Abstract
Apyrimidinic/apurinic endonuclease (APE) is a key protein involved in the base excision DNA repair (BER) pathway of oxidative DNA lesions. Using a novel oligonucleotide substrate, we demonstrate that APE activity in the frontal/parietal cortex (F/PCTX), cerebellum, brainstem, midbrain and hypothalamus declined with age in rats on an ad libitum (AL) diet. In contrast, APE activity for these brain regions was ~1.5-3 times higher in young, caloric restricted (CR) rats. Despite continuous CR treatment in all animals since six weeks of age, APE activity in the CR group started to decline by middle-age and continued into old age. However, CR maintained APE activity at a level that was significantly higher than that in AL rats across age and in the brain regions examined. Because Western analysis of APE, DNA polymerase β and DNA ligase III levels in the F/PCTX of both CR and AL rats remained unchanged with age, this suggests that the increased APE activity in CR rats is the result of differential post-translational modification of APE.
Keywords: Exonuclease, apyrimidinic/apurinic endonuclease (APE), DNA polymerase β, DNA ligase III, frontal/parietal cortex, 8-oxodeoxyguanosine
Introduction
Aging is a complex biological process that is characterized by the progressive decline of physiological and biochemical properties of individual tissues and organs, leading to senescence and related diseases. There is ample evidence in the brain that with aging and disease, cellular energy levels decline while reactive oxygen species increase, causing damage to DNA, proteins, lipids and disruption of mitochondrial electron transport (Markesbery, 1997; Rehman et al., 1999), reviewed in (Holmes et al., 1992). In support of the importance of oxidative DNA damage in the aging human brain, a study has shown increased DNA damage was accompanied by a reduction in transcription and mitochondrial function and an increase in stress response and DNA repair genes, an effect mimicked in vitro in neuroblastoma cells subjected to oxidative stress (Lu et al., 2004). Moreover, the senescence-accelerated mouse (SAM), a mutant with a deficiency in DNA repair, has learning and memory deficits, a shortened lifespan and peripheral tissues that exhibit biochemical changes (e.g. mitochondrial dysfunction, accumulation of single-strand breaks) indicative of oxidative stress (Butterfield and Poon, 2005; Choi et al., 1999; Hosokawa et al., 2000; Nishikawa et al., 1998).
In mammals, oxidative DNA damage is repaired primarily by the base-excision repair (BER) pathway (Maynard et al., 2009). Since oxidized DNA nucleobases and apurinic (AP) sites, are mutagenic or cytotoxic, they must be corrected to maintain genetic stability and cell viability (Retel et al., 1993). The steps and proteins involved in BER pathway include: (i) DNA glycosylases for removal of the oxidative DNA lesion; (ii) apurinic/apyrimidinic endonuclease (APE) for cleaving the phosphodiester backbone 5′ to the abasic site; (iii) DNA polymerase to fill in the gap; and (iv) sealing of the gap by a DNA ligase. Although APE is an abundant repair protein, this enzyme appears to be the rate-limiting step in BER (Barzilay et al., 1996). Following oxidative stress, APE is induced 3- to 5-fold and this cellular response appeared to protect cells from the ensuing cytotoxicity and DNA damage (Izumi et al., 1996). In contrast, reducing cellular APE levels either by an antisense or a ’knock-out’ gene strategy or silencing RNA methodology sensitizes non-neuronal (Ono et al., 1994) or neuronal cells (Vasko et al., 2005) to oxidative stress and DNA damaging agents. APE is also found in both the nucleus and mitochondria of cells (Tell et al., 2001; Tomkinson et al., 1988), suggesting that this enzyme has a vital role in protecting both the nuclear and mitochondrial genome from oxidative DNA damage.
Caloric restriction (CR) is an experimental manipulation that consistently delays the aging process in animals (Bordone and Guarente, 2005), but its longevity-enhancing mechanism is poorly understood, particularly in the central nervous system. However, it has been proposed that CR preserves mitochondrial function and/or increases the resistance or response of aging tissues to oxidative stress-induced injury (Barja, 2004a). Increasing the repair efficiency of oxidative damage to the nuclear and/or mitochondrial genome is one possible mechanism by which CR may reduce the age-dependent increase in DNA damage, mutations and subsequent oxidative stress (The Free Radical Hypothesis of Aging; Barja, 2004b). Consistent with this hypothesis, rodents maintained on a CR diet exhibit an increase or preservation of the fidelity of DNA repair for damaged genes (Guo et al., 1998). Therefore, CR appears to reduce DNA damage and mutations in proliferative tissues of aging animals by increasing DNA repair capacity. CR also appears to trigger similar processes in the aging nervous system by maintaining BER activity at youthful levels (Cabelof et al., 2003).
Because of the pivotal role that APE plays in the BER pathway, this study explored the influence of aging on this repair enzyme in the brain. To aid in the accurate assay for APE activity, we developed a unique oligonucleotide probe that was used to assess endonuclease activity in the brain and other tissues of various species. Use of this novel probe was necessary because extensive degradation of a traditional 5′-3′-oligonucleotide probe was found when brain tissue extracts were examined for APE activity, an indicator of high levels of 3′-5′ exonuclease activity. To specifically assay for APE activity, an oligonucleotide probe was developed that did not possess a 3′ terminus, but contained a unique tetrahydrofuran that was resistant to exonuclease degradation. This probe has been used to accurately assess APE activity in human lymphocytes after in vivo or in vitro exposure to organophosphate pesticides (Muniz et al., 2008). The effect of CR on APE activity in the brains of aging rats was explored in a cross-sectional study. To help interpret any effect of CR on APE activity, the levels of APE and other BER enzymes in the brain were also measured to determine the amount of individual enzymes in the pathway. These studies indicate that CR modulates APE activity and this may be an important component of the life extension properties of CR.
Materials and Methods
Animals
Male Fischer-344 (F344) rats were bred and reared in a vivarium at the Gerontology Research Center (GRC, Baltimore, MD). From weaning (2 wks), the rats were housed individually in standard plastic cages, with beta chip wood bedding. Rats were then divided between ad libitum (AL) and caloric restricted (CR) rats. CR rats were fed a reduced amount based on the food intake of AL animals. Intake was gradually reduced 10% per week starting at six weeks of age [note: the young animals would have to be 10 weeks old (2.5 mo) to achieve 40% reduction and are presented as “2 mo” olds in the results] until there was a 40% difference between AL and CR groups and the rats maintained on this diet until removed for experiments or euthanized. Animals were weighed on a weekly basis. AL animals were fed a standard NIH-31 diet, while at 1 month of age CR animals were provided a vitamin and mineral fortified version of the same diet, but at a level of 60% less food (per weight) than that of AL rats. Filtered and acidified water were available ad libitum for both groups. The vivarium was maintained at a temperature of 25°C, relative humidity 70% and on a 12/12-h light/dark cycle (lights on at 6:00 a.m.) All animals were sacrificed between 9:00 am, 11:00 a.m. after an overnight fast.
Tissue
Human and Primate
CNS tissue (i.e., frontal cortex) was obtained from a 77 yr old non-neurological male (gift from Dr. C. Cotman, Institute for Brain Aging and Dementia Tissue Repository, Irvine, CA) or 2 yr, 13 yr, and 29 yr old rhesus macaques (Macaca mulatta). These samples were collected and preserved in fresh frozen form and processed for exonuclease and APE activity.
Rat
The brains from ad libitum controls and rats maintained on constant CR, were collected from 2-mo, 13-mo, 22-mo and 25-mo old animals (n= 6/age group). Brains were dissected on ice and the frontal/parietal cerebral cortex, hypothalamus, midbrain, cerebellum, and brainstem were immediately frozen in powdered dry ice, and stored at −90°C.
Sample Preparation
Freshly frozen rodent, non-human primate or human brain tissue was thawed and each brain region homogenized in lysis buffer [50 mM Tris (pH 7.5), 2 mM EDTA, 0.1 M NaCl, 1 mM dithiothreitol, and 200 μM phenylmethylsufonyl fluoride] as previously described (Kisby et al., 1997). The tissue homogenate was diluted with buffer and aliquots were taken for determination of protein concentration (Bradford) before storing at −90°C. All tissue samples were coded and sent blinded for analysis of APE activity.
APE Activity
AP endonuclease (APE) activity was determined by measuring the amount of cleavage product from the 32P-labeled 5′-5′ oligonucleotide substrate containing a unique tetrahydrofuran as previously described (Muniz et al., 2008). The duplex oligonucleotide substrate used for assaying APE activity contained a unique tetrahydrofuran, a chemically and thermally stable analog for AP site, and had the nucleotide sequence: 5′-ATATCCTTCCGTXACTTTCCTCTATCGATTCA-3′-3′-C-5′ (5′-5′-substrate, 32-mer), where X = tetrahydrofuran as previously described (Figure 1A). Briefly, the reaction was performed in 20 μl reaction buffer (10 mM NaCl, 10 mM Tris, 2 mM MgCl2, pH 8.0) containing 100 fmol of labeled substrate and 0.1 μg of protein extract from brain and was incubated for 10 min at 37°C. Equal volume of gel loading buffer (0.05 % bromophenol blue, 0.05 % xylene, and 10 mM EDTA) was added to stop the reaction and immediately heated to 90°C for 10 minutes. The product and substrate were separated by electrophoresis on a 12.5% denaturing polyacrylamide gel and the dried gels analyzed for band intensity using a STORM™ phosphoImager (Molecular Dynamics).
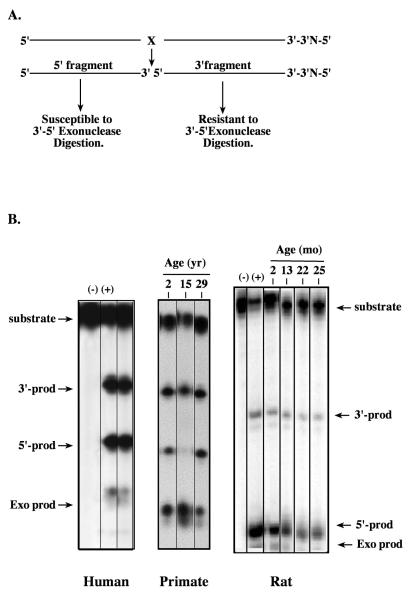
Figure 1. Exonuclease activity in extracts prepared from rodent, primate and human brain tissue.
Tissue homogenates of freshly frozen cortical tissue from 2 month to 25 month-old male rats, 2 yr, 15 yr and 29 yr old male rhesus monkeys (Maccaca mulatta) and a 77 yr male (PMI 2.25h) were prepared according to previously published methods (Kisby et al., 1997). A. An aliquot of each extract (0.1 μg protein) was incubated at 37°C for 10 min with a double-stranded 5′-5′ oligonucleotide substrate (50 fmol) containing a tetrahydrofuran site that had been end labeled with 32P-ATP (Muniz et al., 2008). B. Representative gels showing bands for both APE activity (i.e., 5′ and 3′ cleavage products) and exonuclease activity (exo product). (+) Purified E. coli endonuclease IV; (−) no endonuclease IV.
The relative labeling efficiency of the two termini was determined by using E. coli endonuclease IV (Figure 1B). As described earlier, the 20-mer fragment (3′ fragment) resulting from cleavage by APE has two 5′ termini and is resistant to tissue exonuclease activity, which is predominantly 3′-5′ exonuclease. Under conditions where little exonuclease activity was observed in lysates, the smaller APE cleavage fragment (5′ fragment) was used to determine activity, because this fragment is usually labeled more efficiently. However, under conditions where the lysates contained excessive 3′-5′ exonuclease activities (as indicated by the loss of 5′ fragment and the appearance of degradation products), the larger fragment (20-mer, 3′ fragment) that is resistant to 3′-5′ exonuclease digestion was used to determine APE activity (Figure 1B).
Western Blotting
Apurinic/apyrimidinic endonuclease (APE) levels were determined in AL and CR rat F/P cortex tissue extracts (n=4/age group). Protein extracts (10 μg) were applied to a 12%, 1.5 mm SDS-PAGE-gel and electrophoresed for 4 hours at 200V, and proteins were electroeluted onto a nitrocellulose membrane. The blotted membranes were treated with blocking solution [5% nonfat milk, 100 mM PBS (pH 7.4), 1% Tween-20] and the blots incubated for 1h with blocking solution containing polyclonal anti-APE (1:2000; a gift from Dr. Mark Kelley, Indiana University). Specific binding of the primary antibody to APE was visualized using a HRP-conjugated goat anti-rabbit antibody and Enhanced Chemiluminescence (ECL™, Amersham, Piscataway, NJ) according to the manufacturer’s instructions. Bands were detected, quantified and the background subtracted from each sample using a STORM™ phosphorimager (Molecular Dynamics).
Immunohistochemistry
AL or CR treated rats were perfused with 4% buffered paraformaldehyde, the brain cryoprotected in sucrose, and the tissue rapidly frozen and sectioned. Free-floating sagittal brain tissue sections (25 μm) were rinsed with phosphate-buffered saline (PBS), permeabilized with PBS containing 0.2% TX-100, rinsed with PBS and incubated overnight at 4°C with blocking solution or blocking solution containing a polyclonal antibody to APE (HAP1; 1:6000) as previously described (Kisby et al., 1997). After removal of the primary antibody, the tissue sections were washed with PBS, incubated for 30 min with anti-mouse Alexa 488 (1:500; Invitrogen) and then the sections examined by epifluorescence microscopy using a Zeiss Axioskop 2™.
Statistical Analysis
Data are expressed as the mean ± S.E.M. All data obtained were evaluated for statistical significance by one-way analysis of variance (ANOVA) and Scheffe’s method of comparison (StatView™). A probability value of p < 0.05 was considered significant unless otherwise noted.
Results
Exonuclease Activity in Brain Tissue
To examine the usefulness of the novel substrate (Figure 1A), we first determined the APE activity of extracts derived from the frontal cortex of various species (human, primate, rodent). Figure 1 shows that extracts from the frontal cortex of a 77 yr old adult male or the frontal/parietal cortex of adult male non-human primates and male rats of various ages (Figure 1B) exhibited a variable degree of degradation of the 5′ fragments of the APE cleavage products. All extracts exhibited high levels of 3′-5′ exonuclease activity as indicated by the appearance of fast migrating species (Figure 1B, arrow pointing to Exo prod) and a concomitant reduction in the amount of 5′ cleavage product (Figure 1B, arrow pointing to 5′-prod). Analysis of several brain regions from two other males revealed a different pattern with either high exonuclease activity (87 yr old, PMI 2.0h) or high activity in only the hippocampus and parietal and pre-frontal cortex (data not shown), suggesting that exonuclease activity is also highly variable in the human brain. This variability was also observed in the frontal cortex of primates and similar brain regions (hippocampus, cerebellum; data not shown). In contrast, the 3′ fragment derived from the APE cleavage reaction was relatively constant for all the brain tissue extracts. By using the 3′ fragment, we can accurately estimate APE activity in each brain region. Thus, these studies demonstrate that there is considerable variability in exonuclease activity in the brain across species, and that the 3′ fragment is the most reliable product for assessing APE activity in brain tissue.
Effect of CR on Survival and body weight
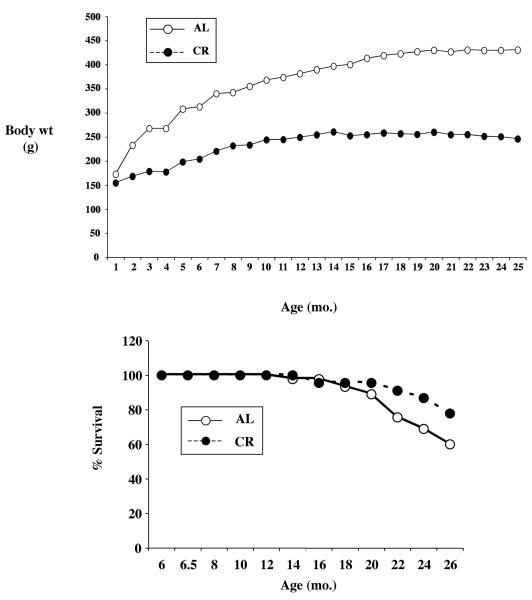
As expected, CR resulted in an immediate slowing in the gain of body weight, compared to ad libitum controls (Figure 2A). The influence of CR on body weight became evident at 14 months of age, such that at 26 months the survival rate for controls and CR treated animals was 60% and 78%, respectively (Figure 2B).
Figure 2. Effect of CR on the survival of male rats.
CR was initiated starting at 6 weeks of age, starting at a reduced intake of 10% per week until there was a 40% difference between ad lib controls and CR treated rats. For the later, the increase in weight with age was dramatically slowed (Figure 2A), whereas controls continued to gain weight over time. The effect of CR on longevity is noted in Figure 2B, where the %survival is maintained better than ad libitum animals started after one year of continuous treatment.
APE Activity in the Aging Brain
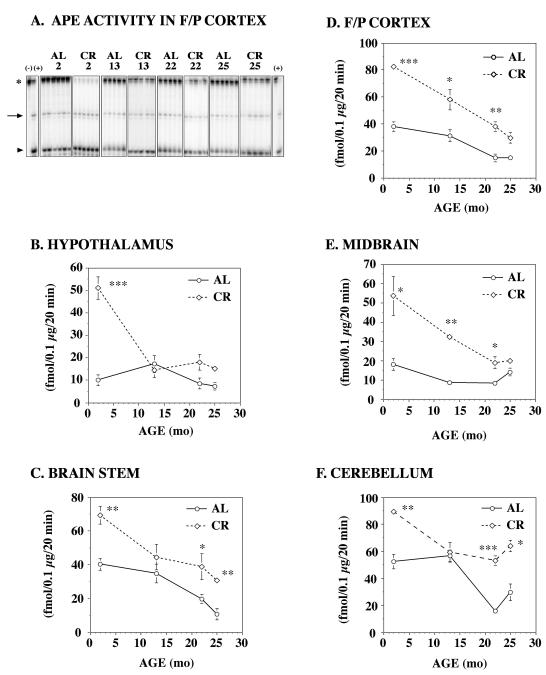
Our next objective was to use the novel 5′-5′ oligo probe to determine if CR, a dietary regimen that is known to extend the lifespan of animals, also influences brain tissue oxidative DNA repair capacity. For these studies, tissue homogenates from five different rat brain regions [frontal/parietal (F/P) cortex, hypothalamus, midbrain, cerebellum and brain stem] were prepared from each age group on either an AL or CR diet and analyzed for APE activity. A significant difference in APE activity was observed in all brain regions between the CR and AL rats at 2 months of age (Figure 3). A representative gel (Fig. 3A) shows that the loss of the labeled 5′-5′ oligo probe (star) and the increased production of the 3′ fragment (arrow) is greater in the F/P Cortex of CR than AL rats (likewise, consumption of substrate is increased in CR). Regional APE activity ranged from 2-fold higher in the cerebellum (Fig. 3F) to as much as 5-fold higher in the hypothalamus (Fig. 3B) of CR rats when compared to AL rats. It is also interesting to note that APE activity in each brain region declined with age for both CR and AL rats. By 25 months of age, the difference in APE activity between these two groups was reduced ~2-fold, although in some instances (e.g. F/P cortex), APE activity was still significantly higher in CR than AL rats. Interestingly, the age-dependent decline in APE activity was much more pronounced in CR rats when compared to AL rats. By 25-months of age, there was a 2-3 fold decrease in APE activity in the F/P cortex, hypothalamus, midbrain and brainstem of CR rats. In contrast, APE activity was much more variable in the hypothalamus and cerebellum of CR and AL rats. APE activity was higher in CR young (2-mo) and old (25 mo) rats, but comparable to AL rats at 13 mo. In comparison to other brain regions, the cerebellum of CR rats retained higher levels of APE activity in the oldest animals. As in Figure 1B, these studies demonstrate that APE activity varies with age and it is influenced by CR.
Figure 3. AP endonuclease (APE) activity in various brain regions of aging CR rats.
Homogenates of freshly frozen brain tissue from 2mo, 13 mo, 22 mo, and 25 mo F344 rats on an ab libitum (AL) or caloric restricted (CR) diet (n=6/age group) were prepared as previously described by Kisby et al. (1997) and examined for APE activity using the 5′-5′ oligonucleotide probe (50 fmol) described in Fig 1. A. A representative autoradiogram showing AP endonuclease activity in the F/P cortex of rats on an AL or CR diet. The arrow denotes the 3′ cleavage product, the star denotes the substrate and the arrowhead denotes the 5′ cleavage product. Negative control (lane 1) and positive control (2nd and last lanes). AP endonuclease activity was measured in the hypothalamus (B), brain stem (C), frontal/parietal (F/P) cortex (D), midbrain (E) and cerebellum (F). In general, APE activity declined with age, but CR significantly retarded the rate of decline. Significantly different from AL (*p<0.05, ** p < 0.01, *** p <0.001).
Analysis of APE Levels in F/P Cortex
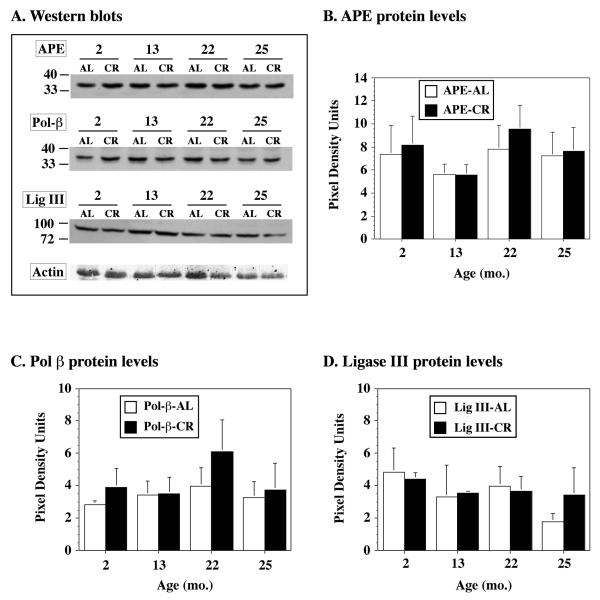
Out of the five brain regions examined for APE activity, the F/P cortex and midbrain of CR and AL rats showed the most significant differences. Therefore, the F/P cortex of AL and CR rats were examined further for the level of APE and other BER proteins (polymerase β and DNA ligase III) by Western analysis. Interestingly, despite significant differences in APE activity between CR and AL rats, Western blotting showed that the protein levels of APE were not significantly different in 2 month-old CR or AL rats (Figure 4). Similar trends were also noted for polymerase β and DNA ligase III. Furthermore, no significant differences in these BER enzymes were observed in older animals (i.e. 13-mos, 22-mos, 25-mos of age). Brain tissue sections from 2- and 13-mos old AL and CR rats were probed with an antibody to APE to determine the distribution of APE in the F/P cortex (Figure 5). APE was uniformly expressed in the F/P cortex and other brain regions (data not shown) of both young and middle aged AL and CR rats. These findings are consistent with previous work demonstrating that BER capacity and not BER protein levels in rodents varies as a function of brain region (i.e. caudate nucleus, frontal cortex, hippocampus, cerebellum, and brainstem) and age (Gredilla et al., 2008; Imam et al., 2005).
Figure 4. BER protein levels in the frontal/parietal cortex of aging CR rats.
Protein extracts (50 μg) prepared from the frontal/parietal cortex of 2 mo, 13 mo, 22 mo or 25 mo AL or CR rats (n=4-5/treatment) were analyzed by Western blotting for APE, DNA polymerase β (Pol β) and DNA ligase III (Ligase III) levels using a polyclonal antibody (1:2000) to human APE or monoclonal antibodies to Pol β (1:1000), DNA Ligase III (1:1000) and γ-actin (loading control, 1:2000). A. Representative blots of the F/P cortex of AL or CR rats probed with antibodies to various BER proteins. Densitometric analysis of the blots indicated that there was no effect of age or CR on protein levels of APE (B), Pol β (C), or Ligase III (D).
Figure 5. Distribution of APE in the frontal/parietal cortex of aging CR rats.
Photomicrographs of representative sections from the frontal/parietal cortex of 2 mo and 13 mo AL and CR rats probed with an antibody to APE. Note that the distribution of immunopositive cells is uniform in AL and CR rats. Magnification 40×.
Oxidative DNA Damage in the F/P Cortex
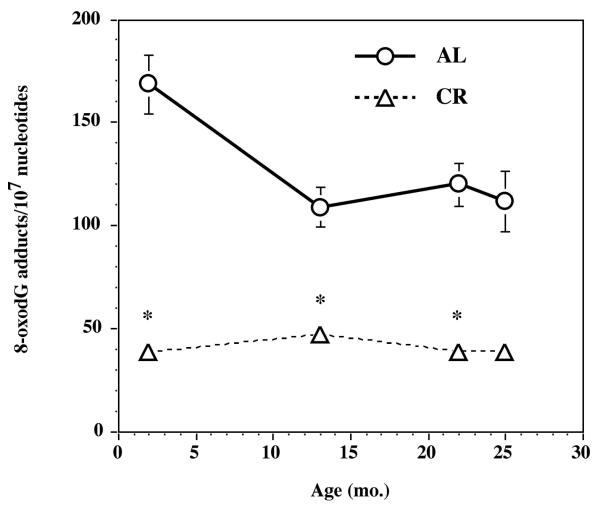
As expected, F/P cortex tissue levels of 8-oxodG were also significantly higher (p < 0.05) in AL than CR rats (Figure 6). This demonstrates that oxidative DNA damage is lower in the F/P cortex of CR rats suggesting that oxidative DNA repair is more efficient in the F/P cortex of a CR rat. These findings are consistent with the increased APE activity (Figure 3) in the F/P cortex of CR rats.
Figure 6. Oxidative DNA damage in the frontal/parietal cortex of aging CR rats.
DNA was isolated from the frontal/parietal cortex of 2 mo, 13 mo, 22 mo or 25 mo AL or CR rats (n=6/age group) and the purified DNA analyzed for 8-oxodeoxyguanosine levels by LC-ES/MS/MS as previously described (Churchwell et al., 2002). Significantly different from AL (*p<0.02).
Discussion
Although there is a wealth of information about the influence of CR on oxidative damage in peripheral tissues during aging, understanding of similar mechanisms in the aging brain is limited. One possibility is that CR provides neuroprotection to the aging brain by increasing the efficiency of oxidative DNA repair (Heydari et al., 2007; Hiona and Leeuwenburgh, 2004). Therefore, one of the major objectives of the present studies was to determine if the neuroprotective properties of a CR diet are related to its influence on brain tissue DNA repair. Although CR has been shown to increase BER in the aging brain (Cabelof et al., 2003), these are the first studies to investigate the regional and temporal changes in BER induced by CR. These two factors are considered to be critical for understanding the selective vulnerability of neurons in the aging brain or age-related neurodegenerative disease (Stuart et al., 2004).
Since BER is considered the primary cellular pathway for repairing oxidative DNA damage (Cadet et al., 2000) and AP endonuclease (APE) is the pivotal enzyme (Izumi et al., 2005), we chose to examine the level and activity of this repair enzyme in the aging brain. We noticed in our initial studies with traditional APE substrates (e.g. depurinated plasmid DNA, uracil or tetrahydrofuran containing 5′-3′ oligonucleotides) that the activity of this repair enzyme is considerably variable in human tissues (i.e., brain, lymphocytes). Based upon previous work in the rat brain (Krishna et al., 2004), we reasoned that tissues contain different amounts of 3′-5′ exonucleases, and this enzyme could readily degrade the more traditional APE substrates leading to an incorrect assessment of cellular APE activity. This could also explain why APE activity is variable in the brain of ALS subjects (Kisby et al., 1997) and there is an observed discrepancy for the age-associated changes in mitochondrial or nuclear APE activity (Chen et al., 2002). To address these potential problems, a novel 5′-5′ oligonucleotide probe was developed so that we could simultaneously measure APE and 3′-5′ exonuclease activity in brain tissue. The results of this effort is clearly demonstrated in Figure 1 where we show that brain tissue of various species (human, primate, rodent) produced variable amounts of the 5′ fragment, but little or no degradation of the 3′ fragment or the substrate. This is due to the fact that the 5′ fragment contains a free 3′ terminus that is susceptible to degradation by 3′-5′ exonucleases while the 3′-fragment is relatively resistant. Thus, this novel oligonucleotide probe allowed us to accurately assess the influence of a CR diet on brain tissue APE activity, a tissue with variable exonuclease activity. Data shown in Figure 1 further illustrate this point by demonstrating that this novel probe resisted degradation by tissues from various species with variable 3′-5′ exonuclease activities.
The CR rodent model has become particularly useful for identifying the molecular mechanisms of brain aging and neurodegenerative disease (Mattson et al., 2001). Results from these studies indicate that the subtle morphological and functional changes that develop in the aging brain appear to be strongly linked with disturbances in both the immune and oxidative stress response. Since preserving the genome from oxidative damage appears to be a major factor in longevity and cell viability (Hasty et al., 2003), DNA repair capacity may be less efficient in the aging brain or in the brain of subjects with neurodegenerative disease. Our primary objective in the present studies was to determine if the anti-aging properties of CR are associated with an increase in brain tissue oxidative DNA repair. Using a novel assay, we demonstrate that APE activity, like the downstream BER enzyme DNA polymerase β (Prapurna and Rao, 1996), declines with increasing age, but the magnitude of this effect was region specific. A steady decline in APE was observed in the cortex, midbrain and brainstem, while the decline was more rapid in the hypothalamus and cerebellum of aging rats (Figure 3). These findings indicate that there are regional differences in the rate of decline in oxidative DNA repair during aging. Consistent with these results, the accumulation of oxidative DNA damage (i.e. 8-oxodG) and its repair are not uniform across the brain of aging mice. Analysis of six brain regions (cortex, caudate, hippocampus, midbrain, pons/medulla, and cerebellum) of aging mice indicated that only three regions exhibited increased 8-oxodG levels (Cardozo-Pelaez et al., 1999) and this was inversely correlated with oxidative DNA capacity (i.e, 8-oxodG DNA glycosylase, OGG1) (Cardozo-Pelaez et al., 2000). In more recent studies of comparably aged mice, four of these brain regions (cortex, caudate, hippocampus and cerebellum) also exhibited a differential pattern of mitochondrial and nuclear OGG1 activity indicating that the regional differences in BER also vary at the sub-cellular level (Gredilla et al., 2008; Imam et al., 2005). A similar pattern of oxidative DNA damage was also found to occur across different brain regions of aging rats (Hamilton et al., 2001) indicating that the rate of DNA damage and decline in DNA repair capacity are a consistent feature of the aging brain. These regional differences in BER may also explain why the subtle morphological and functional changes that develop in the aging brain are not uniform. An alternative explanation is that the spectrum of oxidative DNA lesions may differ among brain regions as previously recognized for the analysis of human brain tissue using similar procedures (Wang et al., 2005).
Few studies have examined the aging brain of CR rodents for regional differences in oxidative DNA repair. Moreover, previous studies have focused on later steps of the BER pathway (e.g., DNA polymerase β) or assessed overall changes in pathway function. We demonstrate for the first time that APE activity is significantly increased in the brain of CR rats and that the magnitude of the effect is both age- and region-dependent. These findings are also consistent with previous work demonstrating that DNA polymerase β activity was moderately increased by CR in the brain of aging Fischer-344 rats (Cabelof et al., 2003), and this effect induced regional changes (e.g, hippocampus, hypothalamus) (Prapurna and Rao, 1996). However, Cabelof and colleagues (2003) noted that DNA polymerase β protein levels were increased by CR, an effect not observed in the present study. This disparity can be explained by differences in the tissue samples used in both studies. Notably, Cabelof and colleagues (2003) used nuclear (vs. tissue) extracts from the whole brain (vs. cortical) of AL and CR rats to measure pol β levels. Moreover, the increases in DNA polymerase β activity reported by Prapurna and Rao (1996) in the hypothalamus and hippocampus of CR-treated rats is consistent with regional changes in APE activity in the present study.
Western blotting and immunohistochemistry (in this study), however, indicate that the regional increase in APE activity was not simply due to an up-regulation in the amount of BER proteins. Imam and colleagues (Imam et al., 2005) noted that the activity of three different DNA glycosylases (OGG1, UDG, NTH) varied with age and brain region, even though protein levels remained unchanged. They examined five different brain regions (caudate nucleus, frontal cortex, hippocampus, cerebellum, brainstem) for mitochondrial or nuclear APE and DNA ligase III protein levels and found no significant age- or region-dependent changes. However, the activity of all three enzymes in the mitochondria declined with age for all brain regions, but activity in the nucleus was variable. Their studies demonstrate that the age-related decline in DNA glycosylase activity in different brain regions is not due to a change in protein levels. These findings and those of the present studies suggest that CR increases brain tissue APE activity by another mechanism other than by inducing gene or protein expression. Since the activity of APE (and other BER enzymes) is increased in neurons after oxidative stress (Harrison et al., 2005) and this has been shown to be associated with the targeting of BER proteins to different sub-cellular compartments (Duguid et al., 1995), it is possible that CR influenced the sub-cellular compartmentalization of APE. Support for this mechanism comes from studies demonstrating that mitochondrial APE activity and mtDNA damage are increased in rat cerebellar neurons, but not in corresponding astrocytes after treatment with the oxidant menadione (Harrison et al., 2005). The translocation of APE among different compartments has also been shown to depend upon the efficient transport of modified forms of APE (e.g. truncation of mitochondrial targeting sequence) or its post-translational processing (e.g. acetylation, phosphorylation) (Tell et al., 2005). Since one of the characteristic features of aging tissues is an increase in oxidative DNA damage and a decline in BER capacity (Cabelof et al., 2003) without a corresponding change in protein or mRNA levels (Imam et al., 2005)(Figure 4), it is conceivable that the decline of APE activity we observed in the different brain regions of the aging rats could be due to a perturbation in these transport mechanisms. It appears that the transport of APE and other BER proteins is disturbed in the liver of 20 month-old rats (Szczesny et al., 2004), but whether similar changes occur in the aging brain is currently unknown.
It is generally believed that the anti-aging properties of CR are mediated by two key mechanisms: (1) an improvement in energy metabolism (i.e, the rate of mitochondrial electron transport) via a reduction in reactive oxygen species (Lopez-Lluch et al., 2006) and (2) an increased resistance to stressors (hormesis) (Koubova and Guarente, 2003; Rattan, 2008). The latter mechanism was the subject of the present studies. More specifically, preservation of the genome appears to be a major factor in longevity and cell viability (Hasty et al., 2003), and DNA repair appears to play a key role. This is especially important in the brain because of the demand for oxidative metabolism and of long-lived cells. Aging studies with transgenic mice that are defective in DNA repair have revealed the importance of these cellular pathways in genome maintenance and longevity. Targeted disruption of certain NER genes has produced mice with an accelerated aging phenotype (including the brain) (de Boer et al., 2002) and demonstrated the importance of DNA repair in the aging process. The failure of BER knockout mice (e.g, APE, DNA polymerase β) to thrive beyond fetal development is evidence that this particular pathway is essential for normal development. Consistent with this idea, findings from the present studies have demonstrated that the activity of APE declines with increasing age and that CR significantly retards this age-dependent process in several regions of the aging rat brain. Collectively, these studies suggest that CR has a positive global effect on the DNA repair status of an organism. The ability of CR to increase maximal lifespan, reduce oxidative DNA damage and increase oxidative DNA repair in an organism is further evidence supporting a role for oxidative stress in the aging process.
Acknowledgments
We thank Dr. Vilhelm Bohr for his valuable comments on the manuscript. This work is supported by NIH CA90860 (YWK), NIEHS PO1 ES011163 (YWK), RR00163 (SGK) and DAMD 17-98-1-8625 (GK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barja G. Aging in vertebrates, and the effect of caloric restriction: a mitochondrial free radical production-DNA damage mechanism? Biol. Rev. Camb. Philos. Soc. 2004a;79:235–251. doi: 10.1017/s1464793103006213. [DOI] [PubMed] [Google Scholar]
- Barja G. Free radicals and aging. Trends Neurosci. 2004b;27:595–600. doi: 10.1016/j.tins.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Barzilay G, Walker LJ, Rothwell DG, Hickson ID. Role of the HAP1 protein in repair of oxidative DNA damage and regulation of transcription factors. Br. J. Cancer. 1996;74:S145–S150. [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat. Rev. Mol. Cell. Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005;40:774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Cabelof DC, Yanamadala S, Raffoul JJ, Guo Z, Soofi A, Heydari AR. Caloric restriction promotes genomic stability by induction of base excision repair and reversal of its age-related decline. DNA Repair (Amst) 2003;2:295–307. doi: 10.1016/s1568-7864(02)00219-7. [DOI] [PubMed] [Google Scholar]
- Cadet J, Bourdat AG, D’Ham C, Duarte V, Gasparutto D, Romieu A, Ravanat JL. Oxidative base damage to DNA: specificity of base excision repair enzymes. Mutat. Res. 2000;462:121–8. doi: 10.1016/s1383-5742(00)00022-3. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, J. BP, Stedeford T, Song S, Sanchez-Ramos J. DNA damage, repair, and antioxidant systems in brain regions: A correlative study. Free Radical Biol. Med. 2000;28:779–785. doi: 10.1016/s0891-5849(00)00172-6. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Song S, Parthasarathy A, Hazzi C, Naidu K, Sanchez-Ramos J. Oxidative DNA damage in the aging mouse brain. Movement Disorders. 1999;14:972–980. doi: 10.1002/1531-8257(199911)14:6<972::aid-mds1010>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Chen D, Cao G, Hastings T, Feng Y, Pei W, O’Horo C, Chen J. Age-dependent decline of DNA repair activity for oxidative lesions in rat brain mitochondria. J. Neurochem. 2002;81:1273–84. doi: 10.1046/j.1471-4159.2002.00916.x. [DOI] [PubMed] [Google Scholar]
- Choi JY, Kim HS, Kang HK, Lee DW, Choi EM, Chung MH. Thermolabile 8-hydroxyguanine DNA glycosylase with low activity in senescence-acclerated mice due to a single-base mutation. Free Radical Biol. Med. 1999;27:848–854. doi: 10.1016/s0891-5849(99)00141-0. [DOI] [PubMed] [Google Scholar]
- Churchwell MI, Beland FA, Doerge DR. Quantification of multiple DNA adducts formed through oxidative stress using liquid chromatography and electrospray tandem mass spectrometry. Chem. Res. Toxicol. 2002;15:1295–301. doi: 10.1021/tx0101595. [DOI] [PubMed] [Google Scholar]
- de Boer J, Andressoo JO, de Wit J, Huijmans J, Beems RB, van Steeg H, Weeda G, van der Horst GT, van Leeuwen W, Themmen AP, Meradji M, Hoeijmakers JH. Premature aging in mice deficient in DNA repair and transcription. Science. 2002;296:1276–9. doi: 10.1126/science.1070174. [DOI] [PubMed] [Google Scholar]
- Duguid JR, Eble JN, Wilson TM, Kelley MR. Differential cellular and subcellular expression of the human multifunctional apurinic/apyrimidinic endonuclease (APE/ref-1) DNA repair enzyme. Cancer Res. 1995;55:6097–6102. [PubMed] [Google Scholar]
- Gredilla R, Garm C, Holm R, Bohr VA, Stevnsner T. Differential age-related changes in mitochondrial DNA repair activities in mouse brain regions. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Heydari A, Richardson A. Nucleotide excision repair of actively transcribed versus nontranscribed DNA in rat hepatocytes: effect of age and dietary restriction. Exp. Cell. Res. 1998;245:228–238. doi: 10.1006/excr.1998.4269. [DOI] [PubMed] [Google Scholar]
- Hamilton ML, Van Remmen H, Drake JA, Yang H, Guo ZM, Kewitt K, Walter CA, Richardson A. Does oxidative damage to DNA increase with age? Proc. Natl. Acad. Sci. U S A. 2001;98:10469–74. doi: 10.1073/pnas.171202698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison JF, Hollensworth SB, Spitz DR, Copeland WC, Wilson GL, LeDoux SP. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;33:4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–9. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- Heydari AR, Unnikrishnan A, Lucente LV, Richardson A. Caloric restriction and genomic stability. Nucleic Acids Res. 2007;35:7485–96. doi: 10.1093/nar/gkm860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiona A, Leeuwenburgh C. Effects of age and caloric restriction on brain neuronal cell death/survival. Ann. N. Y. Acad. Sci. 2004;1019:96–105. doi: 10.1196/annals.1297.018. [DOI] [PubMed] [Google Scholar]
- Holmes GE, Bernstein C, Bernstein H. Oxidative and other DNA damages as the basis of aging: a review. Mutat. Res. 1992;275:305–315. doi: 10.1016/0921-8734(92)90034-m. [DOI] [PubMed] [Google Scholar]
- Hosokawa M, Fujisawa H, Ax S, Zahn-Daimler G, Zahn RA. Age-associated DNA damage is accelerated in the senescence-accelerated mice. Mech. Ageing Dev. 2000;118:61–70. doi: 10.1016/s0047-6374(00)00158-5. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA-repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol. Aging. 2006;27:1129–1136. doi: 10.1016/j.neurobiolaging.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Izumi T, Brown DB, Naidu CV, Bhakat KK, Macinnes MA, Saito H, Chen DJ, Mitra S. Two essential but distinct functions of the mammalian abasic endonuclease. Proc. Natl. Acad. Sci. U S A. 2005;102:5739–43. doi: 10.1073/pnas.0500986102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Henner WD, Mitra S. Negative regulation of the major human AP-endonuclease, a multifunctional protein. Biochemistry. 1996;35:14679–14683. doi: 10.1021/bi961995u. [DOI] [PubMed] [Google Scholar]
- Kisby GE, Milne J, Sweatt C. Evidence of reduced DNA repair in amyotrophic lateral sclerosis brain tissue. NeuroReport. 1997;8:1337–1340. doi: 10.1097/00001756-199704140-00004. [DOI] [PubMed] [Google Scholar]
- Koubova J, Guarente L. How does calorie restriction work? Genes Dev. 2003;17:313–21. doi: 10.1101/gad.1052903. [DOI] [PubMed] [Google Scholar]
- Krishna TH, Hemkal A, Rao KS. The presence of 3′-5′ exonuclease activity in rat brain neurons and its role in template-driven extension of 3′-mismatched primers by DNA-polymerase beta in aging neurons. Neurochem. Res. 2004;29:761–70. doi: 10.1023/b:nere.0000018848.58358.95. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, Hilmer S, Cascajo MV, Allard J, Ingram DK, Navas P, de Cabo R. Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc. Natl. Acad. Sci. U S A. 2006;103:1768–73. doi: 10.1073/pnas.0510452103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;24:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Rad. Biol. Med. 1997;23:134–147. doi: 10.1016/s0891-5849(96)00629-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Lee J, Guo Z. Suppression of brain aging and neurodegenerative disorders by dietary restriction and environmental enrichment: molecular mechanisms. Mech. Ageing Dev. 2001;122:757–78. doi: 10.1016/s0047-6374(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muniz JF, McCauley L, Scherer J, Lasarev M, Koshy M, Kow YW, Nazar-Stewart V, Kisby GE. Biomarkers of oxidative stress and DNA damage in agricultural workers: a pilot study. Toxicol. Appl. Pharmacol. 2008;227:97–107. doi: 10.1016/j.taap.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Takahashi JA, Fujibayashi Y, Fujisawa H, Zhu B, Nishimura Y, Ohnishi K, Higuchi K, Hashimoto N, Hosokawa M. An early stage mechanism of the age-associated mitochondrial dysfunction in the brain of SAMP8 mice; an age-associated neurodegeneration animal model. Neurosci. Lett. 1998;254:69–72. doi: 10.1016/s0304-3940(98)00646-6. [DOI] [PubMed] [Google Scholar]
- Ono Y, Furuta T, Ohmoto T, Akiyama K, Seki S. Stable expression in rat glioma cells of sense and antisense nucleic acids to a human multifunctional DNA repair enzyme, APEX nuclease. Mutat. Res. 1994;315:55–63. doi: 10.1016/0921-8777(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Prapurna DR, Rao KS. Long-term effects of caloric restriction initiated at different ages on DNA polymerases in rat brain. Mech. Ageing Dev. 1996;92:133–142. doi: 10.1016/s0047-6374(96)01815-5. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res. Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rehman A, Nourooz-Zadeh J, Moller W, Tritschler H, Pereira P, Halliwell B. Increased oxidative damage to all DNA bases in patients with type II diabetes mellitus. F.E.B.S. Lett. 1999;448:120–2. doi: 10.1016/s0014-5793(99)00339-7. [DOI] [PubMed] [Google Scholar]
- Retel J, Hoebee B, Braun JEF, Lutgerink JT, van den Akker E, Wanamarta AH, Joenje H, Lafleur MVM. Mutational specificity of oxidative DNA damage. Mutat. Res. 1993;299:165–182. doi: 10.1016/0165-1218(93)90094-t. [DOI] [PubMed] [Google Scholar]
- Stuart JA, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA base excision repair are affected differently by caloric restriction. FASEB J. 2004;18:595–7. doi: 10.1096/fj.03-0890fje. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Bhakat KK, Mitra S, Boldogh I. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech. Ageing Dev. 2004;125:755–65. doi: 10.1016/j.mad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Tell G, Crivellato E, Pines A, Paron I, Pucillo C, Manzini G, Bandiera A, Kelley MR, Loreto CD, Damante G. Mitochondrial localization of APE/Ref-1 in thyroid cells. Mutat. Res. 2001;485:143–152. doi: 10.1016/s0921-8777(00)00068-9. [DOI] [PubMed] [Google Scholar]
- Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid. Redox. Signal. 2005;7:367–84. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- Tomkinson AE, Bonk RT, Linn S. Mitochondrial endonuclease activities specific for apurinic/apyrimidinic sites in DNA from mouse cells. J. Biol. Chem. 1988;263:12532–12537. [PubMed] [Google Scholar]
- Vasko MR, Guo C, Kelley MR. The multifunctional DNA repair/redox enzyme Ape1/Ref-1 promotes survival of neurons after oxidative stress. DNA Repair. 2005;4:367–79. doi: 10.1016/j.dnarep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Xiong S, Xie C, Markesbery WR, Lovell MA. Increased oxidative damage in nuclear and mitochondrial DNA in Alzheimer’s disease. J. Neurochem. 2005;93:953–62. doi: 10.1111/j.1471-4159.2005.03053.x. [DOI] [PubMed] [Google Scholar]