Abstract
Harman’s free radical theory of aging posits that oxidized macromolecules accumulate with age to decrease function and shorten life-span. However, nutritional and genetic interventions to boost antioxidants have generally failed to increase life-span. Furthermore, the free radical theory fails to explain why exercise causes higher levels of oxyradical damage, but generally promotes healthy aging. The separate anti-aging paradigms of genetic or caloric reductions in the insulin signaling pathway is thought to slow the rate of living to reduce metabolism, but recent evidence from Westbrook and Bartke suggests metabolism actually increases in long-lived mice. To unify these disparate theories and data, here, we propose the epigenetic oxidative redox shift (EORS) theory of aging. According to EORS, sedentary behavior associated with age triggers an oxidized redox shift and impaired mitochondrial function. In order to maintain resting energy levels, aerobic glycolysis is upregulated by redox-sensitive transcription factors. As emphasized by de Grey, the need to supply NAD+ for glucose oxidation and maintain redox balance with impaired mitochondrial NADH-oxidoreductase requires the upregulation of other oxidoreductases. In contrast to the 2% inefficiency of mitochondrial reduction of oxygen to the oxyradical, these other oxidoreductases enable glycolytic energy production with a deleterious 100% efficiency in generating oxyradicals. To avoid this catastrophic cycle, lactate dehydrogenase is upregulated at the expense of lactic acid acidosis. This metabolic shift is epigenetically enforced, as is insulin resistance to reduce mitochondrial turnover. The low mitochondrial capacity for efficient production of energy reinforces a downward spiral of more sedentary behavior leading to accelerated aging, increased organ failure with stress, impaired immune and vascular functions and brain aging. Several steps in the pathway are amenable to reversal for exit from the vicious cycle of EORS. Examples from our work in the aging rodent brain as well as other aging models are provided.
Keywords: ROS, oxyradical, redox, mitochondria, aging
Introduction
The free radical theory of aging of Harman proposes that oxidized macromolecules accumulate with age to decrease cell function and shorten life-span (Harman, 1968). However, nutritional and genetic interventions to boost antioxidants have generally failed to increase life-span. The overall result of 19 clinical trials finds supplementation with the lipid-soluble antioxidant vitamin E failed to reduce mortality (Miller et al., 2005). The water soluble antioxidant vitamin C is also generally ineffective in reducing all-cause mortality (Bjelakovic et al., 2007). An even more important test of the free radical theory of aging involves genetic overexpression of antioxidant enzymes. To date, increases in SOD, or catalase or a combination, while lowering oxidized macromolecules, fail to increase lifespan in mice (Perez et al., 2009). Only overexpression of the peroxide and redox active thioredoxin 1 (Mitsui et al., 2002) and mitochondrial targeted catalase (Schriner et al., 2005) have been shown to increase mouse lifespan. In their review of aging theories, Jang and Van Remmen conclude that these and other studies “challenge” the mitochondrial and free-radical theories of aging (Jang et al., 2009) while Howes enumerates a list of failures of the free radical theory (Howes, 2006). A more complex regulation is suggested by experiments in C. elegans, in which careful titration with RNAi against mitochondrial function revealed a middle dose that promoted life-span extension that was not correlated with oxidative stress (Rea et al., 2007). In addition, the free radical theory fails to explain why higher levels of oxyradical damage occur with exercise (Powers et al., 2008), which generally promotes healthy human aging (Nakamura et al., 1996) and extends lifespan in mice (Navarro et al., 2004) and extends survival in rats (Holloszy et al., 1985). Table 1 summarizes this dilemma.
Table 1.
Two prominent current theories of aging and a proposed unifying theory.
| A) Dilemmas of current aging theories | ||
|---|---|---|
| Aging Paradigm/Observations |
Impact | Dilemmas |
| 1) Free radical damage to macromolecules | Cumulative cellular damage leads to organ failure, shortened life-span | Exercise is healthy but produces ROS. Ignores insulin signaling |
| 2) Genetic reductions in the insulin signaling path or caloric restriction | Increased life-span, decreased metabolism = “slower” rate of living | Metabolism actually increases. Ignores role of ROS |
| 3) Both | Ignore healthy aging and sustained cause of ROS | |
| B) Proposed reconciliation as a metabolic shift imposed by an epigenetically enforced oxidizing redox state | ||
| 1) Free radical damage to macromolecules | Cumulative cellulardamage leads to organ failure | Pathologic aging is a metabolic shift to aerobic glycolysis & lacticacid acidosis due to an oxidized redox state that produces more ROS than mitochondrial electron transport |
| 2) Genetic or caloric reductions in the insulin signaling path | Increased life-span, increased mitochondrial metabolism at higher efficiency | Healthy aging or caloric restriction is forced dependence on highly efficient mitochondrial oxidative phosphorylation which requires less insulin signaling and less ROS |
The separate anti-aging paradigm of genetic or caloric reductions in the insulin signaling pathway is thought to slow the rate of living to reduce metabolism and oxyradical production (Weindruch et al., 2001; Carter et al., 2002; Heilbronn et al., 2006; Al-Regaiey et al., 2007). The decrease in animal size from genetic or caloric interventions and lower oxyradical damage could reflect a slower rate of living (Pearl, 1928). However, recent evidence from Westbrook and Bartke suggests metabolism as VO2 and heat/gm body mass actually increases in genetically long-lived mice with decreased insulin signaling (Westbrook et al., 2009). This unexpected finding conflicts with a slower rate of living theory in terms of lower lifetime oxygen input to generate lower oxyradicals. Further, although the resting metabolic rate seems to decrease with age in humans, a study of 28 long-lived people (>95 yr old) indicated an actual increase in metabolic rate compared to 27 aged subjects (66–94 yr old) (Rizzo et al., 2005). And yeast grown in low glucose to increase lifespan actually respire at higher rates with less ROS than normal glucose (Barros et al., 2004). Numerous caloric or dietary restriction studies in rodents that result in increased longevity are also associated with lower activities of the insulin signaling pathway that regulates much of glucose energy intake (Masternak et al., 2005). However, the lower levels of ROS observed from this intervention are not easily explained if the rate of metabolism is not decreased, but increased (Westbrook et al., 2009) (Table 1).
The epigenetic oxidative redox shift (EORS) theory of aging
I propose that a metabolically initiated redox shift is upstream of the commonly observed increase in ROS damage to macromolecules. This shift with age occurs in the oxidized direction of the relative levels of important reductants and oxidants. It manifests as an extracellular decrease in the ratio of cysteine/cystine and an intracellular decrease in the ratio of GSH/GSSG and NAD(P)H/NAD(P). The oxidized redox shift is initiated by low demand for bursts of energy produced by mitochondria. The low demand for energy accompanies low levels of physical and mental activity. This initiates a vicious cycle of oxidized membrane receptors, signaling molecules, transcription factors and epigenetic transcriptional regulators. Epigenetic factors modulated by aging include the histone deacetylase family which includes the sirtuins as well as histone acetylases and DNA methyltransferases. Together, the epigenetic mediators impose the metabolic shift away from use of mitochondrial energy toward reliance on glycolysis. This metabolic shift is further mediated by insulin resistance since a sedentary life does not require the metabolic demands that need to be regulated by insulin. Together, this EORS in aging results in a downward spiral of inability to respond to energy demands or stress which leads to stress-induced catastrophic initiation of cellular death pathways and organ failure.
Why redox state is more important than ROS
In order to begin to resolve these paradoxes, I propose that an oxidized redox state is upstream of the commonly observed ROS damage. Certainly, ROS damage is affected by the balance of oxyradical generation and anti-oxidant defenses. Numerous sources of oxyradical generation have been documented (Cohen, 1994), but less appreciation exists for the essential role that ROS or redox signaling plays in metabolism (Droge, 2002; Finkel, 2003). The common impression that the mitochondrial electron transport chain is the major source of oxyradical generation often overlooks other sources in the cytoplasm and plasma membrane (Morre et al., 2000; Hyun et al., 2006) and an upstream oxidized redox state (Jones, 2006). Redox state is the energetic force for electron transfer, much as pH is a measure of the strength of proton transfer. I.e., redox state measures the ability of a compound to donate or receive electrons, just as pH is a measure of the ability of a compound to donate or receive protons. Technically, redox state, E, is the electromotive force in mV relative to the standard state of hydrogen as follows. An example is also shown for the most abundant intracellular reductant, glutathione:
| [1] |
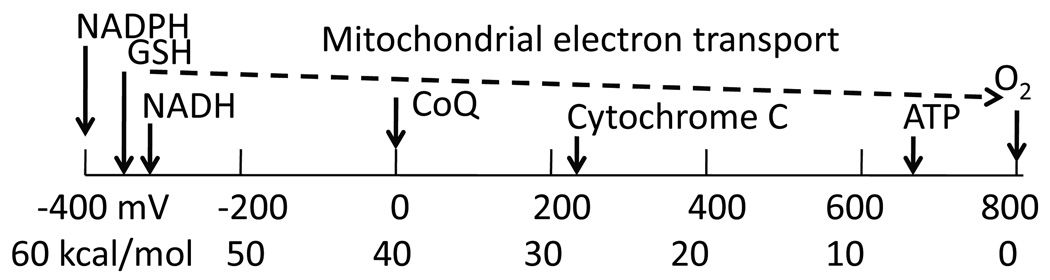
where Eo is the standard reduction state at pH 7 (−264 mV for glutathione (Jones, 2002), R is the gas constant, T is temperature (oK), n is the number of transferred electrons and F is Faraday’s constant. To appreciate the biological significance of this redox scale, consider the strongest common intracellular reductant, NADH with a standard state of −320 mV (equivalent to the biologist’s ΔG = 14.8 kcal/mol) and molecular oxygen as a strong oxidant at +800 mV (Fig. 1). The mitochondrial electron transport chain efficiently oxidizes NADH with molecular oxygen over numerous smaller steps to siphon about 98% of the energy as a proton and electrical gradient across the mitochondrial inner membrane. We commonly think of ATP as a high energy molecular currency, but hydrolysis of the phosphate bond produces only −7.3 kcal/mol, compared to almost −60 kcal/mol of oxidized NADH. Since this reducing energies of NADH, NADPH and GSH are so large, they can provide the power for a large number of reduction reactions in the cell including glycolysis, ATP generation, disulfide bond formation in numerous enzymes, transporters, signaling molecules and transcription factors. The driving force for these reactions depends on the relative concentrations of the oxidized and reduced forms of each redox couple, according to equation 1. Both NADH and glutathione play important roles in preventing ROS damage (Petrat et al., 2003; Drake et al., 2003).
Fig 1.
Biological redox energy scale. The highly reduced energy levels of NADPH, GSH and NADH are used to power catabolism with molecular oxygen as the terminal electron acceptor. The step-wise electron transport in mitochondria is used to generate ATP, a lower denomination of energy currency.
Evidence for an oxidizing shift in redox state with age
Dean Jones at Emory University was the first to show that human plasma GSH/GSSG is controlled at a relatively constant redox state of −137 mV in 740 healthy adults through age 50 (Jones, 2006). However, an oxidative shift of about 7 mV/decade occurs over the next two decades, followed by a further decline to −110 mV in the 70 to 85-year-old group. In a longitudinal study, patients with age-related macular degeneration were observed at age 72 to decline from −121 mV to −118 mV over a four-year period, but the decline was largely prevented in patients given an antioxidant supplement containing vitamins C, E and beta-carotene (Moriarty-Craige et al., 2005). Other diseases associated with an oxidized plasma glutathione redox state include type 2 diabetes at −110 mV (Samiec et al., 1998) and carotid artery thickening at values more oxidized than −130 mV (Ashfaq et al., 2006). In human plasma, concentrations of cysteine are higher than glutathione, suggesting greater importance of the cys/cySS redox couple than GSH/GSSG in plasma, while the millimolar concentrations of GSH and NADH inside cells suggest greater intracellular importance of these redox couples. In mice, the lifespan of the C57BL/6 strain is extended by caloric restriction that reverses the age-related oxidized redox shift of glutathione, but less so in the DBA/2 mouse without lifespan extension (Ferguson et al., 2008). An age-related decline in rat neuron intracellular GSH and NADH indicates a relationship to aging (Parihar et al., 2008), but whether this reflects a causal oxidative shift or a by-product of a more seminal cause of aging remains to be established.
Dependence of metabolism on redox state and age
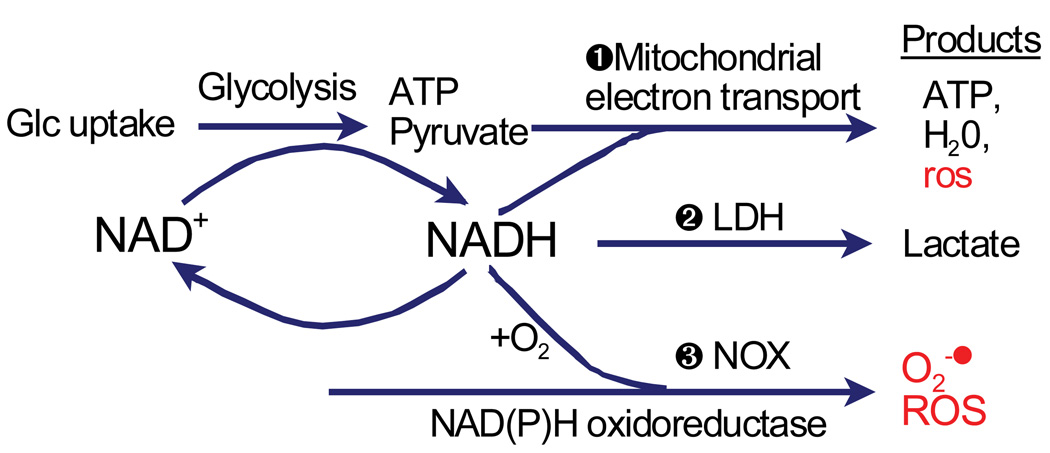
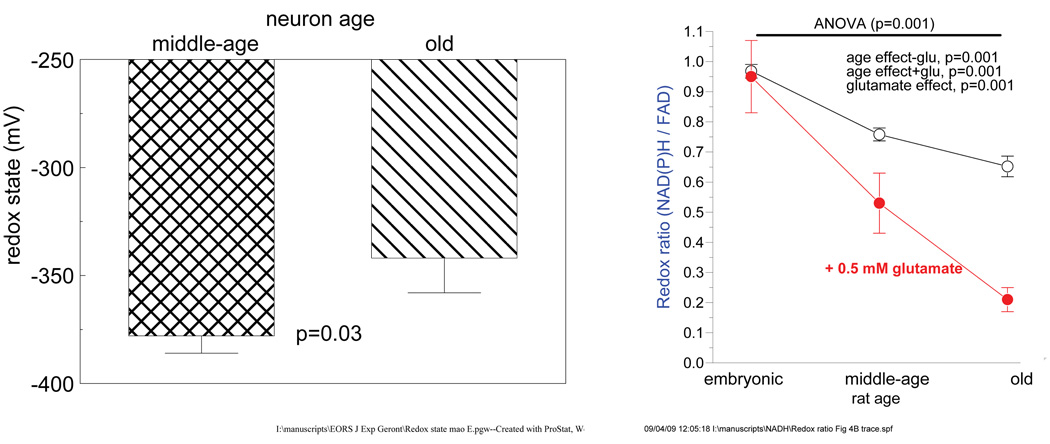
The obligate requirement for the oxidizing power of NAD+ to produce 2 ATP/glucose during glycolysis must be accompanied by a corresponding regeneration of NADH to NAD+ (Figure 2). Since cellular energy is largely used to maintain ionic homeostasis, the large demands for ATP are most easily met by mitochondrial consumption of NADH at complex I (NADH oxidoreductase) generating up to 36 more ATP/glucose while regenerating NAD+ oxidizing power. However, in periods of high energy demand, oxygen becomes rate limiting for mitochondrial electron transport, causing NADH levels to rise to activate lactate dehydrogenase (pathway 2) while regenerating NAD+ oxidizing power. But brain, muscle and other tissues can only tolerate limited amounts of lactic acid before the onset of acidosis, requiring yet another pathway to be activated. Pathway 3 in Figure 2 suggests how the plasma membrane and other NAD(P)H oxidoreductases can regenerate oxidizing power (DeGrey, 2005; Hyun et al., 2006). Now consider the energy generating efficiency of these enzymes in terms of oxyradical byproducts. The mitochondrial electron chain reduces oxygen to water at complex IV, but leaks about 2% of the oxygen to oxyradical formation at complexes I and III. In contrast, the plasma membrane and other NAD(P)H oxidoreductases regenerate oxidizing power with a concomitant 100% generation of oxyradicals (DeGrey, 2005). This level of oxyradical production can only be sustained for short periods with good tissue perfusion, but is likely to damage nearby cells and the vascular endothelium. Since we have observed an age-related decline in mitochondrial membrane potential in neurons from rat brains together with increased oxyradical production (Parihar et al., 2007) and lower NADH levels (Parihar et al., 2008), we propose that aging is associated with a metabolic shift from oxidative phosphorylation toward oxidative glycolysis, as shown in Figure 2. A possible signal for this shift would be the observed oxidative shift in the redox couple NADH/NAD+ toward a lower ratio of NADH/NAD+ as we have seen (Figure 3).
Fig. 2.
Three alternative mechanisms for energy production that maintain NAD+ levels for glycolysis and redox balance. 1) NADH is consumed by complex one of the electron transport chain with low levels of oxyradical production. In aerobic glycolysis, 2) lactate dehydrogenase reduces pyruvate to lactic acid and 3) the plasma membrane NAD(P)H oxidoreductase (NOX) stoichiometricly produces one oxygen radical anion for every NADH reoxidized, relying on antioxidant defenses.
Fig. 3.
Oxidative shift in NADH/NAD redox levels with age in old rat brains and in neurons isolated from them. Adapted from (Parihar et al., 2008).
Redox dependence of insulin signaling
The well-known insulin signaling system leads to insertion of GluT4 glucose transporters into the plasma membrane to stimulate glucose uptake outside the brain (Figure 4). In the brain, a different glucose transporter with a higher affinity for glucose, GluT3, ensures essential glucose uptake into the brain even during fasting. Surprisingly, the brain is also sensitive to endogenous insulin signaling through a similar pathway (Uemura et al., 2006). Further downstream in insulin signaling, receptor binding activates IRS and PI3K (step 4), which activates PIdP to PItP (step 5) to signal Akt to stimulate neuron GluT3 (Uemura et al., 2006) or muscle/vascular GluT4 (Klip, 2009) insertion from vesicles into the plasma membrane for increased neuronal glucose uptake (step 6).
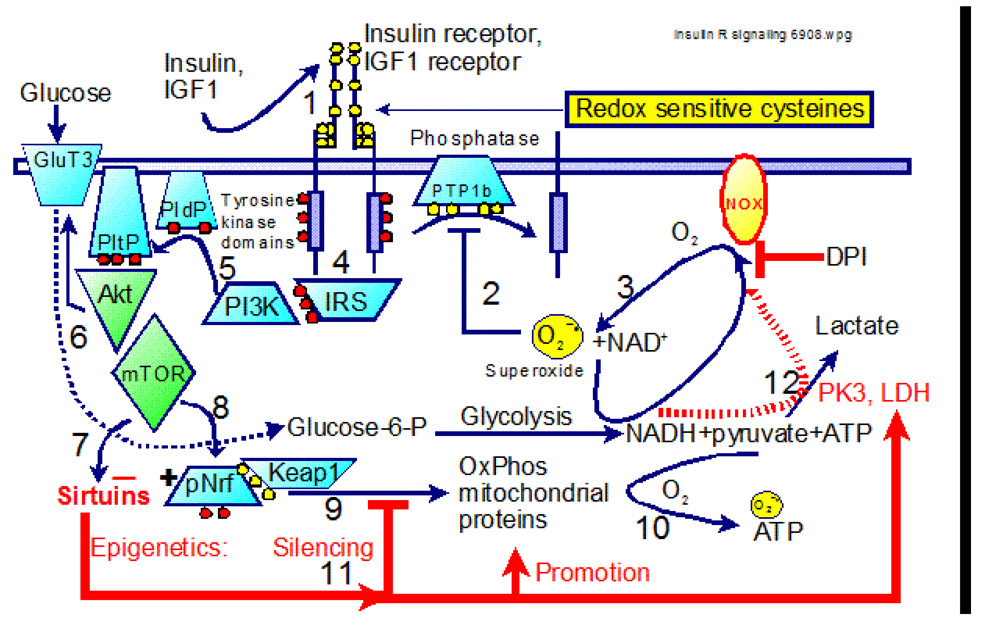
Figure 4.
Insulin and IGF1 signaling pathway to promote glucose uptake by GluT3 in neurons (shown) or GlutT4 in muscle (not shown). Note redox-sensitive cysteines (yellow dots) in the insulin and IGF1 receptors, in protein tyrosine phosphatase (PTP1b) and in Keap1. In insulin resistance, constitutive phosphorylation (red dots) activates the NOX enzyme which generates higher levels of oxyradicals (3) and NADH is recycled by lactate dehydrogenase (LDH, broken red line). See text for more details.
To link the observed oxidative shift in redox state to insulin signaling, consider the abundance of oxidizable cysteine residues on the insulin and IGF1 receptors (Ullrich et al., 1986) (step 1 in Figure 4). Furthermore, the requisite phosphatase PTP1b that reactivates the insulin receptor, does so by an obligatory oxidation of its cysteine residues (step 2) (Droge et al., 2007) in turn controlled by activation of NOX to generate physiologically normal and required oxyradicals. Akt also stimulates mTOR to activate pNrf, a prime transcription factor for upregulation of mitochondrial proteins of oxidative phosphorylation. Notice that pNrf is held in an inactive reserve state in the cytoplasm binding to Keap-1, protected from degradation by redox-sensitive cysteines (step 9) (Zhang et al., 2004). Thus, in the oxidizing environment of aging, the insulin/IGF1 receptor may be constitutively oxidized into a dimer of a continual on state (step 4) which is enforced by the failure of oxidized PTP1b to reactivate the receptor. In this on-state, it is unclear whether subsequent signaling elements would limit the insertion of glucose transporters based on energetic demands, but additional stimulation by insulin would be blocked. Without stimulation by insulin, glucose levels will rise, stimulating more insulin production, but without effect. This state of high glucose, high insulin and inability of more insulin to stimulate glucose uptake is the definition of insulin-resistance in the age-related onset of type 2 diabetes. Furthermore, the oxidized state of Keap-1 would block release of Nrf to signal mitochondrial biogenesis. With decreased mitochondrial synthesis, cells would be forced to rely more on aerobic glycolysis with less energy available for high stress needs. In support of an effect of aging on this process, we have observed normal resting glucose uptake in neurons in a uniform culture environment, but an age-related deficit in upregulation of glucose uptake in neurons in response to stress (Patel et al., 2003).
Reductions in several elements in the insulin signaling pathway are known to promote longevity in yeast, worms, mice and man (Carter et al., 2002; Bluher et al., 2003; van Heemst et al., 2005; Kurosu et al., 2005; Taguchi et al., 2007; Pawlikowska et al., 2009). The converse of overexpression of insulin signaling elements is less frequently studied. Overexpression of IRS1 in mice on a klotho−/− background decreases life-span (Kurosu et al., 2005). Overexpression of IRS1, 2, 3 or 4 in adipose cells stimulates Glut4 translocation independent of insulin (Zhou et al., 1999). Overexpression of the insulin receptor itself promotes a tumorigenic phenotype in mammary epithelial cells (Frittitta et al., 1995). These studies suggest that continuous activation of insulin signaling by an oxidized redox state is not likely to be beneficial, but controlled experiments are needed to measure this effect directly.
Caloric restriction
Mostly studied in mice and rats, caloric restriction with full vitamin and mineral supplementation prolongs lifespan, reduces ROS damage, reduces inflammation, increases insulin sensitivity and decreases the incidence of cancer, even in human studies (Heilbronn et al., 2006; Fontana & Klein, 2007). Two landmark studies in mice showed strong evidence for increased mitochondrial function with caloric restriction in muscle (Desai et al., 1996) and fat tissue (Higami et al., 2004), which were extended to humans (Civitarese et al., 2007). This work led Anderson and Weindruch (2007) to propose the mechanism of caloric restriction to involve a metabolic reprogramming for more efficient use of fats, carbohydrates and protein. Thus, control for the metabolic shift could be effected by transcription factors with redox sensitivities, first proposed by Merry (2004). Even higher levels of control could be exerted at the level of epigenetics.
Epigenetic control imposed by a metabolic shift toward an oxidized redox state
Epigenetic controls are the histone acetylations, methylations, phosphorylations and sumolations and cytosine methylations that control gene expression (Fraga et al., 2007; Feinberg, 2008; Sedivy et al., 2008). In general, histone acetylation facilitates the removal of transcription-blocking histone tails from DNA. Methylation of CpG islands, often in the upstream regulatory sequences of a gene, blocks the binding of RNA polymerase. Both processes control differentiation and contribute to organ-specific gene expression. Since aging can be considered the last stage of development, deregulation of epigenetic controls is thought to contribute to age-related diseases including cancer (Kondo, 2009), type 2 diabetes (Villeneuve et al., 2008), cardiovascular disease (Turunen et al., 2009), memory (Guan et al., 2009) and possibly neurodegenerative diseases (Wang et al., 2008; Mastroeni et al., 2009). Evidence is beginning to accumulate for epigenetic control of aging, but no studies yet describe epigenetic changes in metabolism associated with aging. A diverse set of observations will be described first, followed by suggestive evidence from our specific aging neuron model.
Calorie restriction upregulates the class II HDAC, SIRT1 deacetylase (Cohen et al., 2004), thus linking calorie restriction to an epigenetic control of metabolism. Histone deacetylase inhibitors (Hede, 2006) and cytosine demethylase activators or methyltransferase inhibitors (Fandy, 2009; Spannhoff et al., 2009) are under active investigation for treatment of cancers and heart disease (Turunen et al., 2009) but may find application to ameliorate aging. Genetically identical twins are a powerful source for associating differential phenotypes with epigenetic regulation. For a human complex I gene of the respiratory chain, NDUFB6, mRNA and protein expression that declines with age correlates with increased promoter methylation (Ling et al., 2007). In a broader approach with 80 monozygoous twins, global methylation in lymphocytes was decreased with age along with decreases in histone H3 acetylation, which in one individual was associated with overexpression of 3800 genes (Fraga et al., 2005). In one twin who contracted Alzheimer disease, DNA methylation was decreased compared to the twin without Alzheimer disease (Mastrooeni et al., 2009).
Second, our group has tried to dissect an aging environment that also influences gene transcription from a possibly intrinsic level of epigenetic control. To reduce complications from many variables such as the aging hormonal vascular and immune systems, we have studied brain aging with rat neurons extracted from the brain and placed in a common culture medium. We were surprised to see that neurons retained a number of aspects of an aging brain phenotype (Brewer, 2000) in culture after extraction from old, compared to middle-age rat brains. Despite equal survival and regeneration of dendrites and axons, compared to neurons isolated from middle-age rat brain, the first important characteristic of old neurons discovered was an increased sensitivity to stressors common to the brain including glutamate, lactic acid and beta-amyloid, the protein fragment that accumulates in the brain of Alzheimer disease patients (Brewer, 1998). Incidentally, this observation met a common definition of aging as increased susceptibility to stress (Miller, 2009). Follow-up studies indicated increased sensitivity in old neurons to the inflammatory mediator TNFα (Patel et al., 2008a; 2008b), impaired glucose uptake (Patel et al., 2003), elevated intracellular calcium (Brewer et al., 2006), decreased respiratory capacity (Jones et al., 2009) and a partially depolarized mitochondrial membrane potential associated with increased ROS production (Parihar et al., 2007). Small age-related decrements in the redox-active NADH and glutathione were catastrophically lowered in old neurons following treatment with glutamate to depolarize the plasma membrane and increase intracellular calcium (Parihar et al., 2008). Measures of NADH and NAD in old rat brains indicate a large shift toward oxidation from more reduced levels in middle-age rat brain. It remains to be determined whether this oxidative redox shift is an upstream factor to all the above. But something upstream of these age-related physiological changes must be intrinsic to the neurons and not readily changed by a common culture medium.
Although age-related mutations have been proposed as an explanation for aging, evidence is lacking to support age-related accumulation of mutations. Furthermore, the ability to massively reverse these characteristics of old neurons to those of middle-age by estrogen (Brewer et al., 2006; Jones et al., 2009), blueberry extract (Brewer et al., 2009) or cell division (Jones et al., 2009) suggest that the genetic program of these neurons have characteristics more consistent with epigenetic regulation than genetic mutation. We have preliminary evidence for epigenetic histone acetylation in old neurons that can partially reverse their susceptibility to beta-amyloid toxicity.
How aging could induce an oxidized metabolic redox shift that drives epigenetic changes, insulin resistance and ROS damage in a downward spiral
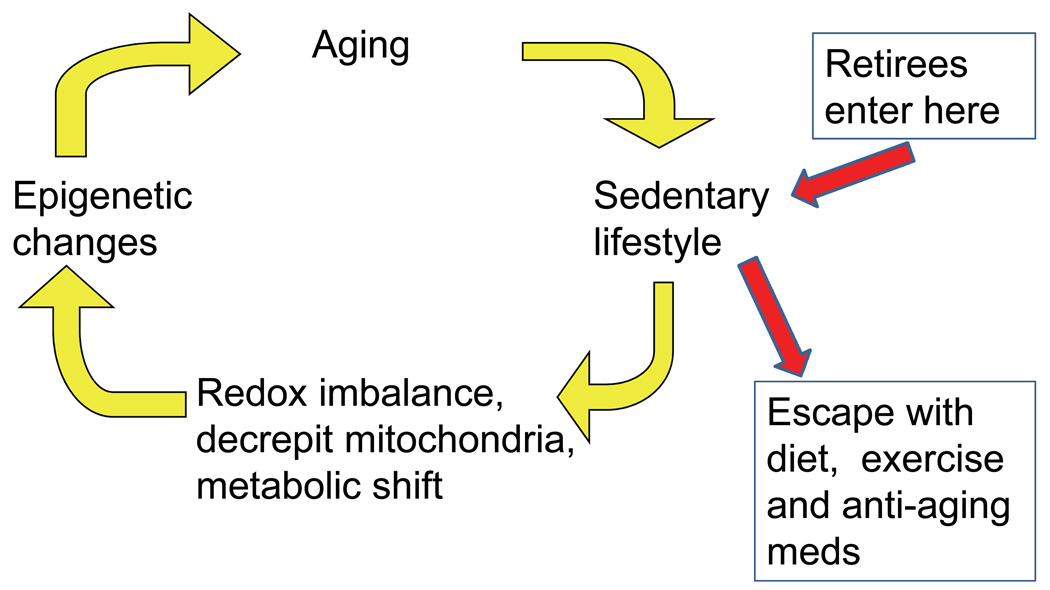
Aging is often associated with a sedentary life style (Figure 5). If there are no demands for the extra energy that can be produced by aerobic oxidative phosphorylation, then cells and organs may down-regulate the electron transport chain components and survive adequately on glycolysis (Figure 2). Increased consumption of sugar in beverages (Sanchez-Lozada et al., 2008; Johnson et al., 2009) may also enforce reliance on glycolysis. An oxidative shift is proposed to ensure ample supplies of the requisite NAD+. Such an oxidative shift has been recorded in old rat brain and in plasma from healthy humans. Declines in cytochrome C oxidase of mitochondria with age are widely reported (Brewer, 2000; Jones et al., 2009). The oxidative redox shift would change the activities of numerous redox-sensitive transcription factors, enzymes, transport and signaling proteins with redox-active cysteines (e.g. insulin receptor, Figure 4). The NMDA receptor for glutamate (Sucher et al., 1991; Lipton, 2008) and the Ca+2-ATPase in the brain have long been known to be sensitive to oxidation (Zaidi et al., 2003). Examples of transcription factors with demonstrated redox sensitivity include NfkB (Bykov et al., 2009), SP1 (Ammendola et al., 1994), HOXB5 (Galang et al., 1993) and USF (Pognonec et al., 1992). Eventually, signaling would reach the level of enzymes that control epigenetic marks such as histone acetylases, deacetylases (with their substrate requirement for NAD+) and methyltransferases. We have preliminary evidence to support histone acetylation-dependent susceptibility in old rat brain neurons, but the robustness and generality of age-related epigenetic controls remain largely to be established. Such a last step of epigenetic development could constitute the driving force for aging that enforces a downward spiral of less and less mitochondrial capacity (Figure 5) and increased sedentary behavior (Rimbert et al., 2004; Figueiredo et al., 2009). Anti-aging changes in gene expression elicited by caloric restriction are consistent with such a metabolic shift (Anderson et al., 2007). Other interventions that force mitochondrial turnover and biogenesis may prolong lifespan by selecting for more efficient mitochondrial generation of energy (Rea et al., 2007). Diet and exercise are inexpensive ways to reduce the effects of aging (Nakamura et al., 1996; Kalmijn et al., 1997; Milgram et al., 2005; Scarmeas et al., 2006; Aliev et al., 2009). Anti-aging drugs remain to be developed and validated, but simplistic approaches with anti-oxidant vitamins like vitamin E appear to be ineffectual (Brewer, 2009).
Fig. 5.
Use it or lose it, the downward spiral of aging enforced by an epigenetic oxidized redox shift (EORS).
Acknowledgments
I thank Dean Jones for inspiring discussions on redox energetics. This work was supported in part by the NIA RO1 AG13435, AG032431 and the Kenneth Stark Endowed Chair in Alzheimer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Regaiey KA, Masternak MM, Bonkowski MS, Panici JA, Kopchick JJ, Bartke A. Effects of caloric restriction and growth hormone resistance on insulin-related intermediates in the skeletal muscle. J Gerontol. A Biol. Sci. Med. Sci. 2007;62:18–26. doi: 10.1093/gerona/62.1.18. [DOI] [PubMed] [Google Scholar]
- Aliev G, Liu J, Shenk JC, Fischbach K, Pacheco GJ, Chen SG, Obrenovich ME, Ward WF, Richardson AG, Smith MA, Gasimov E, Perry G, Ames BN. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J. Cell Mol. Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendola R, Mesuraca M, Russo T, Cimino F. The DNA-binding efficiency of Sp1 is affected by redox changes. Eur. J Biochem. 1994;225:483–489. doi: 10.1111/j.1432-1033.1994.t01-1-00483.x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip. Top. Gerontol. 2007;35:18–38. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, Vaccarino V, Harrison DG, Quyyumi AA. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am. Coll. Cardiol. 2006;47:1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- Barros MH, Bandy B, Tahara EB, Kowaltowski AJ. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J Biol Chem. 2004;279:49883–49888. doi: 10.1074/jbc.M408918200. [DOI] [PubMed] [Google Scholar]
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Age-related toxicity to lactate, glutamate, and beta-amyloid in cultured adult neurons. Neurobiol Aging. 1998;19:561–568. doi: 10.1016/s0197-4580(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Neuronal plasticity and stressor toxicity during aging. Exp Gerontol. 2000;35:1165–1183. doi: 10.1016/s0531-5565(00)00121-2. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. Why Vitamin E Therapy Fails for Treatment of Alzheimer's Disease. J. Alzheimers. Dis. 2009 doi: 10.3233/JAD-2010-1238. 6J461328612707J5 [pii];10.3233/JAD-2009-1238 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Lindsey AL, Kunz EZ, Torricelli JR, Neuman A, Fisher DR, Joseph JA. Age-related toxicity of β-amyloid associated with increased pERK and pCREB in primary hippocampal neurons: reversal by blueberry extract. Journal of Nutritional Biochemistry. 2009 doi: 10.1016/j.jnutbio.2009.08.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer GJ, Reichensperger JD, Brinton RD. Prevention of age-related dysregulation of calcium dynamics by estrogen in neurons. Neurobiol Aging. 2006;27:306–317. doi: 10.1016/j.neurobiolaging.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Bykov VJ, Lambert JM, Hainaut P, Wiman KG. Mutant p53 rescue and modulation of p53 redox state. Cell Cycle. 2009;8:2509–2517. doi: 10.4161/cc.8.16.9382. [DOI] [PubMed] [Google Scholar]
- Carter CS, Ramsey MM, Sonntag WE. A critical analysis of the role of growth hormone and IGF-1 in aging and life-span. Trends Genet. 2002;18:295–301. doi: 10.1016/S0168-9525(02)02696-3. [DOI] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie Restriction Increases Muscle Mitochondrial Biogenesis in Healthy Humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann. N. Y. Acad. Sci. 1994;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, De Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- DeGrey A. The plasma membrance redox system: a candidate source of aging-relarted oxidative stress. Age. 2005;27:129–138. doi: 10.1007/s11357-005-1630-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai VG, Weindruch R, Hart RW, Feuers RJ. Influences of age and dietary restriction on gastrocnemius electron transport system activities in mice. Arch. Biochem. Biophys. 1996;333:145–151. doi: 10.1006/abbi.1996.0375. [DOI] [PubMed] [Google Scholar]
- Drake J, Sultana R, Aksenova M, Calabrese V, Butterfield DA. Elevation of mitochondrial glutathione by gamma-glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J Neurosci Res. 2003;74:917–927. doi: 10.1002/jnr.10810. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Droge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandy TE. Development of DNA methyltransferase inhibitors for the treatment of neoplastic diseases. Curr. Med. Chem. 2009;16:2075–2085. doi: 10.2174/092986709788612738. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA. 2008;299:1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Rebrin I, Forster MJ, Sohal RS. Comparison of metabolic rate and oxidative stress between two different strains of mice with varying response to caloric restriction. Exp. Gerontol. 2008;43:757–763. doi: 10.1016/j.exger.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo PA, Powers SK, Ferreira RM, Amado F, Appell HJ, Duarte JA. Impact of lifelong sedentary behavior on mitochondrial function of mice skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:927–939. doi: 10.1093/gerona/glp066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. JAMA. 2007;297:986–994. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Esteller M. Epigenetics and aging: the targets and the marks. Trends Genet. 2007;23:413–418. doi: 10.1016/j.tig.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Frittitta L, Vigneri R, Stampfer MR, Goldfine ID. Insulin receptor overexpression in 184B5 human mammary epithelial cells induces a ligand-dependent transformed phenotype. J. Cell. Biochem. 1995;57:666–669. doi: 10.1002/jcb.240570411. [DOI] [PubMed] [Google Scholar]
- Galang CK, Hauser CA. Cooperative DNA binding of the human HoxB5 (Hox-2.1) protein is under redox regulation in vitro. Mol. Cell Biol. 1993;13:4609–4617. doi: 10.1128/mcb.13.8.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging: effect of free radical reaction inhibitors on the mortality rate of male LAF mice. J Gerontol. 1968;23:476–482. doi: 10.1093/geronj/23.4.476. [DOI] [PubMed] [Google Scholar]
- Hede K. Histone deacetylase inhibitors sit at crossroads of diet, aging, cancer. J. Natl. Cancer Inst. 2006;98:377–379. doi: 10.1093/jnci/djj120. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18:415–417. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK, Vining M, Adams S. Effect of voluntary exercise on longevity of rats. J. Appl. Physiol. 1985;59:826–831. doi: 10.1152/jappl.1985.59.3.826. [DOI] [PubMed] [Google Scholar]
- Howes RM. The free radical fantasy: a panoply of paradoxes. Ann. N. Y. Acad. Sci. 2006;1067:22–26. doi: 10.1196/annals.1354.004. [DOI] [PubMed] [Google Scholar]
- Hyun DH, Emerson SS, Jo DG, Mattson MP, De Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl. Acad. Sci. U. S. A. 2006;103:19908–19912. doi: 10.1073/pnas.0608008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang YC, Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp. Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, Roncal C, Nakagawa T. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr. Rev. 2009;30:96–116. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid. Redox. Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- Jones TT, Brewer GJ. Critical age-related loss of cofactors of neuron cytochrome C oxidase reversed by estrogen. Exp. Neurol. 2009;215:212–219. doi: 10.1016/j.expneurol.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann. Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- Klip A. The many ways to regulate glucose transporter 4. Appl. Physiol Nutr. Metab. 2009;34:481–487. doi: 10.1139/H09-047. [DOI] [PubMed] [Google Scholar]
- Kondo Y. Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med. J. 2009;50:455–463. doi: 10.3349/ymj.2009.50.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Poulsen P, Simonsson S, Ronn T, Holmkvist J, Almgren P, Hagert P, Nilsson E, Mabey AG, Nilsson P, Vaag A, Groop L. Genetic and epigenetic factors are associated with expression of respiratory chain component NDUFB6 in human skeletal muscle. J Clin. Invest. 2007;117:3427–3435. doi: 10.1172/JCI30938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA. NMDA receptor activity regulates transcription of antioxidant pathways. Nat. Neurosci. 2008;11:381–382. doi: 10.1038/nn0408-381. [DOI] [PubMed] [Google Scholar]
- Masternak MM, Al Regaiey KA, Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Bartke A. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp. Gerontol. 2005;40:679–684. doi: 10.1016/j.exger.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Mastroeni D, McKee A, Grover A, Rogers J, Coleman PD. Epigenetic differences in cortical neurons from a pair of monozygotic twins discordant for Alzheimer's disease. PLoS. ONE. 2009;4:e6617. doi: 10.1371/journal.pone.0006617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram NW, Head E, Zicker SC, Ikeda-Douglas CJ, Murphey H, Muggenburg B, Siwak C, Tapp D, Cotman CW. Learning ability in aged beagle dogs is preserved by behavioral enrichment and dietary fortification: a two-year longitudinal study. Neurobiol Aging. 2005;26:77–90. doi: 10.1016/j.neurobiolaging.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Miller ER, III, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:179–182. doi: 10.1093/gerona/gln072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, Ishizaki-Koizumi S, Hirakawa T, Inoue T, Yodoi J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid. Redox. Signal. 2002;4:693–696. doi: 10.1089/15230860260220201. [DOI] [PubMed] [Google Scholar]
- Moriarty-Craige SE, Adkison J, Lynn M, Gensler G, Bressler S, Jones DP, Sternberg P., Jr. Antioxidant supplements prevent oxidation of cysteine/cystine redox in patients with age-related macular degeneration. Am. J Ophthalmol. 2005;140:1020–1026. doi: 10.1016/j.ajo.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Morre DM, Lenaz G, Morre DJ. Surface oxidase and oxidative stress propagation in aging. J Exp Biol. 2000;203(Pt 10):1513–1521. doi: 10.1242/jeb.203.10.1513. [DOI] [PubMed] [Google Scholar]
- Nakamura E, Moritani T, Kanetaka A. Effects of habitual physical exercise on physiological age in men aged 20–85 years as estimated using principal component analysis. Eur. J. Appl. Physiol Occup. Physiol. 1996;73:410–418. doi: 10.1007/BF00334417. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, Lopez-Cepero JM, Boveris A. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am. J Physiol Regul. Integr. Comp Physiol. 2004;286:R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Brewer GJ. Simultaneous age-related depolarization of mitochondrial membrane potential and increased mitochondrial reactive oxygen species production correlate with age-related glutamate excitotoxicity in rat hippocampal neurons. J Neurosci. Res. 2007;85:1018–1032. doi: 10.1002/jnr.21218. [DOI] [PubMed] [Google Scholar]
- Parihar MS, Kunz EA, Brewer GJ. Age-related decreases in NAD(P)H and glutathione cause redox declines before ATP loss during glutamate treatment of hippocampal neurons. J Neurosci. Res. 2008;86:2339–2352. doi: 10.1002/jnr.21679. [DOI] [PubMed] [Google Scholar]
- Patel JR, Brewer GJ. Age-related changes in neuronal glucose uptake in response to glutamate and beta-amyloid. J Neurosci Res. 2003;72:527–536. doi: 10.1002/jnr.10602. [DOI] [PubMed] [Google Scholar]
- Patel JR, Brewer GJ. Age-related changes to tumor necrosis factor receptors affect neuron survival in the presence of beta-amyloid. J Neurosci. Res. 2008a;86:2303–2313. doi: 10.1002/jnr.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JR, Brewer GJ. Age-related differences in NFkappaB translocation and Bcl-2/Bax ratio caused by TNFalpha and Abeta42 promote survival in middle-age neurons and death in old neurons. Exp. Neurol. 2008b;213:93–100. doi: 10.1016/j.expneurol.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R. The Rate of Living. New York: Alfred A. Knopf; 1928. [Google Scholar]
- Perez VI, Van RH, Bokov A, Epstein CJ, Vijg J, Richardson A. The overexpression of major antioxidant enzymes does not extend the lifespan of mice. Aging Cell. 2009;8:73–75. doi: 10.1111/j.1474-9726.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F, Pindiur S, Kirsch M, de Groot H. NAD(P)H, a Primary Target of 1O2 in Mitochondria of Intact Cells. J. Biol. Chem. 2003;278:3298–3307. doi: 10.1074/jbc.M204230200. [DOI] [PubMed] [Google Scholar]
- Pognonec P, Kato H, Roeder RG. The helix-loop-helix/leucine repeat transcription factor USF can be functionally regulated in a redox-dependent manner. J Biol. Chem. 1992;267:24563–24567. [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea SL, Ventura N, Johnson TE. Relationship between mitochondrial electron transport chain dysfunction, development, and life extension in Caenorhabditis elegans. PLoS. Biol. 2007;5:e259. doi: 10.1371/journal.pbio.0050259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimbert V, Boirie Y, Bedu M, Hocquette JF, Ritz P, Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18:737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- Rizzo MR, Mari D, Barbieri M, Ragno E, Grella R, Provenzano R, Villa I, Esposito K, Giugliano D, Paolisso G. Resting metabolic rate and respiratory quotient in human longevity. J Clin Endocrinol Metab. 2005;90:409–413. doi: 10.1210/jc.2004-0390. [DOI] [PubMed] [Google Scholar]
- Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P, Jr., Reed RL, Jones DP. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic. Biol. Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lozada LG, Le M, Segal M, Johnson RJ. How safe is fructose for persons with or without diabetes? Am J Clin. Nutr. 2008;88:1189–1190. doi: 10.3945/ajcn.2008.26812. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Stern Y, Tang MX, Mayeux R, Luchsinger JA. Mediterranean diet and risk for Alzheimer's disease. Ann. Neurol. 2006;59:912–921. doi: 10.1002/ana.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of Murine Lifespan by Overexpression of Catalase Targeted to Mitochondria. Science. 2005 doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Sedivy JM, Banumathy G, Adams PD. Aging by epigenetics-A consequence of chromatin damage? Exp. Cell Res. 2008;314:1909–1917. doi: 10.1016/j.yexcr.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spannhoff A, Hauser AT, Heinke R, Sippl W, Jung M. The Emerging Therapeutic Potential of Histone Methyltransferase and Demethylase Inhibitors. ChemMedChem. 2009 doi: 10.1002/cmdc.200900301. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Lipton SA. Redox modulatory site of the NMDA receptor-channel complex: Regulation by oxidized glutathione. J Neurosci Res. 1991;30:582–591. doi: 10.1002/jnr.490300316. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317:369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim. Biophys. Acta. 2009;1790:886–891. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Uemura E, Greenlee HW. Insulin regulates neuronal glucose uptake by promoting translocation of glucose transporter GLUT3. Exp. Neurol. 2006;198:48–53. doi: 10.1016/j.expneurol.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le BT, Kathuria S, Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RG. Reduced insulin/IGF-1 signalling and human longevity. Aging Cell. 2005;4:79–85. doi: 10.1111/j.1474-9728.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. U. S A. 2008;105:9047–9052. doi: 10.1073/pnas.0803623105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Oelze B, Schumacher A. Age-specific epigenetic drift in late-onset Alzheimer's disease. PLoS. ONE. 2008;3:e2698. doi: 10.1371/journal.pone.0002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Kayo T, Lee CK, Prolla TA. Microarray Profiling of Gene Expression in Aging and Its Alteration by Caloric Restriction in Mice. J Nutr. 2001;131:918S–923S. doi: 10.1093/jn/131.3.918S. [DOI] [PubMed] [Google Scholar]
- Westbrook R, Bonkowski MS, Strader AD, Bartke A. Alterations in Oxygen Consumption, Respiratory Quotient, and Heat Production in Long-Lived GHRKO and Ames Dwarf Mice, and Short-Lived bGH Transgenic Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009;64:443–451. doi: 10.1093/gerona/gln075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A, Barron L, Sharov VS, Schoneich C, Michaelis EK, Michaelis ML. Oxidative inactivation of purified plasma membrane Ca(2+)-ATPase by hydrogen peroxide and protection by calmodulin. Biochemistry. 2003;42:12001–12010. doi: 10.1021/bi034565u. [DOI] [PubMed] [Google Scholar]
- Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol. Cell Biol. 2004;24:10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chen H, Xu P, Cong LN, Sciacchitano S, Li Y, Graham D, Jacobs AR, Taylor SI, Quon MJ. Action of insulin receptor substrate-3 (IRS-3) and IRS-4 to stimulate translocation of GLUT4 in rat adipose cells. Mol Endocrinol. 1999;13:505–514. doi: 10.1210/mend.13.3.0242. [DOI] [PubMed] [Google Scholar]