Abstract
Event-related potentials (ERPs) were employed to investigate the relationship between the familiarity strength of recognition memory test items (pictures of animate and inanimate objects) and a putative ERP correlate of familiarity, the mid-frontal ‘old/new’ effect. A modified Remember/Know task was used in which subjects endorsed items as ‘remembered’ if any detail of the study presentation could be retrieved and, if not, judged the old/new status of the item using a 4-point confidence scale (‘confident old’ to ‘confident new’). Studied test items elicited a mid-frontal old/new effect that varied according to the rated familiarity of the eliciting item. Thus, prior findings that the mid-frontal effect is graded according to familiarity strength are not attributable to the confounding influence of study status, as has been suggested. ERPs elicited by studied and unstudied items that were rated equally familiar differed in the same latency range as that occupied by the mid-frontal old/new effect. Furthermore, the scalp topography of this repetition effect differed significantly from the topography of the mid-frontal effect. The findings suggest that ERPs elicited by recognition memory test items are modulated during the 300-500 ms latency range both by the familiarity strength of the item and, separately, by an implicit memory process that acts independently of the processes supporting familiarity-driven recognition judgments.
Keywords: recognition memory, event-related potential, remember-know, confidence, familiarity, conceptual priming
1. Introduction
There is substantial evidence that recognition memory is supported by two processes, namely, familiarity and recollection. In what is probably the most widely accepted ‘dual-process’ account of recognition memory (Yonelinas, 1994; Yonelinas, 2001; see Yonelinas and Parks, 2007 for a review of this and several other models), these processes are held to be qualitatively distinct. Familiarity reflects an acontextual, graded memory signal that does not provide qualitative details about the study episode. By contrast, recollection has a threshold-like character, and involves retrieval of consciously accessible details of the study event.
Despite the apparent success of dual-process models in accounting for a diverse range of findings in both experimental psychology and neuropsychology (e.g. Diana et al., 2006; Yonelinas, 2002), these models have been challenged on the grounds that the findings can also be accommodated by more parsimonious ‘single-process’ models (Donaldson, 1996; Dunn, 2004; Wixted and Stretch, 2004). In such models, familiarity and recollection are viewed either as expressions of a common memory signal (e.g. Dunn, 2004), or as being combined into a single source of evidence before a recognition judgment is made (Wixted, 2007). Either way, single-process models incorporate the idea that recognition decisions are based on assessment of a single, continuously varying memory signal. These models can be distinguished from dual-process accounts, which propose that decisions can be based on either of two qualitatively distinct signals, one of which is continuous (familiarity), and the other thresholded (recollection).
It has proven difficult to adjudicate between dual- and single-process models of recognition memory on the basis of behavioral findings alone. Thus, findings from healthy subjects vary in how well they can be accommodated by each of the competing models (see, for example, Dunn, 2004; Heathcote et al., 2006; Parks and Yonelinas, 2009; Yonelinas and Parks, 2007), and studies of brain-lesioned patients have yielded conflicting results (e.g. Wais et al., 2006; Yonelinas et al., 2002; see Cipolotti and Bird, 2006 for review). The inconsistent behavioral evidence has given additional impetus to studies seeking convergent evidence by investigating the neural correlates of recognition memory. The assumption motivating these studies is that, to the extent that recollection and familiarity are qualitatively distinct memory signals, their neural correlates should likewise be qualitatively distinct. By contrast, if the two forms of memory are merely expressions of a common memory signal, one might expect their neural correlates to differ in degree (that is, quantitatively) but not in kind. From this perspective, it is noteworthy that findings from both functional magnetic resonance imaging (fMRI) and event-related potential (ERP) studies suggest that recollection and familiarity are indeed qualitatively distinct (see Eichenbaum et al., 2007 for review of relevant fMRI findings, and Rugg and Curran, 2007 for review of ERP studies).
Here, we focus on evidence that has been used to argue that recollection and familiarity are dissociated by event-related potentials (ERPs). On the basis of a now-substantial number of reports, it has been argued that ERP effects elicited by items recognized on the basis of familiarity versus recollection differ on a combination of temporal and topographical criteria (for reviews see Mecklinger, 2006; Rugg and Curran, 2007). Both classes of effects take the form of a positive-going ERP modulation relative to ERPs elicited by correctly rejected new items. However, whereas familiarity is associated with a relatively early (ca. 300-500 ms), frontally distributed ‘old/new’ effect (the ‘mid-frontal’ old/new effect), recollection is reflected by a later (ca. 400-800 ms) effect often maximal over the left parietal scalp (the ‘left parietal’ old/new effect). To the extent that it is correct to associate these two ERP effects with the constructs of familiarity and recollection, these findings offer strong support for the proposal that the two memory signals are neurally, and hence likely functionally, distinct.
A difficulty in the interpretation of these putative ERP correlates of recollection and familiarity arises because recollection- and familiarity-based recognition judgments are typically associated with different levels of confidence. Whereas recollection-based responses are almost invariably made with high confidence, familiarity-based judgments can vary markedly in confidence depending on the strength of the familiarity signal elicited by the test item (Yonelinas, 1994). Thus, other things being equal, the neural correlates of familiarity and recollection are confounded by differences in the confidence of the ensuing recognition decisions. To obviate this problem, Yonelinas et al., (2005) introduced a modified version of the well known ‘Remember/Know’ procedure (Tulving, 1985). In this modified procedure, subjects respond ‘remember’ when a test item elicits a subjective sense of recollection, but in the absence of recollection they provide a rating of their confidence that the item is studied or unstudied, rather than merely make a ‘know’ or a ‘new’ judgment. It is typically assumed that recognition confidence is a monotonic function of the amount of evidence favoring an ‘old’ judgment (MacMillan and Creelman, 1991). Hence, in the procedure of Yonelinas et al. (2005), confidence that an unrecollected item was old is assumed to co-vary with level of familiarity (the familiarity ‘strength’) of the item.
Woodruff et al. (2006) employed this modified Remember/Know procedure in an ERP study. Consistent with prior proposals that the mid-frontal old/new effect is a neural correlate of familiarity (see above), the magnitude of the effect covaried with confidence that the eliciting item was old, but did not further vary according to whether or not the item was recollected. By contrast, the parietal old/new effect was elicited by items endorsed as recollected relative to those confidently judged old, but was not evident for the contrast between ‘confident old’ and ‘confident new’ responses. These findings are consistent with the proposal that the mid-frontal and parietal old/new effects do indeed dissociate familiarity and recollection, but they are subject to an important caveat. In order to have sufficient trials to form stable ERP waveforms, Woodruff et al. (2006) were forced to collapse their data across the factor of study status. Since studied items were more likely to attract confident old judgments than unstudied items, while unstudied items were more likely than studied items to be confidently judged new, collapsing over study status led to a confound between confidence rating and study status. Hence, effects attributed to familiarity might instead merely have reflected simple repetition effects that masqueraded as graded familiarity effects (see Kirwan et al., 2009, for a similar argument in respect of fMRI findings). In response to this problem, Woodruff et al., (2006) conducted an additional analysis in which they assessed whether the mid-frontal old/new effect was evident in a contrast between ERPs elicited by studied and unstudied items that had been rated as equally familiar. Although no old/new effect was identified, implying that the mid-frontal effect was indeed responding primarily to familiarity strength, the statistical power of this analysis has been called into question (Paller et al., 2007).
The present experiment replicates the study of Woodruff et al. (2006), but with a procedure modified to permit the relationship between rated familiarity strength and the mid-frontal old/new effect to be assessed for studied items only, removing the confound between strength and study status discussed above. This was achieved by employing pictorial rather than verbal stimulus materials, so as to boost overall recognition performance1, and by employing a larger number of study items than were presented in the prior experiment. To the extent that the effects of recognition confidence on the mid-frontal old/new effect reported by Woodruff et al. (2006) do indeed reflect the sensitivity of the effect to familiarity strength, similar effects should be evident in ERPs elicited exclusively by studied items.
2. Results
In the ANOVAs reported below, F ratios associated with factors containing more than two levels were corrected for nonsphericity by the Greenhouse-Geisser procedure (Greenhouse and Geisser, 1959).
2.1. Behavioral data
Table 1 shows the proportion of studied and unstudied items given each of the five possible responses. There is a systematic relationship between response category and study status, such that studied items predominated in the recollect and confident old categories and unstudied items predominated in the confident new and unconfident new categories. ANOVA (factors of study status and response category) revealed a main effect of response category (F2.2, 48.9 = 16.55, P < 0.001) along with a significant interaction between study status and response category (F2.8, 61.7 = 122.62, P < 0.001). The interaction reflected the cross-over between the proportions of recollected, confident old and confident new responses accorded to studied and unstudied items (see Table 1).
Table 1.
Proportions of old and new items assigned to each response category and the weighted RT associated with each category.
| Recollect | Conf old | Unconf old | Unconf new | Conf new | |
|---|---|---|---|---|---|
| Studied | 0.33 | 0.26 | 0.18 | 0.14 | 0.09 |
| Unstudied | 0.02 | 0.03 | 0.10 | 0.31 | 0.54 |
| RT (ms) | 1024 | 1260 | 1424 | 1396 | 1179 |
Table 1 also shows the reaction times (RTs) for each response category, collapsed (weighted means) across study status (there were too few responses in several cells to permit RTs to be analyzed according to study status). As is evident from the table, the means demonstrate an inverted U function across the four categories of non-recollected responses. ANOVA revealed a main effect of response category (F3.0, 65.4 = 57.72, P < 0.001). This effect remained in a second ANOVA in which remember responses were dropped from the analysis (F2.5 54.2 = 30.14, P < 0.001).
2.2. ERPs
Analyses of the ERP data were conducted in several stages. First, we describe the old/new effects elicited by studied items, the main focus of the present experiment. More briefly, we then describe an analysis of the old/new effects elicited by items from the different response categories collapsed across study status, replicating the key analyses of Woodruff et al. (2006). Finally, we address the possibility that effects of old/new status may modulate ERPs independently of the effects of familiarity strength, again following procedures adopted by Woodruff et al. (2006).
2.2.1. Mid-frontal old/new effect elicited by studied items
We begin with analyses that characterize the relationship between the familiarity strength of studied test items and the mid-frontal old/new effect2 elicited by these items. As in prior studies (e.g. Ecker et al., 2007; Nyhus and Curran, 2009; Woodruff et al., 2006), the mid-frontal effect was quantified as the mean amplitude (with respect to the mean of the pre-stimulus baseline) of the waveforms between 300 and 500 ms post-stimulus. Sufficient trials (15 or more) to allow a contrast between waveforms elicited by studied items accorded confident old, unconfident old and unconfident new judgments were available from 19 subjects. Too few trials were available to allow ERPs to be formed for studied items confidently endorsed as new. The mean numbers of trials (and ranges) comprising the ERPs in each condition were 38 (15-74) for confident old, 28 (15-49) for unconfident old, and 23 (17-29) for unconfident new. We contrasted the mean amplitudes of the 300-500 ms latency region of the ERPs elicited by items hypothesized to differ maximally according to familiarity strength, that is, items attracting confident old vs. unconfident new judgments. The grand averages of these waveforms from left and right hemisphere frontal electrode sites are shown in Fig. 1, where it can be seen that an old/new effect is evident from around 200-600 ms post-stimulus, especially over the left frontal quadrant. To establish whether this effect was statistically significant, an initial ANOVA was conducted on the mean amplitudes of the 300-500 ms latency region of the ERPs from the clusters of four electrodes located over each quadrant of the scalp (see Fig. 1). The ANOVA employed the factors of response category (confident old vs. unconfident new), hemisphere, location (frontal vs. parietal) and electrode site. It revealed significant interactions between response category and location (F1, 18 = 6.04, P < 0.025), and response category and hemisphere (F1, 18= 5.45, P < 0.05). Follow-up ANOVAs conducted on the data from each electrode quadrant revealed a main effect of response category for the left frontal quadrant (F1, 18 = 10.94, P < 0.005), but no significant effects for the other quadrants.
Figure 1.
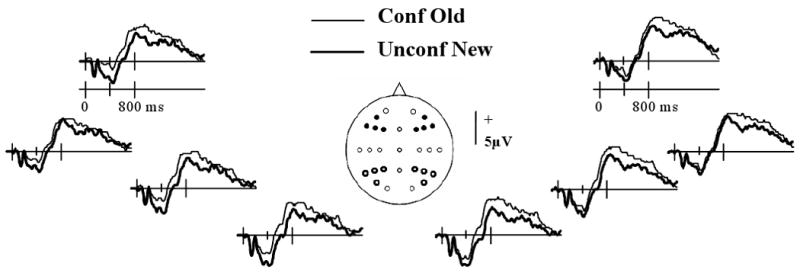
Grand average ERP waveforms from the left and right frontal electrode sites (indicated on the scalp schematic by solid black circles) elicited by studied items accorded confident old or unconfident new judgments. Also highlighted are the left and right parietal electrode sites that were incorporated into the initial analyses of old/new effects (see text).
We then went on to assess whether the mid-frontal effect was graded as a function of familiarity strength, as was reported by Woodruff et al. (2006). Fig. 2A illustrates the grand average waveforms, collapsed across the four left frontal electrodes, elicited by studied items accorded confident old, unconfident old or unconfident new judgments. As is evident from the figure, the amplitude of the 300-500 ms latency region of the waveform associated with unconfident old judgments occupies an intermediate position between the confident old and unconfident new response categories, suggestive of a graded effect. To assess whether this effect was reliable across subjects we performed an additional analysis directly analogous to that employed by Woodruff et al. (2006) to address the same question (see also Yonelinas et al., 2005). For each subject, and for the four electrode sites belonging to each scalp quadrant, we calculated the regression coefficient between the mean amplitude of the 300-500 ms latency region and a dummy vector representing the three confidence levels. The coefficients were then averaged to give a single value for each quadrant. Under the null hypothesis of no relationship between amplitude and confidence, the expectation for the across subject mean of the regression coefficient is zero. The coefficient derived from the left frontal electrodes differed significantly from zero (mean (sd) = 0.87 (1.15), t18 = 3.31, 1 tailed P <.005). The regression coefficients derived from the remaining three quadrants did not significantly differ from zero.
Figure 2.

A: Above: Grand average ERP waveforms, collapsed across the four left frontal quadrant electrode sites, elicited by studied items accorded ‘confident old’, ‘unconfident old’, or ‘unconfident new’ judgments. Below: Plot of mean amplitudes of the ERP waveforms between 300-500 ms from the collapsed left frontal electrode sites according to type of recognition response. Error bars represent standard errors of the mean. B: Above: Grand average ERP waveforms, collapsed across left frontal quadrant sites, elicited by ‘confident old’, ‘unconfident old’, unconfident new’ or ‘confident new’ judgments collapsed across the factor of study status. Below: Plot of mean 300-500 ms amplitudes, averaged across the four left frontal electrodes, of the ERP waveforms collapsed across study status.
Finally, we contrasted the mid-frontal effect elicited by recollected items with the effect elicited by items confidently endorsed old. ANOVA of the 300-500 ms data from the left frontal quadrant for these two response categories revealed a significant response category by site interaction (F2.1, 38.1 = 3.61, P < 0.05). The interaction reflected a tendency for greater positivity in the ERPs elicited by confident old items relative to recollected items, the effect increasing in size from midline to lateral sites. Pair-wise contrasts on the data from each site were, however, uniformly non-significant.
2.2.2. Mid-frontal old/new effect collapsed across study status
In a second set of analyses, we determined whether the present findings replicated the results reported by Woodruff and colleagues (2006). Thus, these analyses were conducted on the ERP waveforms elicited by test items associated with each response category (i.e. confident old, unconfident old, unconfident new, confident new) collapsed across the factor of study status. The analyses were performed on the data from 23 subjects who had sufficient trials in the critical conditions (15 or more). The number of trials (and ranges) comprising each of the waveforms for recollect, confident old, unconfident old, unconfident new and confident new conditions were 52 (16-103), 42 (16-94), 34 (15-66), 44 (27-76), and 52 (19-112), respectively. The grand average waveforms are illustrated in Fig. 3. As is evident from the figure, left frontal waveforms between around 200 to 600 ms post-stimulus are more positive for items rated confident old than confident new. ANOVA (factors of response category and electrode site) of the mean 300-500 ms amplitudes for the left frontal quadrant revealed a significant effect of response category (F1, 22 = 27.48, P < 0.001). Fig. 2B illustrates the grand average waveforms (averaged across the four left frontal electrodes) associated with each confidence category. As is evident from the figure, the mean amplitudes of these waveforms in the 300-500 ms latency region varied monotonically with confidence. Regression coefficients expressing the relationship in each subject between the amplitude of the left frontal ERPs and confidence (see above) differed significantly from zero (mean (sd) = 0.83 (0.71), t22 = 5.87, 1 tailed P <.001). Thus, as in the case of the waveforms elicited by studied items alone (see above), there was a reliable monotonic relationship between amplitude of the 300-500 ms latency region and confidence.
Figure 3.
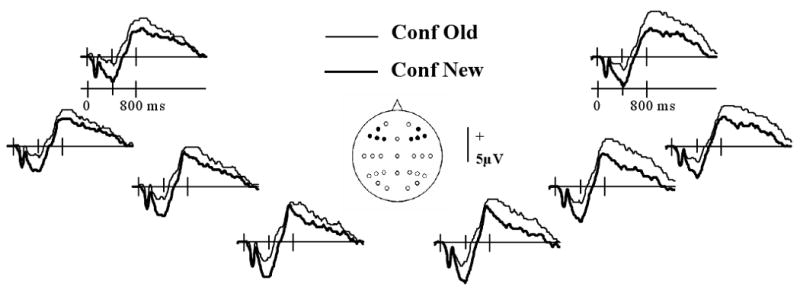
Grand average ERP waveforms from the left and right frontal electrodes elicited by test items collapsed across study status accorded either ‘confident old’ or ‘confident new’ judgments.
2.2.3. Effects of study status
The finding that ERPs elicited by studied items demonstrate a familiarity-sensitive mid-frontal old/new effect provides unequivocal evidence that the effect does not arise because of a confound between study status and familiarity (see Introduction). Nonetheless, the question remains whether the mid-frontal effect is sensitive to study status independently of familiarity strength – that is, whether the effect is also sensitive to mere item repetition. Thus we investigated whether ERPs elicited by studied and unstudied items that had been equated for familiarity strength manifested a mid-frontal effect. We accomplished this using the procedure first adopted by Woodruff et al. (2006) to form, for each subject, ERPs elicited by studied and unstudied items that had been equated for familiarity strength. For each level of confidence, we first identified the type of test item (studied or unstudied) with the fewest trials. We then randomly selected an equivalent number of trials associated with the alternate item type at that confidence level. [For example, a subject might have endorsed 5 unstudied items but 20 studied items as ‘confident old’. By randomly selecting only 5 of those 20 items, the bias in favor of studied items for that confidence level is eliminated]. After equating studied and unstudied trial numbers for each confidence level in this manner, separate ERPs for studied and unstudied items were created by collapsing across confidence level. Because the numbers of studied and unstudied items were equated at each confidence level, any differences between these ERPs must reflect the influence of study status rather than confidence (i.e. familiarity strength).
The mean numbers (and range) of trials forming the ERPs for studied and unstudied items were 39 (24-64) and 39 (23-63), respectively. Fig. 4A illustrates the left frontal ERPs, collapsed across electrode site, associated with studied and unstudied items equated for familiarity as just described. ANOVA contrasting the mean amplitudes of the 300-500 ms data from these electrodes revealed a main effect of item type (F1, 22= 5.12, P < 0.05). For the purposes of comparison, the waveforms associated with confident old (familiar) and confident new (unfamiliar) judgments are shown in Fig. 4B. As already reported, these latter waveforms differed significantly in the 300-500 ms latency region. As is evident in Fig. 3C, the effects of study status and familiarity differ markedly in their magnitudes, with familiarity yielding the greater effect. A pairwise t-test on confirmed that the two classes of effect do indeed differ significantly (t22= 2.56, 2 tailed P < 0.025).
Figure 4.
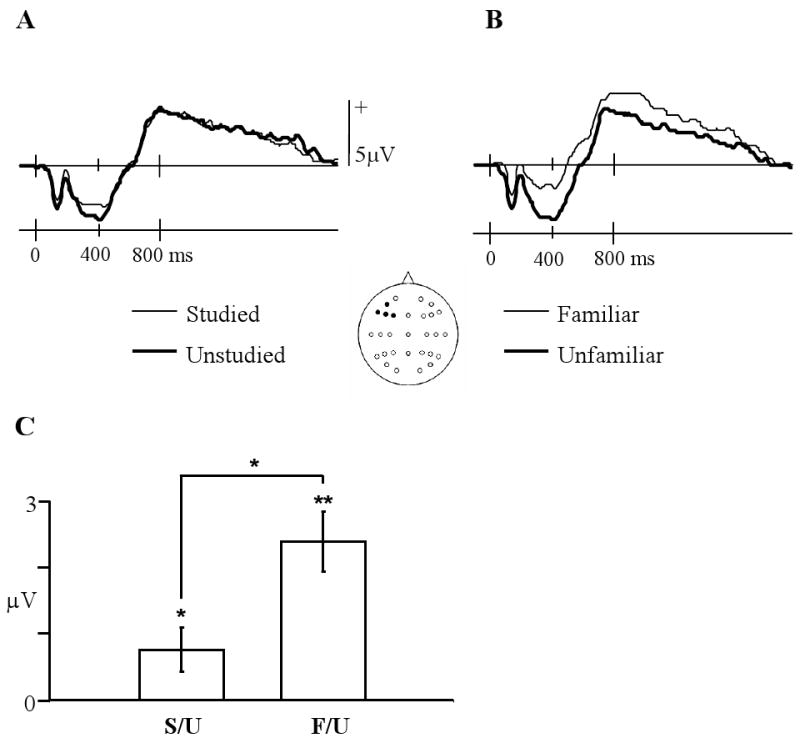
A: ERP waveforms, averaged over the left frontal electrodes, elicited by studied and unstudied items equated for familiarity strength. B: Left frontal ERP waveforms elicited by items accorded confident old (familiar) or confident new (unfamiliar) judgments collapsed across study status. C: Mean left frontal amplitude differences in the 300-500 ms latency region for familiar minus unfamiliar, and studied minus unstudied contrasts. (** = p<.01, * = p<.05). Error bars represent standard errors of the mean.
2.2.4 Parietal old/new effect
Figure 5 illustrates the grand average waveforms from the left and right parietal quadrants, in each case collapsed across the four electrode sites. As is evident from the figure, the waveforms begin to diverge around 400 ms post-stimulus, with the ERPs elicited by recollected items demonstrating a marked positivity relative to the other item types (see Fig. 6D for a depiction of the scalp distribution of this effect). As in prior studies (e.g. Rugg et al., 1998, Woodruff et al., 2006), the parietal old/new effect was quantified by measuring the mean amplitude of the 500-800 ms latency region. An initial ANOVA contrasted these data from the left and right parietal electrode quadrants, employing the factors of response category (recollect, confident old, confident new), hemisphere, and electrode site. The ANOVA revealed a significant main effect of response category (F2, 44= 17.96, P < 0.001) and a significant three-way interaction between this factor and the other two factors (F3.3, 71.7= 2.66, P = 0.05). A follow-up ANOVA restricted to the data from the left parietal quadrant contrasting the recollected and confident old response categories revealed a significant effect of category (F1, 22= 61.84, P < 0.001) and a significant interaction between response category and site (F1.8, 40.0= 4.91, P < 0.025). These effects reflected the greater positivity of the ERPs elicited by recollected items, along with a tendency for this effect to be smallest at the most posterior of the sites. ANOVA of the left parietal data contrasting recollected and confident new responses revealed the same pattern of effects (main effect of response category: F1, 22= 23.69, P < 0.001; response category by site interaction: F1.6, 35.8= 17.55, P < 0.001). By contrast, ANOVA of the data for the confident old and confident new response categories gave rise to no significant effects.
Figure 5.
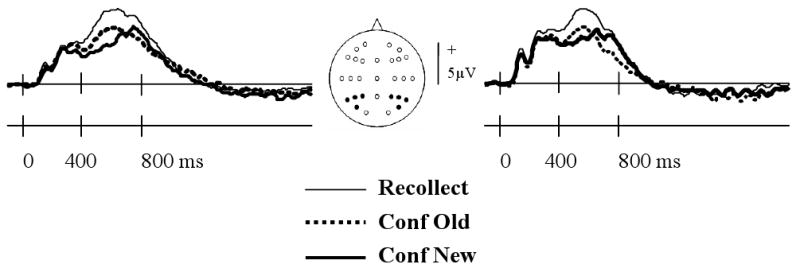
A: Grand average left and right parietal ERP waveforms elicited by test items accorded recollect, confident old, or confident new judgments.
Figure 6.
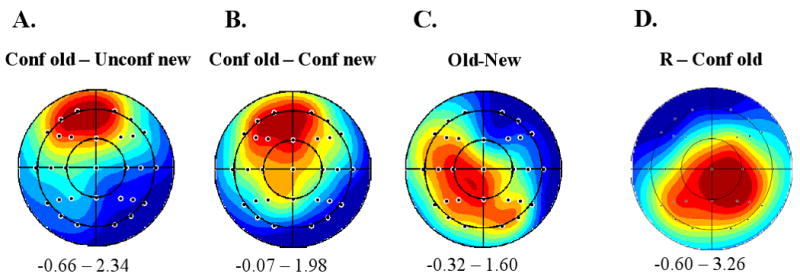
A: Scalp topography of the mean amplitude difference in the 300-500 ms latency region between ERPs associated with ‘confident old’ vs. ‘unconfident new’ judgments made on studied test items; B: Topography of the equivalent effect for test items collapsed across study status; C: Topography of the amplitude difference in the 300-500 ms latency region between ERPs elicited by studied and unstudied items equated for familiarity; D: Topography of the mean amplitude difference in the 500-800 ms latency range between ERPs associated with ‘recollect’ and ‘confident old’ judgments. Plots are range-normalized (red = positive, blue = negative) with the range in microvolts displayed under each plot. The nose is located at the top of each plot.
2.2.5. Topographic analyses
The question arises whether the mid-frontal old/new effects elicited by studied items, by items collapsed across study status, and by studied versus unstudied items equated for familiarity strength share the same scalp distributions and hence, perhaps, the same intra-cerebral generators. To address this question, we contrasted the scalp distributions of the following effects: i.) studied items endorsed as confident old vs. unconfident new; ii.) items collapsed across study status endorsed as confident old vs. confident new; and iii.) studied and unstudied items equated for familiarity strength. These analyses were based on the data from the 19 subjects employed in the analysis of the ERPs elicited by studied items, described above. The scalp topographies of each of these effects for the 300-500 ms latency region are shown in Fig. 6. As can be seen from Fig. 6A and 6B, the topographies of the two recognition memory effects both show frontal maxima, whereas the topography of the study status effect has a left central maximum (see Fig. 6C).
These topographies were contrasted by ANOVA of difference scores from the 29 electrodes located over the lateral scalp, employing the factors of type of effect (studied items: confident old – unconfident new, all items: confident old – unconfident new; equated for familiarity: studied – unstudied items), hemisphere, and site. The scores were range-normalized prior to analysis to remove the confounding effects of overall differences in effect magnitude (McCarthy and Wood, 1985)3. The ANOVA revealed a significant interaction between type of effect and electrode site (F2.8, 49.7= 3.55, P < 0.025), indicating that the three topographies were not statistically equivalent. Follow-up pair-wise ANOVAs revealed that while the two recognition memory effects did not differ in their topographies (Fs < 1 for all interactions between effects and the factors of site and/or hemisphere), each of these effects demonstrated a significant effect by site interaction when its topography was contrasted with that of the old-new effect (F2.5, 44.3= 4.59, P < 0.025 and F2.2, 40.4= 3.31, P < 0.05 for studied items and all items respectively). Thus, consistent with the impression given by Fig. 5, the topography of the familiarity-equated old-new effect differs from those of the two recognition memory effects.
The scalp topography of the recollection effect (recollect – confident old) in the 500-800 ms latency region is illustrated in Fig. 6D. ANOVA contrasting the topography of this effect with that of the familiarity effect (confident old – confident new) revealed a significant interaction between effect and electrode site (F2.1, 45.4= 7.53, P < 0.01).
3. Discussion
The aim of this experiment was to assess whether the mid-frontal ERP old/new effect remains sensitive to familiarity strength when strength is unconfounded from study status. We addressed this issue by modifying the design of our prior study (Woodruff et al., 2006) so that ERPs elicited by studied but unrecollected recognition memory test items could be contrasted according to the confidence of the recognition judgment they attracted. Consistent with proposals that the mid-frontal old/new effect is a neural correlate of familiarity (Curran, 2000; Rugg et al., 1998; see Mecklinger, 2006, and Rugg and Curran, 2007 for review), we found that the effect was modulated by recognition confidence (and hence familiarity strength) even when elicited exclusively by studied items. Thus, the relationship between the mid-frontal effect and familiarity strength described by Woodruff et al. (2006) cannot be attributed to the confounding effects of study status as was suggested by Paller et al., 2007.
Before discussing the ERP findings in detail, we turn briefly to the behavioral findings. These closely resembled those reported by Woodruff et al. (2006), demonstrating the typically reported relationship between confidence and accuracy (e.g. Wixted and Stretch, 2004). Crucially, as in our prior study, RTs demonstrated a non-monotonic, ‘inverted U’ pattern as a function of confidence. This contrasts with the monotonic relationship we observed between the magnitude of the mid-frontal effect and confidence (see Fig. 2), and indicates that the ERP findings are not merely a consequence of such factors as decisional uncertainty or difficulty.
As just noted, the present findings – along with prior reports that the mid-frontal old/new effect is not elicited by studied test items misclassified as new (Curran and Hancock, 2007; Rugg et al., 1998; Tsivilis et al., 2001; Woolams et al., 2008) – demonstrate that the sensitivity of the mid-frontal effect to familiarity strength is not due to the confounding influence of study status. This finding is difficult to reconcile with the proposal that the mid-frontal effect is a neural correlate of conceptual priming rather than familiarity (Voss and Paller, 2006; Yovel and Paller, 2004). According to this proposal, studies linking the mid-frontal effect with familiarity have employed contrasts that confounded differences in the familiarity of recognition test items with differences in the degree to which the items had been primed by virtue of their study presentation. The present finding that the mid-frontal effect is graded according to the rated familiarity strength of studied items (Fig. 2) adds to other evidence that contradicts this proposal (see, for example, Groh-Bordin et al., 2006; Stenberg et al., 2009). Specifically, given that conceptual priming and familiarity are supported by independent processes (Stenberg et al., 2009), no association would be expected between the degree to which a test item is primed by a study presentation, and the amount of familiarity it accrues. Hence, studied test items separated according to familiarity strength should on average be primed to equal extents, and thus should not be differentiated by a neural correlate of priming. As a rejoinder, it could be argued that some level of correlation between familiarity and priming will always exist because of the influence of encoding factors that impact both types of memory (to take an extreme example, if a test item was presented while a subject's eyes were shut, neither its familiarity nor its level of priming would be enhanced). Although it is conceivable that such a mechanism might allow a priming account to accommodate the failure of recognition misses to elicit a mid-frontal effect, this account would have to stretch considerably further to explain the graded pattern of the effects illustrated in Fig. 2 and reported by Woodruff et al. (2006).
This is not to say that we were unable to find evidence of an ERP old/new effect that was independent of rated familiarity. Unlike in the case of Woodruff et al. (2006), here the contrast between studied and unstudied items that had been equated for familiarity strength revealed a reliable ERP modulation in the same latency region as the mid-frontal effect (see Fig. 4). This finding adds to other evidence suggesting that ERPs are sensitive to item repetition independently of effects that co-vary with concurrent explicit memory judgments (Rugg et al., 1998; Woolams et al., 2008). Importantly, while the present repetition effect may have contributed in a minor way to the graded mid-frontal effects observed in the ERPs elicited by test items collapsed across study status, it cannot have been the sole determinant of these effects. First, the repetition effect was significantly smaller in magnitude than the familiarity-driven mid-frontal effect (Fig. 4). If the mid-frontal effect merely reflected the confounding influence of study status this finding would not have been possible; rather, the differences in the magnitude of the two effects would have been reversed. Second, and arguably more important, the scalp topographies of the repetition and mid-frontal effects significantly differed (Fig. 6; for analogous findings see Woolams et al., 2008; Rugg et al., 1998). Rugg et al. (1998) suggested that such repetition effects are a reflection of implicit memory processes that are distinct from the processes that support familiarity-driven recognition judgments and there seems no reason why this account should not also be applicable to the present findings. Our experimental design does not however permit a determination of whether the present ERP repetition effects reflect overlap at the conceptual or perceptual level. Thus, the question whether these effects should be construed as neural correlates of conceptual or perceptual priming cannot be addressed.
Also consistent with prior results (Woodruff, et al., 2006), recollected items did not elicit a larger mid-frontal effect than did items accorded a confident old judgment. As has been noted previously (Rugg and Curran, 2007), these findings are difficult to reconcile with the proposal that items endorsed as recollected differ from items endorsed as ‘familiar’ merely because they possess greater memory strength (e.g., Donaldson, 1996; Dunn, 2004). Were this so, the mid-frontal effect for recollected items should have exceeded that for items confidently endorsed as familiar. On the contrary, the ERPs elicited by recollected items tended to demonstrate slightly smaller effects than did the waveforms for highly familiar items. Although tentative, this finding is compatible with the long-standing and widely held assumption that recollection and familiarity act independently to support recognition memory (Mandler, 1980). According to this assumption, whereas items confidently endorsed as old should have uniformly high levels of familiarity strength, the familiarity of recollected items is more variable and, on average, lower. In contrast to items accorded a confident judgment, however, recollected items did elicit a robust parietal old/new effect, as would be expect in light of numerous prior findings (see Introduction). This double dissociation between the mid-frontal and parietal old/new adds to the evidence that familiarity and recollection are not merely weak and strong forms of a common memory signal.
3.1. Conclusions
In conclusion, we found that the mid-frontal ERP effect is modulated by the familiarity strength of studied test items. We further found that while study status modulated ERPs in the same latency range as the mid-frontal effect, the effects of study status were topographically dissociable. We conclude that the mid-frontal old/new effect is a relatively direct correlate of the familiarity strength of a recognition test item, and is not a consequence of the confounding effects of study status and associated variables such as level of conceptual (or perceptual) priming.
4. Methods
4.1. Subjects
Twenty-six subjects (seventeen females) participated in return for payment of $15/h. The subjects ranged in age between 18 yrs and 22 yrs (mean 19.4 yrs), and were right-handed, native English speakers from the University of California, Irvine community who were free from neurological and psychiatric disorder according to self-report. Prior to participating, all subjects gave their informed consent in accordance with the UCI Institutional Review Board guidelines. In total, three subjects were rejected from analysis. One subject was rejected because of an almost total absence of ‘confident old’ or ‘unconfident old’ responses. Two other subjects were rejected because there were insufficient trials (<16) for the ERPs from one or more of the critical experimental conditions.
4.2. Stimuli
A pool of 324 colored pictures of animals and everyday objects constituted as the critical experimental stimuli. A study list of 222 items was randomly selected from this pool for each subject. The remaining 102 items served as new pictures for a test list composed of all 324 critical items. For each subject, new pictures were intermixed and pseudo-randomized with studied pictures such that the test list did not contain more than three items of the same study status occurring consecutively. Two buffer trials were added to the beginning and end of the study list, and two buffer trials were added to the beginning of the test list. Seventy-five pictures from a different picture pool were employed for the practice phase (50 items for the study list and 75 items for the test list).
4.3. Procedure
All experimental procedures were performed in a sound-attenuated room with subjects sitting 1 m from the display monitor. Experimental items were displayed on the monitor in a gray frame superimposed on a black background. The frame subtended a visual angle of 4.6° × 4.6° at the 1 m viewing distance. Subjects were given practice with both the study and test tasks prior to the experimental study and test phases.
Following the practice session, subjects were fitted with the EEG cap and electrodes (see below). Instructions for the study phase were verbally repeated again before the administration of the study list. The task requirement was to judge whether each picture represented an animate or inanimate object, responding with the left or right index finger accordingly. Each study trial consisted of the presentation of a red fixation cross (+) at the center of the gray frame for 500 ms, followed by a picture for 500 ms, followed by a black fixation cross for 1500 ms, during which time the subject made their judgment. The entire study list consisted of 226 trials. A short rest break was given after approximately every 75 trials.
Prior to the test phase, subjects were reminded of the instructions. A modified Remember/Know procedure was employed in which subjects indicated the status of each test item with one of five responses. Instructions were to make a ‘remember’ response (a button press with the thumb) when an item was judged old and a specific detail or details about the study episode could be recollected. If no details could be recollected, the requirement was to make one of four responses using the fingers on the other hand according to the confidence with which the item was judged as studied or unstudied [(‘confident old’ (thumb), ‘unconfident old’ (index finger), ‘unconfident new’ (middle finger) and ‘confident new’ (ring finger)]. The mapping of hand to the ‘remember’ and confidence judgments was counterbalanced across subjects. Each test trial consisted of the presentation of a red fixation cross for 500 ms, followed by a picture for 500 ms, followed by a black fixation cross for 2150 ms during which time the subject signaled one of the five judgments described above. In the event of a failure to respond during this interval, the next test trial began. The entire test comprised of 326 trials. A short rest break was given after approximately every 82 trials. Subjects were instructed to respond as quickly as possible without sacrificing accuracy. They were also reminded to remain as still as possible and to maintain fixation at the center of the screen at all times.
4.4. ERP recording and analysis
EEG was recorded continuously from 32 silver/silver chloride electrodes, 30 of which were attached to an elasticated head cap. The electrodes were situated in accordance with the extended 10-20 system (American Electroencephalographic Society, 1994) at sites FP1, AF7, F7, F5, F3, T7, C5, C3, P7, P5, P3, PO7, O1, PZ, Fz, FP2, AF8, F8, F6, F4, T8, C6, C4, P8, P6, P4, PO8, O2, Cz, and mid-way between Fz and Cz. The remaining two electrodes were placed on the left and right mastoid processes. EEG was recorded using Cz as a common reference, with the electrode situated between Fz and Cz serving as ground. Electro-oculographic (EOG) data were recorded from two bipolar electrode pairs, situated above and below the left eye, and on the outer canthi, respectively. Data were acquired continuously at a sampling rate of 256 Hz with an amplifier bandwidth of 0.01-40 Hz (-3 dB points). Inter-electrode impedances were maintained below 5 kΩ. EEG data were later epoched (duration: 2048 ms; pre-stimulus baseline: 102 ms), downsampled to a 125 Hz sampling rate, and algebraically re-referenced to averaged mastoids. Single epochs were visually inspected, and any epoch that contained artifact due to movement, horizontal eye-movement, vertical eye-movement not attributable to blinks, or excessive baseline drift were rejected. Averaged ERPs for each subject were digitally smoothed (-3 dB at 19.4 Hz, zero phase-shift) and blink artifacts removed using a previously described linear regression method (e.g. Henson et al., 2004).
Acknowledgments
This research was supported by NIMH grant 5R01MH072966.
Footnotes
A reviewer queried whether one might expect the shift from verbal to pictorial materials to alter the scalp distributions of the respective old/new effects. In light of the null findings of a prior study that contrasted ERP old/new effects elicited by words and pictures (Schloerscheidt and Rugg, 2004) there is no reason to expect major differences in the scalp distributions evident in the present study and that of Woodruff et al. (2006), and none were readily apparent.
Although the effect is left-lateralized in the present study, we will refer to it as the ‘mid-frontal’ effect so as to remain consistent with the prior literature. The analogous effect described by Woodruff et al. (2006) was similarly left-lateralized.
The practice of amplitude normalization prior to topographic analysis has been criticized (Urbach and Kutas, 2002). The criticisms of these authors – that normalization results in error due to baseline artifact and the effects of residual noise – apply only when data are normalized with respect to root mean square amplitude and are not relevant to range-normalized data (Wilding, 2006).
References
- American Electroencephalographic Society. Guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1994;11:111–113. [PubMed] [Google Scholar]
- Cipolotti L, Bird CM. Amesia and the hippocampus. Current Opinion in Neurology. 2006;19:593–598. doi: 10.1097/01.wco.0000247608.42320.f9. [DOI] [PubMed] [Google Scholar]
- Curran T. Brain potentials of recollection and familiarity. Memory and Cognition. 2000;28:923–938. doi: 10.3758/bf03209340. [DOI] [PubMed] [Google Scholar]
- Curran T, Hancock J. The FN400 indexes familiarity-based recognition of faces. NeuroImage. 2007;36:464–471. doi: 10.1016/j.neuroimage.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Reder LM, Arndt J, Park HY. Models of recognition: a review of arguments in favor of a dual-process account. Psychonomic Bulletin and Review. 2006;13:1–21. doi: 10.3758/bf03193807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson W. The role of decision processes in remembering and knowing. Memory and Cognition. 1996;28:523–533. doi: 10.3758/bf03200940. [DOI] [PubMed] [Google Scholar]
- Dunn JC. Remember-know: a matter of confidence. Psychological Review. 2004;111:524–542. doi: 10.1037/0033-295X.111.2.524. [DOI] [PubMed] [Google Scholar]
- Ecker UK, Zimmer HD, Groh-Bordin C. The influence of object and background color manipulations on the electrophysiological indices of recognition memory. Brain Research. 2007;1185:221–230. doi: 10.1016/j.brainres.2007.09.047. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Groh-Bordin C, Zimmer HD, Ecker UK. Has the butcher on the bus dyed his hair? When color changes modulate ERP correlates of familiarity and recollection. NeuroImage. 2006;32:1879–1890. doi: 10.1016/j.neuroimage.2006.04.215. [DOI] [PubMed] [Google Scholar]
- Heathcote A, Raymond F, Dunn J. Recollection and familiarity in recognition memory : Evidence from ROC curves. Journal of Memory and Language. 2006;55:495–514. [Google Scholar]
- Henson RN, Rylands A, Ross E, Vuilleumeir P, Rugg MD. The effect of repetition lag on electrophysiological and haemodynamic correlates of visual object priming. NeuroImage. 2004;21:733–743. doi: 10.1016/j.neuroimage.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Shrager Y, Squire LR. Medial temporal lobe activity can distinguish between old and new stimuli independently of overt behavioral choice. Proceedings of the National Academy of Science. 2009;106:14617–14621. doi: 10.1073/pnas.0907624106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan NA, Creelman CD. Detection theory: A user's guide. New York: Cambridge University Press; 1991. [Google Scholar]
- Mandler G. Recognizing: the judgment of previous occurrence. Psychological Review. 1980;87:252–271. [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of variance models. Electroencephalography and Clinical Neurophysiology. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Mecklinger A. Electrophysiological measures of familiarity memory. Clinical EEG and Neuroscience. 2006;37:292–299. doi: 10.1177/155005940603700406. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Semantic and perceptual effects on recognition memory: Evidence from ERP. Brain Research. 2009;1283:102–114. doi: 10.1016/j.brainres.2009.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. Trends in Cognitive Neuroscience. 2007;11:243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Parks CM, Yonelinas AP. Evidence for a memory threshold in second-choice recognition memory responses. Proceedings of the National Academy of Science. 2009;106:11515–11519. doi: 10.1073/pnas.0905505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Sciences. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allen K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Schloerscheidt AM, Rugg MD. The impact of change in stimulus format on the electrophysiological incides of recognition. Neuropsychologia. 2002;42:451–466. doi: 10.1016/j.neuropsychologia.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Stenberg G, Hellman J, Johansson M, Rosén I. Familiarity or conceptual priming: event-related potentials in name recognition. Journal of Cognitive Neuroscience. 2009;21:447–460. doi: 10.1162/jocn.2009.21045. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Otten LJ, Rugg MD. Context effects on the neural correlates of recognition memory: an electrophysiological study. Neuron. 2001;31:497–505. doi: 10.1016/s0896-6273(01)00376-2. [DOI] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Urbach TP, Kutas M. The intractability of scaling scalp distributions to infer neuroelectric sources. Psychophysiology. 2002;39:791–808. doi: 10.1111/1469-8986.3960791. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Fluent conceptual processing and explicit memory for faces are electrophysiologically distinct. Journal of Neuroscience. 2006;26:926–933. doi: 10.1523/JNEUROSCI.3931-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wais PE, Wixted JT, Hopkins RO, Squire LR. The hippocampus supports both the recollection and the familiarity components of recognition memory. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding EL. On the practice of rescaling scalp-recorded event-related potentials. Biological Psychology. 2006;72:325–332. doi: 10.1016/j.biopsycho.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Wixted JT. Dual-process theory and signal-detection theory of recognition memory. Psychological Review. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychonomic Bulletin and Review. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama HR, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Brain Research. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Woolams AM, Taylor JR, Karayanidis F, Henson RN. Event-related potentials associated with masked priming of test cues reveal multiple potential contributions to recognition memory. Journal of Cognitive Neuroscience. 2008;20:1114–1129. doi: 10.1162/jocn.2008.20076. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1351. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Components of episodic memory: the contribution of recollection and familiarity. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2001;356:1363–1374. doi: 10.1098/rstb.2001.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. The nature of recollection and familiarity: a review of 30 years of research. Journal of Memory and Language. 2002;46:411–517. [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: a review. Psychological Bulletin. 2007;133:800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauvé MJ, Widaman KF, Knight RT. Effects of extensive temporal lobe damage or mild hypoxia on recollection and familiarity. Nature Neuroscience. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- Yovel G, Paller KA. The neural basis of the butcher-on-the-bus phenomenon: when a face seems familiar but is not remembered. NeuroImage. 2004;21:789–800. doi: 10.1016/j.neuroimage.2003.09.034. [DOI] [PubMed] [Google Scholar]


