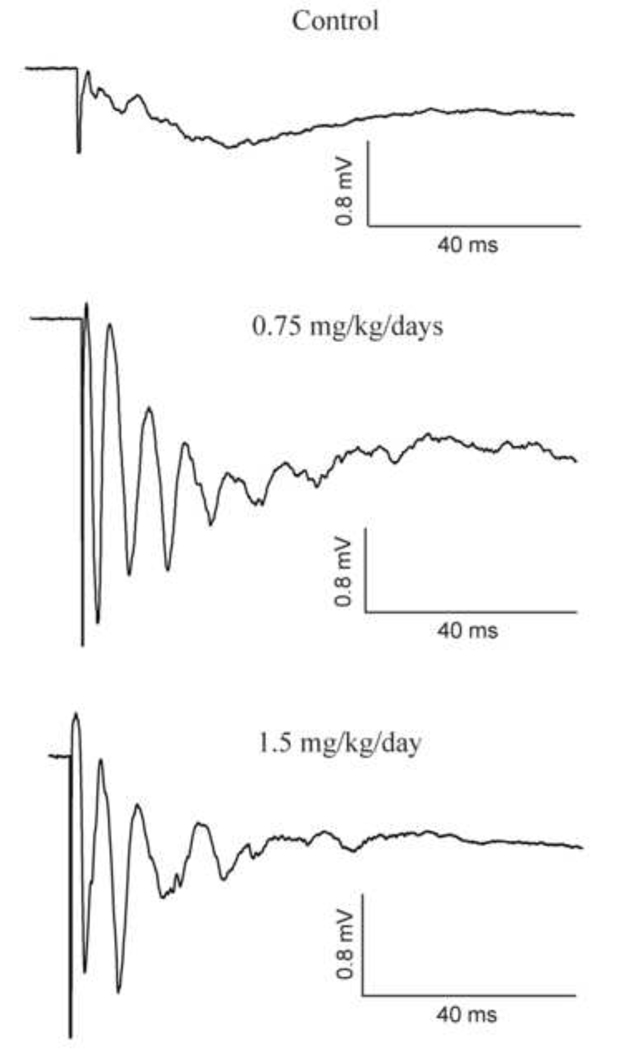
It has been previously shown that bath application of MeHg preferentially blocked GABAergic synaptic responses and induced an epileptiform activity that was similar to that caused by GABAA receptor antagonist bicuculline in hippocampal slices (Yuan and Atchison, 1997). Therefore, one possibility is that the hyperexcitability in slices from MeHg-treated animals could result from reduced GABAergic inhibition (disinhibition) following MeHg exposure. If this is the case, then neuronal excitability in slices prepared from MeHg-exposed animals should be more sensitive to bicuculline than slices from control animals. To test this, we next compared effects of bicuculline on field potentials in slices from control and MeHg-treated animals. Three representative recordings in slices prepared from rats that were treated, respectively, with 0.9% NaCl (Control), 0.75 mg/kg/day or 1.5 mg/kg/day MeHg for 30 days plus extra 30 days of MeHg “clearance” are shown in Figure 2. In control slices, 10 µM bicuculline induced a giant, long depolarization response with some small fluctuations. This phenomenon was observed in all slices prepared from control animals. In contrast, the same concentration of bicuculline not only induced a giant depolarization response, but also caused multiple spikes superimposed on the depolarization responses in slices from MeHg-treated animals. These results suggest that MeHg exposure increased the susceptibility of cortical neurons to bicuculline-induced epileptiform activity.
Figure 2.
MeHg exposure increased sensitivity of neurons to bicuculline. fEPSPS were recorded in three representative slices from PND65 rats that were treated with 0.9% NaCl (Control) or 0.75 or 1.5 mg/kg/day MeHg for 30 days plus 30 days of “clearance” in the presence of 10 µM bicuculline in the bath. Again, each trace is the average of 15 – 20 subsequent traces recorded in a given experiment under the same conditions. Each trace is a representative of 4 – 6 individual experiments.
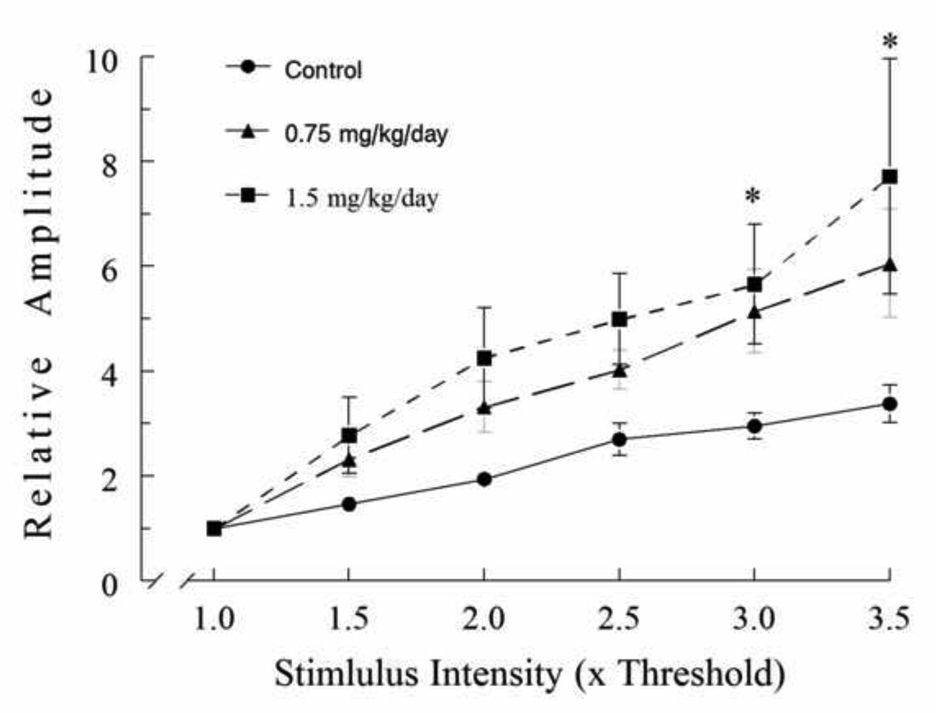
If neuronal excitability is increased following MeHg exposure, then the threshold or sensitivity of neurons to electrical activation should be changed. To further test this, we examined effects of MeHg exposure on the input-output relation of fEPSPs. To do this, fEPSPs were evoked by increasing stimulation intensities gradually from the threshold to maximum levels. Figure 3 shows the input-output curves obtained from PND65 rats treated with 0.9% NaCl, 0.75 or 1.5 mg/kg/day MeHg, respectively. Clearly, the amplitudes of fEPSPs increased proportionally as the stimulus intensity increased. However, increases in amplitudes of fEPSPs are more prominent in slices prepared from MeHg-treated animals than those in slices from control rats. That means that relatively less stimulus intensity was required to evoke a similar level of change in fEPSP amplitude in slices from MeHg-treated animals than those in slice from control animals. Thus, these data again suggest that MeHg exposure enhanced neuronal excitability by reducing the threshold for excitation. However, no similar changes in the input-output curves were seen in slices prepared from animals treated with MeHg for 15 or 30 days, suggesting that this effect may be time-dependent.
Figure 3.
MeHg exposure enhanced the sensitivity of cortical neurons to electrical activation. fEPSPs were recorded in slices from rats that were treated with 0.9% NaCl (Control) or MeHg (0.75 or 1.5 mg/kg/day) for 30 days plus an additional 30 day without any further treatment. fEPSPs were evoked by varying stimulation intensity from sub-threshold that failed to evoke any fEPSP to maximum stimulation that generated maximum fEPSP responses. After normalization as described in Materials and Methods, the input-output curves were constructed as fEPSP responses against stimulation intensities. All values are the mean ± SEM of 5 – 8 individual recordings from 4 animals. The asterisk indicates a statistically significant difference between control and 1.5 mg/kg/day MeHg-treated animals (p < 0.05).
Discussion
The primary finding of the present study is that early postnatal exposure of rats in vivo to MeHg induced hyperexcitability in neurons of rat cortical slices. Consistently, MeHg exposure increased sensitivity of neurons to bicuculline and altered the input-output relation. These changes were MeHg exposure time-dependent and long-lasting.
Previous epidemiological and clinical findings have shown that acute or chronic MeHg exposure caused an increased prevalence rate of seizures or epilepsy in patients with acute or chronic MeHg poisoning (Harada, 1968, 1979, 1995; Marsh et al. 1987). In the Minamata Bay area of Japan, one of the worst MeHg-contaminated regions during 1950s, 8 – 9% of patients with Minamata disease (MD, a neurological syndrome caused by severe MeHg poisoning) had epileptic seizures; whereas only 0.8% of the age and sex-matched controls in a non-MeHg-contaminated area exhibited epilepsy. The frequency of convulsive seizures in congenital MD was even higher (Harada, 1979). Epileptic seizures were seen in about 50 – 83% of congenital MD patients in the Minamata bay area. The mercury content in the hair of children with congenital MD was 40.3 ± 30.3 µg/g, whereas the mercury content in the hair of control individuals was < 7 µg/g (Harada, 1979). Clearly, the incidence of epilepsy among MD patients was MeHg exposure-dependent. Consistently, in episode of MeHg poisoning in Iraq, children with prenatal and postnatal MeHg exposure also had increased incidence of seizures in a MeHg exposure level-dependent manner (Marsh et al., 1987). When the maximum hair mercury contents were <75 ppm, none of the 53 children had seizures; when the maximum hair mercury contents were >75 ppm, 7 of 28 (25%) children had seizures. In addition, in a New Mexico family in the USA, four children with pre- and/or postnatal exposure to MeHg due to consumption of MeHg-contaminated food for three months developed severe neurological disorders (Davis et al., 1994). Three of the four children had seizures. The total mercury (Hg) content in the cerebral cortex from one of the children who died 22 years later was still over 50 times those of the control patient (~1.595 vs 0.038 µg/g). Thus, prenatal and postnatal MeHg exposure appears to enhance the susceptibility of children to epileptic seizures.
Changes in neuronal excitability were also seen in experimental animals following pre-and postnatal MeHg exposure. Exposure of mother rats to 0.375 mg/kg/day of MeHg during mating period, through gestation and lactation period caused a significantly facilitated induction and propagation of epileptic discharges in the brains of 4-weeks-old offspring. Electrocorticographic examinations showed that epileptic activity spread over the whole cortical surface of MeHg-treated animals, suggesting an increased susceptibility of MeHg-treated animals to epileptic activity. Consistent with the MeHg-induced epileptic activity was the brain Hg concentration was higher in the brains of MeHg-treated rats (1.23 ± 0.29 µg/g) than in control rats (0.20 ± 0.15 µg/g) (Szász et al., 1999). In agreement with this finding, similar pattern of prenatal and postnatal exposure of rats to MeHg enhanced neuronal membrane excitability (Világi et al., 2000). MeHg reduced the input resistance and thresholds for evoking the excitatory postsynaptic potentials and spikes. In addition, MeHg also shifted the input-output curve to more negative potentials (i.e. the same stimulus intensity evoked more number of spikes in MeHg-treated rats than in control rats). All these changes indicated a higher neuronal excitability following MeHg exposure. Similarly, the susceptibility of mice offspring to flurothy-induced convulsion (Su and Okita, 1976) and audio-induced seizures (Menashi et al., 1982) were increased significantly following prenatal exposure of mother mice to a single dose (8 or 12 mg/kg body weight) of MeHg on Day 10 or 12 of gestation. Thus, our results are generally consistent with those findings and suggest that early postnatal exposure of rats to MeHg also altered neuronal excitability and induced epileptiform activity in cortical neurons.
Consistent with our previous observations of in vivo effects of MeHg on short-term synaptic plasticity in the visual cortex (Dasari and Yuan, 2009), no clear dose-dependent effect of MeHg on neuronal excitability in the neocortex was observed in the present study. One possible reason is that only two MeHg doses with a limited dose range were used in the present study and both doses might be at the high end of dose-response relationship. Therefore, further confirmation with a wider range of MeHg exposure doses, more time points and lager sample size should be considered in order to determine the dose-dependent effect of MeHg on neuronal excitability in the cortex.
The mechanisms underlying MeHg induced epileptiform activity remain to be determined. One possibility is that MeHg-induced neuronal hyperexcitability could result from its preferential inhibitory effects on GABAergic system because: 1). GABAergic neurons, especially the aspinous or sparsely-spinous stellate interneurons in the layer IV of neocortex, were particularly sensitive to MeHg (Shaw et al., 1975; O'Kusky, 1985); 2) GABAergic inhibitory synaptic responses were more sensitive to MeHg than were glutamtergic excitatory synaptic responses in vitro in hippocampal slices (Yuan and Atchison, 1997; Fountain and Rowan, 2000) and 3) The MeHg-induced multiple spike or epileptiform activity is similar to that seen in control slices that were treated with the GABAA receptor antagonist bicuculline in the present and previous studies (Yuan and Atchison, 1997). However, further investigations are needed to determine if effects of MeHg on GABAergic systems are the primary cause of increased neuronal excitability.
One interesting finding in the present study is that the epileptiform activity did not disappear after MeHg exposure had been terminated for 30 days; the brain total Hg contents had been reduced from 0.86 ± 0.46 and 1.87 ± 0.87 µg/g at the end of the 30-day treatment to 0.08 ± 0.03 and 0.18 ± 0.06 µg/g, respectively, for 0.75 and 1.5 mg/kg/day MeHg groups (Dasari and Yuan, 2009). Instead, it persisted and became observable in 50 – 60 % of animals. This delayed increase in epiliptiform activity is consistent with the characteristic “latent” period seen in patients (Harada, 1995; Nierenberg et al., 1998; Weiss et al., 2002) and monkeys with MeHg poisoning (Rice and Gilbert, 1982). The mechanism for this delayed or long-lasting MeHg effect remains unclear. It has been previously shown that prenatal or neonatal MeHg exposure induced significant changes in synaptic circuits and number and morphology of dendritic spines (O'Kusky, 1985; Stoltenburg-Didinger and Markwort, 1990). Thus, the delayed or long-lasting changes in synaptic responses could be also related to alterations in synaptic circuits or microstructures following MeHg exposure.
In children with MD in Minamata and Niigata area, Japan, the total brain Hg levels varied depending on the types of MD. The total brain Hg contents were 0.04 – 0.7 µg/g in children with prenatal and postnatal exposure, whereas the total brain Hg contents were 1.3 – 14.4 µg/g in children with acute postnatal exposure (Takeuchi and Eto, 1979). It is estimated that a minimum 0.26 µg/g Hg in the brain would lead to affliction, and 5 µg/g or more would lead to death (Takeuchi and Eto, 1979). The question is why no behavioral seizures were observed in animals during MeHg exposure and “clearance” period even the brain total Hg contents were as high as 1.87 ± 0.87 µg/g at the end of 30 days of exposure to 1.5 mg/kg/day MeHg under our experimental condition (Dasari and Yuan, 2009). One possible reason is the differential sensitivity between rat and human, with human more sensitive to MeHg neurotoxicity. Another possible explanation is that the MeHg-induced effects might be still in the so-called “latent” period and not develop into clinical or behavioral seizures yet. Thus, longer period of observation and continuous monitoring methods should be used to confirm whether or not behavioral seizures are inducible under the present experimental conditions.
Seizures occur more frequently in the neonatal period and early childhood than at any other time in life. The high incidence of seizures in the first decade of life and the propensity of children is reflective of increased susceptibility of the immature brain to seizures. If prenatal and/or postnatal MeHg exposure alters neuronal excitability and reduces the threshold for excitation, it will certainly increase the susceptibility of developing brains to seizures or even epilepsy. This may explain why congenital MD in Japan and fetal MeHg poisoning in Iraq had increased rates of seizures or epilepsy (Harada, 1968, 1979, 1995; Marsh et al. 1987). Nowadays, outbreak of acute or chronic MeHg poisoning that was seen in Japan or Iraq during 1950s or 1970s is unlikely to happen again. However, effects of chronic low level MeHg exposure on developing brains remain a major health concern because human exposure to MeHg today is primarily through consumption of MeHg-contaminated fish and seafood. Studies have shown that exposure to MeHg at levels that may not cause detectable signs or symptoms in mothers who consumed MeHg- contaminated fish or food during pregnancy and nursing period may pose a hazard to developing brains and cause subclinical developmental or neurosychological deficits in children (Grandjean et al., 1997, 1998, 1999, 2003; Murata et al., 1999; Counter and Buchanan, 2004; Auger et al., 2005; Murata et al., 2006; Debes et al., 2006; Trasand et al., 2006). One important question is whether or not pre- and/or postnatal exposure to low levels of MeHg plays any role in commonly-seen neonatal seizures including febrile seizures or even epilepsies. Given the fact that MeHg alters neuronal excitability and increases the frequency of seizures or epilepsy in patients or animals following prenatal and/or postnatal MeHg exposure as shown in previous and current studies, MeHg should, at least theoretically, facilitate induction of some types of neonatal seizures. To address this specific question, further investigation is needed.
In conclusion, early postnatal exposure of animals to relatively low levels of MeHg altered neuronal membrane excitability and induced a long-lasting epileptiform activity in cortical neurons in slices.
Acknowledgements
We thank Dr. William D. Atchison for his generous laboratory equipment support during preparation of this manuscript. This work is supported by NIEHS grants R21ES013767 and Michigan State University funding 06-HBRI-II-616.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
A preliminary report of part of these results was presented in an abstract form at the 38th Society for Neuroscience Annual Meeting, Washington D.C. November 15–19, 2008.
References
- Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood MR. Intra-uterine methylmercury poisoning in Iraq. Pediatr. 1974;54:587–595. [PubMed] [Google Scholar]
- Amin-Zaki L, Elhassani S, Majeed MA, Clarkson TW, Doherty RA, Greenwood MR, Giovanoli-Jakubczak T. Perinatal methylmercury poisoning in Iraq. Am. J. Dis. Child. 1976;130:1070–1076. doi: 10.1001/archpedi.1976.02120110032004. [DOI] [PubMed] [Google Scholar]
- Amin-Zaki L, Majeed MA, Elhassani SB, Clarkson TW, Greenwood MR, Doherty RA. Prenatal methylmercury poisoning. Clinical observations over five years. Am. J. Dis. Child. 1979;133:172–177. [PubMed] [Google Scholar]
- Auger N, Kofman O, Kosatsky T, Armstrong B. Low-level methylmercury exposure as a risk factor for neurological abnormalities in adults. Neurotoxicology. 2005;26:149–157. doi: 10.1016/j.neuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Choi BH. The effects of methylmercury on the developing brain. Prog. Neurobiol. 1989;32:447–470. doi: 10.1016/0301-0082(89)90018-x. [DOI] [PubMed] [Google Scholar]
- Cordier S, Garel M, Mandereau L, Morcel H, Doineau P, Gosme-Seguret S, Josse D, White R, Amiel-Tison C. Neurodevelopmental investigations among methylmercury-exposed children in French Guiana. Environ. Res. 2002;89:1–11. doi: 10.1006/enrs.2002.4349. [DOI] [PubMed] [Google Scholar]
- Counter SA, Buchanan LH. Mercury exposure in children: a review. Toxicol. Appl. Pharmacol. 2004;198:209–230. doi: 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Dasari S, Yuan Y. Low level postnatal methylmercury exposure in vivo alters developmental forms of short-term synaptic plasticity in the visual cortex of rat. Toxicol. Appl. Pharmacol. 2009;240:412–422. doi: 10.1016/j.taap.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, Clarkson TW. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann. Neurol. 1994;35:680–688. doi: 10.1002/ana.410350608. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotixicology. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain S, Rowan Effects of sequential exposure to multiple concentrations of methylmerrcury in the rat hippocampal slice. Ecotoxicol Environ Safety. 2000;47:130–136. doi: 10.1006/eesa.2000.1941. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F. Cognitive performance of children prenatally exposed to "safe" levels of methylmercury. Environ. Res. 1998;77:165–172. doi: 10.1006/enrs.1997.3804. [DOI] [PubMed] [Google Scholar]
- Grandjean P, White RF, Nielsen A, Cleary D, de Oliveira Santos EC. Methylmercury neurotoxicity in Amazonian children downstream from gold mining. Environ. Heal. Perspect. 1999;107:587–591. doi: 10.1289/ehp.99107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Budtz-Jørgensen E, Steuerwald U, Heinzow B, Needham LL, Jørgensen PL, Weihe P. Attenuated growth of breast-fed children exposed to increased concentrations of methylmercury and polychlorinated biphenyls. FASEB J. 2003;17:699–701. doi: 10.1096/fj.02-0661fje. [DOI] [PubMed] [Google Scholar]
- Grubb MS, Thompson ID. The influence of early experience on the development of sensory systems. Curr. Opin. Neurobiol. 2004;14:503–512. doi: 10.1016/j.conb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Hablitz J. Picrotoxin-induced epileptiform activity in hippocampus:Role of endogenous versus synaptic factors. J. Neurophysiol. 1984;51:1011–1027. doi: 10.1152/jn.1984.51.5.1011. [DOI] [PubMed] [Google Scholar]
- Harada Y. Study Group of Minamata Disease (eds), Minamata disease. Japan: Kumamoto University; 1968. Congenital (or fetal) Minamata Bay disease; pp. 73–91. [Google Scholar]
- Harada Y. Congenital Minamata Disease. In: Tsubaki T, Irukayama K, editors. Minamata disease: methylmercury poisoning in Minamata and Niigata, Japan. New York: Elsevier; 1979. pp. 209–269. [Google Scholar]
- Harada M. Minamata disease: Methylmercury poisoning in Japan caused by environmental pollution. Crit. Rev. Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Hass U. The need for developmental neurotoxicity studies in risk assessment for developmental toxicity. Reprod. Toxicol. 2006;22:148–156. doi: 10.1016/j.reprotox.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Hochman DW, D’Ambrosio R, Janigro D, Schwartzkroin PA. Extracellular chloride and the maintenance of spontaneous epileptiform activity in rat hippocampal slices. J. Neurophysiol. 1999;81:49–59. doi: 10.1152/jn.1999.81.1.49. [DOI] [PubMed] [Google Scholar]
- Hohnke CD, Sur M. Stable properties of spontaneous EPSCs and miniature retinal EPSCs during the development of ON/OFF sublamination in the ferret lateral geniculate nucleus. J. Neurosci. 1999;19:236–247. doi: 10.1523/JNEUROSCI.19-01-00236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakita A, Wakabayashi K, Su M, Sakamoto M, Ikuta F, Takahashi H. Distinct pattern of neuronal degeneration in the fetal rat brain induced by consecutive transplacental administration of methylmercury. Brain. Res. 2000;859:233–239. doi: 10.1016/s0006-8993(00)01964-8. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Sci. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Lebel J, Mergler D, Branches F, Lucotte M, Amorim M, Larribe F, Dolbec J. Neurotoxic effects of low-level methylmercury contamination in the Amazonian Basin. Environ. Res. 1998;79:20–32. doi: 10.1006/enrs.1998.3846. [DOI] [PubMed] [Google Scholar]
- Majewska AK, Sur M. Plasticity and specificity of cortical processing networks. Trends. Neurosci. 2006;29:323–329. doi: 10.1016/j.tins.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Margineanu DG, Klitgaard H. Caffeine-induced epileptiform field potentials in rat hippocampal slices: a pharmacological characterization. Neuropharmacology. 2004;47:926–934. doi: 10.1016/j.neuropharm.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Marsh DO, Clarkson TW, Cox C, Myers GJ, Amin-Zaki L, Al-Tikriti S. Fetal methylmercury poisoning. Relationship between concentration in single strands of maternal hair and child effects. Arch. Neurol. 1987;44:1017–1022. doi: 10.1001/archneur.1987.00520220023010. [DOI] [PubMed] [Google Scholar]
- Marsh R, Gerber AJ, Perterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childrenhood neuropsychiatric disorders. J Am Acad Child Adoles Psychiatty. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menashi M, Ornoy A, Yanai J. Transplacental effects of methylmercury chloride in mice with specific emphasis on the audiogenic seizure response. Dev. Neurosci. 1982;5:216–221. doi: 10.1159/000112679. [DOI] [PubMed] [Google Scholar]
- Murata K, Weihe P, Renzoni A, Debes F, Vasconcelos R, Zino F, Araki S, Jørgensen PJ, White RF, Grandjean P. Delayed evoked potentials in children exposed to methylmercury from seafood. Neurotoxicol Teratol. 1999;21:343–348. doi: 10.1016/s0892-0362(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Murata K, Sakamoto M, Nakai K, Dakeishi M, Iwata T, Liu X, Satoh H. Subclinical effects of prenatal methylmercury exposure on cardiac autonomic function in Japanese children. Int. Arch. Occup. Environ. Health. 2006;79:379–385. doi: 10.1007/s00420-005-0064-5. [DOI] [PubMed] [Google Scholar]
- Nierenberg DW, Nordgren RE, Chang MB, Siegler RW, Blayney MB, Hochberg F, Toribara TY, Cernichiari E, Clarkson T. Delayed cerebellar disease and death after accidental exposure to dimethylmercury. N. Engl. J. Med. 1998;338:1672–1676. doi: 10.1056/NEJM199806043382305. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR. Synapse elimination in the developing visual cortex: a morphometric analysis in normal and dark-reared cats. Brain Res. 1985;354:81–91. doi: 10.1016/0165-3806(85)90071-9. [DOI] [PubMed] [Google Scholar]
- Penn AA. Early brain wiring: activity-dependent processes. Schizophr. Bull. 2001;27:337–347. doi: 10.1093/oxfordjournals.schbul.a006880. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Early chronic low-level methylmercury poisoning in monkeys impairs spatial vision. Science. 1982;216:759–761. doi: 10.1126/science.7079739. [DOI] [PubMed] [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Heal. Perspect. 2000;108:511S–533S. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier PM. Developing brain as a target of toxicity. Environ. Health Perspect. 1995;103(S6):73–76. doi: 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Kakita A, de Oliveira RB, Pan HS, Takahashi H. Dose-dependent effects of methylmercury administered during neonatal brain spurt in rats. Dev. Brain Res. 2004;152:171–176. doi: 10.1016/j.devbrainres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children's health. Environ. Heal. Perspect. 2000;108:451S–455S. doi: 10.1289/ehp.00108s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw CM, Mottet NK, Body RL, Luschei ES. Variability of neuropathologic lesions in experimental methylmercurial encephalopathy in primates. Am. J. Pathol. 1975;80:451–470. [PMC free article] [PubMed] [Google Scholar]
- Shigematsu J, Yasuda T, Goto Y, Tanaka K, Tobimatsu S. Chronic effects of methylmercury on the cerebral function in rats. J. Neurol. Sci. 2000;182:69–75. doi: 10.1016/s0022-510x(00)00454-8. [DOI] [PubMed] [Google Scholar]
- Stoltenburg-Didinger G, Markwort S. Prenatal methylmercury exposure results in dendritic spine dysgenesis in rats. Neurotoxicol. Teratol. 1990;12:573–576. doi: 10.1016/0892-0362(90)90064-j. [DOI] [PubMed] [Google Scholar]
- Su M-Q, Okita GT. Behavioral effects on the progeny of mice treated with methylmercury. Toxico. Appl. Pharmacol. 1976;38:195–205. doi: 10.1016/0041-008x(76)90173-3. [DOI] [PubMed] [Google Scholar]
- Sur M, Angelucci A, Sharma J. Rewiring cortex: the role of patterned activity in development and plasticity of neocortical circuits. J. Neurobiol. 1999;41:33–43. doi: 10.1002/(sici)1097-4695(199910)41:1<33::aid-neu6>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Sci. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Synder RD. Congenital mercury poisoning. N. Engl. J. Med. 1971;284:1014–1016. doi: 10.1056/NEJM197105062841806. [DOI] [PubMed] [Google Scholar]
- Szász A, Barna B, Szupera Z, De Visscher G, Galbács Z, Kirsch-Volders M, Szente M. Chronic low-dose maternal exposure to methylmercury enhances epileptogenicity in developing rats. Int. J. Dev. Neurosci. 1999;17:733–742. doi: 10.1016/s0736-5748(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Eto K. Congenital Minamata Disease. In: Tsubaki T, Irukayama K, editors. Minamata disease: methylmercury poisoning in Minamata and Niigata, Japan. New York: Elsevier; 1979. pp. 103–141. [Google Scholar]
- Trasand L, Schechter CB, Haynes KA, Landrigan PJ. Mental Retardation and Prenatal Methylmercury Toxicity. Amer. J. Indust. Med. 2006;49:153–158. doi: 10.1002/ajim.20268. [DOI] [PubMed] [Google Scholar]
- Világi I, Dóczi J, Banczerowski-Pelyhe I. Altered electrophysiological characteristics of developing rat cortical neurones after chronic methylmercury chloride treatment. Int. J. Dev. Neurosci. 2000;18:493–499. doi: 10.1016/s0736-5748(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Weiss B, Clarkson TW, Simon W. Silent latency periods in methylmercury poisoning and in neurodegenerative disease. Environ. Health Perspect. 2002;110:851–854. doi: 10.1289/ehp.02110s5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Disruption by methylmercury of membrane excitability and synaptic transmission in hippocampal slices of the rat. Toxicol. Appl. Pharmacol. 1993;120:203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Action of methylmercury on GABA(A) receptor-mediated inhibitory synaptic transmission is primarily responsible for its early stimulatory effects on hippocampal CA1 excitatory synaptic transmission. J. Pharmacol. Exp. Ther. 1997;282:64–73. [PubMed] [Google Scholar]