Abstract
Purpose
This study was performed to investigate whether removal of cholesterol from the plasma membrane and collapse of the acrosome can prevent structural chromosome aberrations of paternal origin in mouse zygotes produced by intracytoplasmic sperm injection (ICSI).
Methods
Mouse spermatozoa were treated with methyl-β-cyclodextrin (MβCD) to remove cholesterol from the plasma membrane and with calcium ionophore A23187 to collapse the acrosome. Chromosomes of zygotes derived from MβCD- and ionophore-treated spermatozoa were analyzed at the first mitotic metaphase.
Results
Both chemical agents effectively induced the acrosome reaction. Incidence of structural chromosome aberrations in ICSI zygotes derived from MβCD-treated spermatozoa was similar to that in zygotes produced by in vitro fertilization (IVF) with the same spermatozoa, but significantly lower compared to ICSI zygotes derived from acrosome-intact spermatozoa. Chromosome aberration rates in ICSI zygotes derived from ionophore-treated spermatozoa were evidently high compared to IVF zygotes.
Conclusions
Induction of the acrosome reaction through cholesterol efflux by MβCD can prevent chromosome aberrations of paternal origin, while use of ionophore to induce the acrosome reaction exerts detrimental effect on paternal chromosomes in ICSI zygotes.
Keywords: Methyl-β-cyclodextrin, Calcium ionophore, Acrosome reaction, ICSI, Chromosome aberrations
Introduction
With fertilization in mammals, spermatozoa undergo the acrosome reaction, in which hydrolytic enzymes help the passage of sperm through the zona pellucida enclosing the oocyte. Prior to the acrosome reaction, spermatozoa undergo capacitation, in which cholesterol dissociates from the plasma membrane overlying the acrosomal region [1]. With the intracytoplasmic sperm injection (ICSI) technique, uncapacitated spermatozoa with intact acrosomes are usually used to produce embryos, so the cholesterol-rich plasma membrane and acrosome enzymes are injected into the ooplasm. These spermatozoal structures have been suggested to affect sperm chromatin remodeling in mice [2], pigs [3] and rhesus monkeys [4–6]. While injection of uncapacitated spermatozoa with intact acrosomes affects development of mouse ICSI embryos [7], simultaneous removal of the plasma membrane and acrosome before ICSI improves embryonic development [8]. Supportive evidence has been presented for the rat [9].
Previous study found that when mouse spermatozoa were used for ICSI shortly after collection from the cauda epididymis, incidences of structural chromosome aberrations in resultant zygotes were high compared to zygotes conventionally produced by in vitro fertilization (IVF) procedure [10]. However, the aberration rate was reduced when spermatozoa were incubated for 2 h or more in bicarbonate-buffered TYH medium, which can effectively induce the capacitation and acrosome reaction in mouse spermatozoa [11]. Furthermore, when sperm incubation was carried out in hepes and phosphate-buffered media that never induce the capacitation and acrosome reaction, the chromosome aberration rate in resultant ICSI zygotes was raised in a time-dependent manner, but the time-dependent increase in chromosome aberrations disappeared when testicular spermatozoa with a low content of cholesterol in the plasma membrane were used [12]. These findings suggest that the membrane cholesterol and acrosome enzymes are involved in generation of structural chromosome aberrations of paternal origin in ICSI zygotes, and removal of cholesterol from the plasma membrane and induction of the acrosome reaction before ICSI can reduce risk of generating chromosome aberrations.
It has been demonstrated that methyl-β-cyclodextrin (MβCD) allows mouse spermatozoa to capacitate by promotion of cholesterol efflux from the plasma membrane [13–15]. Calcium ionophore A23187 is commonly used to induce the acrosome reaction in mammalian spermatozoa. In the present study, attempts were made to examine whether treatment of mouse spermatozoa with MβCD and ionophore before ICSI can prevent structural chromosome aberrations of paternal origin in ICSI zygotes.
Materials and methods
Chemicals
All inorganic reagents and polyvinylpyrrolidone (PVP) were purchased from Nacalai Tesque (Kyoto, Japan). Lipid-rich bovine serum albumin (BSA) (AlbuMax; Gibco BRL, Auckland, New Zealand) was used instead of conventional fraction V albumin. Polyvinyl alcohol (PVA) (cold-water soluble), hyaluronidase, antibiotics, vinblastine sulfate, methyl-β-cyclodextrin (MβCD), FITC-conjugate peanut agglutinin (PNA), and fetal bovine serum (FBS) were products of Sigma–Aldrich (St. Louis, MO, USA). Actinase E (Kaken Pharmaceuticals, Tokyo, Japan) was used as a protease. Calcium ionophore A23187 and Hoechst 33258 were products of Calbiochem (Merck KGaA, Darmstadt, Germany). Paraffin oil (Art. 1.07162.1000) was purchased from Merck Japan (Tokyo) and Vectashield from Vector Laboratories (Burlingame, CA, USA). Gonadotropic hormones eCG and hCG were products of Teikoku–Zoki Pharmaceuticals (Tokyo) and Aska Pharmaceuticals (Tokyo), respectively.
Media
Bicarbonate-buffered TYH [16] and hepes-buffered H-TYH [10] were used as media for preparing spermatozoa. When spermatozoa were treated with ionophore, the concentration of calcium in TYH was doubled to effectively induce the acrosome reaction [17]. This modified TYH with two-fold of Ca2+ was designated as mTYH. Hepes-buffered H-mCZB [18] was used for preparing oocytes where appropriate. Temporary storage of oocytes and cultivation of fertilized ova were performed using bicarbonate-buffered CZB [19] with modification by addition of 5.56 mM glucose. This was designated as mCZB. H-TYH and H-mCZB were used under 100% air, and TYH, mTYH and mCZB were used under 5% CO2 in air.
Animals
Oocytes and spermatozoa were collected from hybrid B6D2F1 mice. Animals were maintained under conditions of 14 h-lighting and a temperature of 23 ± 2°C, and provided with ad libitum access to food and water. All experiments were performed according to the Guidelines for Animal Experiments of Asahikawa Medical College.
Preparation of oocytes and spermatozoa
Female mice at 7–12 weeks old were intraperitoneally injected with 8–10 IU eCG, followed 48 h later with an injection of 8–10 IU hCG to induce superovulation. Approximately 16 h after hCG injection, MII oocytes were collected from the oviducts and placed in H-mCZB with 0.1% hyaluronidase for 3–5 min at 37°C to remove cumulus cells. After thorough washing in mCZB, cumulus-free oocytes were kept in the same medium under paraffin oil at 37°C. Mature spermatozoa were collected from the cauda epididymis of male mice at 7–12 weeks old.
Treatment of spermatozoa with MβCD and ionophore
For treatment with MβCD, spermatozoa were directly collected from the epididymis into a droplet (150 μl) of H-TYH with 1 mM of MβCD under paraffin oil at a concentration of 2–4 × 107 cells/ml. Spermatazoa were incubated for 2 h at 37°C in the experiment for IVF and for 3–3.5 h in the experiment for ICSI.
Treatment of spermatozoa with ionophore was performed under three different sperm incubation conditions: no-incubation, post-incubation and pre-incubation. With no-incubation, spermatozoa were directly released from the epididymis into a droplet (150 μl) of TYH at a concentration of 2–4 × 107 cells/ml under paraffin oil. After dispersing for 5 min, an aliquot (100 μl) of sperm suspension was transferred to 2.5 ml of mTYH with 20 μM of ionophore, and treated for 10 min at 37°C. Spermatozoa were washed twice with normal TYH by centrifugation of 350g and immediately used for ICSI and IVF. With post-incubation, spermatozoa were additionally incubated in TYH for 2 h at 37°C after treatment with ionophore, then used for ICSI and IVF. With pre-incubation, spermatozoa were incubated in TYH for 2 h at 37°C before treatment with ionophore and used for ICSI and IVF without additional incubation.
Immunocytological staining of acrosome
Responsiveness of the acrosome to MβCD was examined after treatment for 3 h. Acrosome status of ionophore-treated spermatozoa was examined immediately before use for IVF or ICSI under the three different sperm incubation conditions. The procedure for staining the acrosome was basically performed as described by Mendosa et al. [20]. In brief, an aliquot (50 μl) of sperm suspension was put in a 15-ml plastic tube, and the same volume of Hoechst 33258 (4 μg/ml) was added to the tube. The staining reaction was continued for 5 min at 37°C to distinguish live spermatozoa from dead spermatozoa. Spermatozoa were washed once with H-TYH, then fixed with 1% paraformaldehyde for 1 h at room temperature (RT). After washing twice with H-TYH, spermatozoa were smeared on a slide glass and treated with 100% methanol for 15 min at RT in the dark. The smear was dried and treated with FITC-PNA (50 μg/ml) for 30 min at RT in the dark. After washing once with distilled water, the smear was dried, covered with Vectashield and immediately examined using a fluorescent microscope. The ratio of live spermatozoa with different acrosome statuses was determined by counting more than 900 cells in five trials.
ICSI using spermatozoa treated with MβCD and ionophore
When spermatozoa were incubated in H-TYH with MβCD for 3–3.5 h, 10 μl of the sperm suspension was first transferred to a droplet (100 μl) of normal H-TYH to dilute MβCD. A small amount (approximately 5 μl) of diluted sperm suspension was then transferred into a droplet (20–30 μl) of H-TYH containing 10% PVP under paraffin oil, which was prepared in a plastic chamber on an inverted microscope with a piezo micromanipulator. A motile spermatozoon with morphologically normal feature was selected under ×200 magnification, and the head was separated from the tail by applying piezo pulses, then immediately injected into an oocyte using a piezo micromanipulator as described by Kimura and Yanagimachi [18]. Injected oocytes were transferred into mCZB for cultivation within 30 min of the manipulation.
The ICSI procedure using ionophore-treated spermatozoa was the same with MβCD-treated spermatozoa.
IVF using spermatozoa treated with MβCD and ionophore
IVF zygotes were conventionally produced to compare the incidence of chromosome aberrations with that of ICSI zygotes. When spermatozoa were incubated in H-TYH with MβCD for 2 h, a small amount (10 μl) of sperm suspension (2–4 × 107 cells/ml) was added to a droplet (100 μl) of H-TYH containing cumulus-free oocytes under paraffin oil. Co-culture of spermatozoa and oocytes was maintained for 4 h at 37°C. Fertilized ova were thoroughly washed with mCZB and transferred into the same medium at 37°C for further cultivation.
In the IVF experiment using ionophore-treated spermatozoa, cumulus-free oocytes were inseminated with spermatozoa at a concentration of approximately 106 cells/ml in TYH and kept for 2 h at 37°C. After being washed with mCZB, fertilized ova were cultured in the same medium at 37°C.
IVF zygotes derived from spermatozoa that underwent capacitation in normal TYH served as control.
Preparation of chromosome slides and analysis
At 6–8 h after ICSI or IVF, zygotes were exposed to vinblastine sulfate (0.02 μg/ml) to prevent syngamy and spindle formation. At the first cleavage metaphase, zygotes were processed for chromosome preparations. In brief, zygotes were treated with 0.5% actinase E to digest the zona pellucida, then placed in hypotonic solution (1:1 mixture of 1% sodium citrate solution and 30% FBS) for 8 min at RT. Chromosome slides were made by the gradual fixation-air drying method [21]. Chromosome slides were stained with 2% Giemsa solution for 8 min. After conventional analysis to detect chromosome break, gap, ring and chromatid exchange, slides were stained using the C-banding technique to detect dicentric aberration and to distinguish between structural chromosome aberration and aneuploidy [22]. In the present study, incidence of chromosome aberrations was represented as that in zygotes, because paternal chromosome complements were not always separated from maternal ones in some chromosome preparations. As described elsewhere [23], the incidence of structural chromosome aberrations of maternal origin in mouse ICSI zygotes remains fairly constant (0–2.8%) despite the occurrence of aberrations in paternal chromosomes. In the present study, therefore, increased incidence of structural chromosome aberrations in zygotes derived from treated spermatozoa was considered to have been caused by the increase of structural chromosome aberrations of paternal origin. Polyploid zygotes due to polyspermy in the IVF experiment and triploid zygotes due to suppression of the second polar body in the ICSI experiment were eliminated from chromosome analysis.
Statistical analysis
The χ2 test or Fisher’s exact probability test was used to compare the percentage of zygotes with chromosome aberrations. Differences in frequency of acrosome-reacted spermatozoa were examined using the Mann–Whitney U-test. When more than one pair of frequencies was to be compared, a non-parametric Kruskal–Wallis one-way analysis of variance and Scheffe’s method were used. Differences were considered significant at the level of P < 0.05.
Results
Induction of the acrosome reaction by MβCD and ionophore
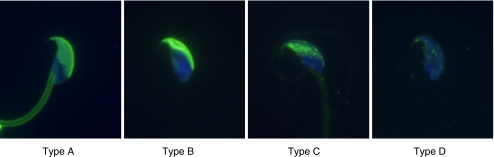
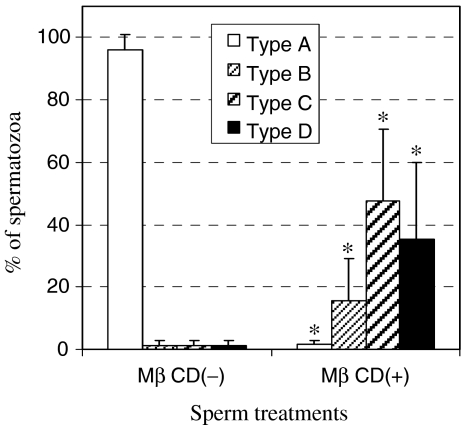
In the present results, acrosome status was divided into four categories: intact (type A); condensation (type B); collapse (type C); and elimination (type D) (Fig. 1). Spermatozoa of types C and D were regarded as acrosome-reacted spermatozoa. Over 95% of spermatozoa incubated in H-TYH without MβCD for 3 h had an intact acrosome (type A) (Fig. 2). When sperm incubation was carried out in H-TYH with MβCD for the same period, the percentage of acrosome-intact (type A) spermatozoa was extremely reduced, and in contrast that of acrosome-reacted (types C and D) spermatozoa was markedly raised. Thus the cholesterol efflux by MβCD was closely coupled with the acrosome reaction in mouse spermatozoa.
Fig. 1.
Classification of acrosome status of mouse spermatozoa after treatment with 1 mM MβCD for 3 h in H-TYH. Type A, intact acrosome; type B, condensed acrosome; type C, collapsed acrosome; and type D, elimination of the acrosome
Fig. 2.
Comparison between incubations in H-TYH with and without MβCD regarding the ratio of spermatozoa with different acrosome statuses. *P < 0.01: significant difference compared to MβCD(−) in each type
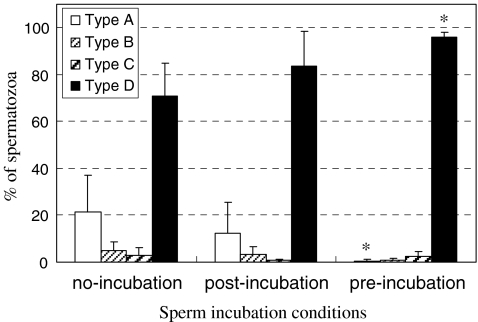
Morphological changes of the acrosome following treatment with ionophore appeared similar to those resulting from treatment with MβCD. As expected, ionophore well induced the acrosome reaction (Fig. 3). Spermatozoa that underwent pre-incubation were most sensitive to ionophore, showing the highest percentage (96%) of completely acrosome-reacted (type D) spermatozoa.
Fig. 3.
Comparison among sperm incubation conditions regarding the ratio of spermatozoa with different acrosome statuses after treatment with ionophore A23187. *P < 0.05, significant difference vs. no-incubation group in each type of acrosome statuses
Chromosome analysis of zygotes derived from MβCD- and ionophore-treated spermatozoa
When IVF were performed with spermatozoa incubated in H-TYH with MβCD for 2 h, 45–65% of oocytes were fertilized. Incidence (2.8%) of structural chromosome aberrations in IVF zygotes derived from MβCD-treated spermatozoa was not significantly different from that (1.6%) in control IVF zygotes (Table 1). When spermatozoa were used for ICSI after incubation in H-TYH with MβCD for 3–3.5 h, incidence (4.5%) of structural chromosome aberrations in resultant zygotes was close to that in IVF zygotes. However, when spermatozoa were incubated in H-TYH without MβCD for the same time, incidence (10.8%) of structural chromosome aberrations in resultant ICSI zygotes was significantly raised. Chromosome breaks, dicentrics and chromatid breaks discernibly occurred. In the present results, no significant increase of aneuploidy was identified in any group.
Table 1.
Chromosome analysis of IVF zygotes and ICSI zygotes derived from MβCD-treated spermatozoa
| Fertilization method | Sperm treatment with MβCD | No. of zygotes analyzed (no. exp.) | No. of aneuploid zygotes (%) | No. of zygotes with structural chromosome aberrations (%) | No. of aberrant chromosomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome type | Chromatid type | ||||||||||||
| Break | Gap | Dicentric | Translocation | Ring | Break | Gap | Exchange | Total | |||||
| IVF | Control | 253 (4) | 2 (0.8) | 4 (1.6) | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 4 |
| IVF | 1 mM, 2 h | 211 (5) | 3 (1.4) | 6 (2.8) | 4 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 6 |
| ICSI | 0 mM, 3–3.5 h | 212 (6) | 6 (2.8) | 23 (10.8)a | 15 | 4 | 16 | 0 | 1 | 10 | 0 | 0 | 46 |
| ICSI | 1 mM, 3–3.5 h | 200 (7) | 5 (2.5) | 9 (4.5)b | 2 | 2 | 4 | 0 | 0 | 0 | 1 | 0 | 9 |
aSignificantly high (P < 0.001) compared to control IVF zygotes derived from spermatozoa incubated without MβCD
bSignificantly low (P < 0.05) compared to ICSI zygotes derived from spermatozoa incubated without MβCD
Ionophore-treated spermatozoa showed high fertilizing capacity, as more than 80% of inseminated oocytes were successfully fertilized in any sperm incubation condition. Incidences of structural chromosome aberrations in IVF zygotes derived from ionophore-treated spermatozoa were low (1.1–2.8%) regardless of sperm incubation conditions (Table 2). The incidences were similar to that in control IVF zygotes (see Table 1). In ICSI zygotes of ionophore-treated sperm origin, however, structural chromosome aberration rates were markedly higher than in IVF zygotes of matched controls. The chromosome aberration rate of ICSI zygotes in the no-incubation condition was significantly higher than that of ICSI zygotes in the other two incubation conditions. Breakage-type aberrations were predominantly observed in any incubation condition. No increase in aneuploidy was seen for any zygotes produced by IVF and ICSI with ionophore-treated spermatozoa.
Table 2.
Chromosome analysis of IVF zygotes and ICSI zygotes derived from ionophore-treated spermatozoa under different sperm incubation conditions
| Fertilization method | Sperm incubation conditions | No. of zygotes analyzed (no. exp.) | No. of aneuploid zygotes (%) | No. of zygotes with structural chromosome aberrations (%) | No. of aberrant chromosomes | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chromosome type | Chromatid type | ||||||||||||
| Break | Gap | Dicentric | Translocation | Ring | Break | Gap | Exchange | Total | |||||
| IVF | No-inc. | 218 (6) | 4 (1.8) | 6 (2.8) | 3 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 8 |
| Post-inc. | 324 (6) | 9 (2.8) | 9 (2.8) | 6 | 0 | 2 | 2 | 0 | 0 | 0 | 0 | 10 | |
| Pre-inc. | 270 (7) | 3 (1.1) | 3 (1.1) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | |
| ICSI | No-inc. | 209 (8) | 4 (1.9) | 49 (23.4)a,b,c | 32 | 16 | 13 | 6 | 0 | 6 | 3 | 3 | 79 |
| Post-inc. | 215 (8) | 6 (2.8) | 34 (15.8)a | 15 | 16 | 6 | 2 | 1 | 4 | 2 | 1 | 47 | |
| Pre-inc. | 201 (8) | 4 (2.0) | 26 (12.9)a | 11 | 14 | 2 | 0 | 0 | 0 | 3 | 0 | 30 | |
aP < 0.001 compared to IVF zygotes of the matched control
bP < 0.05 and cP < 0.01 compared to ICSI zygotes in post-incubation and pre-incubation, respectively
Discussion
There was no significant increase in incidence of structural chromosome aberrations in IVF zygotes derived from MβCD-treated spermatozoa and ICSI zygotes derived from MβCD-treated spermatozoa. These findings demonstrate that MβCD has no detrimental effect on sperm chromatin, and cytogenetically supports the previous finding that IVF embryos derived from MβCD-treated spermatozoa show normal developmental ability [15]. In contrast, the increased incidence of structural chromosome aberrations was found in ICSI zygotes derived from acrosome-intact spermatozoa after incubation in H-TYH without MβCD. The incidence was significantly higher than that in ICSI zygotes derived from MβCD-treated spermatozoa. This indicates that induction of the acrosome reaction through the cholesterol efflux by MβCD before ICSI can effectively prevent structural chromosome aberrations of paternal origin in mouse ICSI zygotes. It is probable that removal of the membrane cholesterol and acrosome before ICSI would enable normal sperm chromatin remodeling within the ooplasm after ICSI. MβCD is known to induce the acrosome reaction in human spermatozoa [24]. If structural chromosome aberrations of paternal origin in human ICSI zygotes are caused by the same mechanism as seen in mouse ICSI zygotes, treatment of human spermatozoa with MβCD may reduce chromosomal risk.
Ionophore effectively induced the acrosome reaction in mouse spermatozoa, and incidences of structural chromosome aberrations in IVF zygotes derived from ionophore-treated spermatozoa were similar to that in control IVF zygotes. However, when ionophore-treated spermatozoa were used for ICSI, incidences of structural chromosome aberrations in resultant zygotes were evidently raised in any sperm incubation condition. The previous studies found that when mouse uncapacitated spermatozoa with intact acrosome were used for ICSI following short incubation (≤0.5 h) in TYH, incidence of structural chromosome aberrations in resultant zygotes was approximately 7%. When spermatozoa underwent capacitation and acrosome reaction following incubation in TYH for 2–2.5 h, the incidence was reduced to 3.8% [10, 23]. In the present study, however, the incidence (23.4%) was unaccountably high when spermatozoa were treated with ionophore under the no-incubation. The chromosomal disorder was not sufficiently conquered even when spermatozoa were incubated in TYH for 2 h before or after the ionophore treatment. Therefore, it seems likely that causal factors other than the membrane cholesterol and the acrosome may be involved in generation of structural chromosome aberrations of paternal origin in mouse ICSI zygotes derived from ionophore-treated spermatozoa.
Endonucleases in mature epididymal spermatozoa of mice can be activated by ionophore, resulting in degradation of sperm DNA [25]. Phosphatidylserine externalization of the plasma membrane, one of the earliest signs of apoptosis, has been reported in human spermatozoa after treatment with ionophore [26]. Assuming that these alterations caused by ionophore are closely linked to generation of paternal chromosome aberrations, a possible explanation for the discrepancy in chromosome damage between IVF zygotes and ICSI zygotes is that the altered spermatozoa fail to pass through the zona pullucida. Chromosome analysis of IVF zygotes from zona-free oocytes fertilized with ionophore-treated spermatozoa and ICSI zygotes derived from ionophore-treated spermatozoa that have passed through the zona pellucida may provide clues to resolving this issue.
In conclusion, MβCD effectively induces the acrosome reaction in mouse spermatozoa through cholesterol efflux from the plasma membrane. This treatment can reduce risk of generating structural chromosome aberrations of paternal origin in resultant ICSI zygotes. Ionophore is also effective in inducing the acrosome reaction, but the treatment exerts detrimental effect on paternal chromosomes in ICSI zygotes.
Acknowledgments
This study was supported by Grant-in-Aid for Scientific Research (C): No. 19591886 from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Conflict of interest statement The author declares that there are no conflicts of interest.
Footnotes
Capsule
Structural chromosome aberrations in ICSI zygotes were prevented when acrosome reaction was induced by methyl-β-cyclodextrin, but generated when ionophore was used to induce the reaction.
References
- 1.Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110:731–736. doi: 10.1172/JCI16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajduk A, Yamauchi Y, Ward MA. Sperm chromatin remodeling after intracytoplasmic sperm injection differs from that of in vitro fertilization. Biol Reprod. 2006;75:442–451. doi: 10.1095/biolreprod.106.053223. [DOI] [PubMed] [Google Scholar]
- 3.Katayama M, Koshida M, Miyake M. Fate of the acrosome in ooplasm in pigs after IVF and ICSI. Hum Reprod. 2002;17:2657–2664. doi: 10.1093/humrep/17.10.2657. [DOI] [PubMed] [Google Scholar]
- 4.Sutovsky P, Hewitson L, Simerly CR, Tengowski MW, Navara CS, Haavisto A, Schatten G. Intracytoplasmic sperm injection for rhesus monkey fertilization results in unusual chromatin, cytoskeletal, and membrane events, but eventually leads to pronuclear development and sperm aster assembly. Hum Reprod. 1996;11:1703–1712. doi: 10.1093/oxfordjournals.humrep.a019473. [DOI] [PubMed] [Google Scholar]
- 5.Hewitson L, Dominko T, Takahashi D, Martinovich C, Ramalho-Santos J, Sutovsky P, Fanton J, Jacob D, Monteith D, Neuringer M, Battaglia D, Simerly C, Schatten G. Unique checkpoints during the first cell cycle of fertilization after intracytoplasmic sperm injection in rhesus monkeys. Nat Med. 1999;5:431–433. doi: 10.1038/7430. [DOI] [PubMed] [Google Scholar]
- 6.Ramalho-Santos J, Sutovsky P, Simerly C, Oko R, Wessel GM, Hewitson L, Schatten G. ICSI choreography: Fate of sperm structures after monospermic rhesus ICSI and first cell cycle implications. Hum Reprod. 2000;15:2610–2620. doi: 10.1093/humrep/15.12.2610. [DOI] [PubMed] [Google Scholar]
- 7.Morozumi K, Yanagimachi R. Incorporation of the acrosome into the oocyte during intracytoplasmic sperm injection could be potentially hazardous to embryo development. Proc Natl Acad Sci USA. 2005;102:14209–14214. doi: 10.1073/pnas.0507005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morozumi K, Shikano T, Miyazaki S, Yanagimachi R. Simultaneous removal of sperm plasma membrane and acrosome before intracytoplasmic sperm injection improves oocyte activation/embryonic development. Proc Natl Acad Sci USA. 2006;103:17661–17666. doi: 10.1073/pnas.0608183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seita Y, Ito J, Kashiwazaki N. Removal of acrosomal membrane from sperm head improves development of rat zygotes derived from intracytoplasmic sperm injection. J Reprod Dev. 2009;55:475–479. doi: 10.1262/jrd.20216. [DOI] [PubMed] [Google Scholar]
- 10.Tateno H, Kamiguchi Y. Evaluation of chromosomal risk following intracytoplasmic sperm injection in the mouse. Biol Reprod. 2007;77:336–342. doi: 10.1095/biolreprod.106.057778. [DOI] [PubMed] [Google Scholar]
- 11.Ward CR, Storey BT. Determination of the time course of capacitation in mouse spermatozoa using a chlortetracycline fluorescence assay. Dev Biol. 1984;104:287–296. doi: 10.1016/0012-1606(84)90084-8. [DOI] [PubMed] [Google Scholar]
- 12.Tateno H. Chromosome aberrations in mouse embryos and fetuses produced by assisted reproductive technology. Mutat Res. 2008;657:26–31. doi: 10.1016/j.mrgentox.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Choi Y-H, Toyoda Y. Cyclodextrin removes cholesterol from mouse sperm and induces capacitation in a protein-free medium. Biol Reprod. 1998;59:1328–1333. doi: 10.1095/biolreprod59.6.1328. [DOI] [PubMed] [Google Scholar]
- 14.Visconti PE, Galantino-Homer H, Ning XP, Moore G, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- 15.Takeo T, Hoshi T, Kondo Y, Toyodome H, Arima H, Yamamura K, Irie T, Nakagata N. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod. 2008;78:546–551. doi: 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- 16.Toyoda Y, Yokoyama M, Hosi T. Studies on the fertilization of mouse eggs in vitro: I. In vitro fertilization of eggs by fresh epididymal sperm (in Japanese) Jpn J Anim Reprod. 1971;16:147–151. [Google Scholar]
- 17.Tateno H, Kamiguchi Y. In vitro fertilization of Chinese hamster oocytes by spermatozoa that have undergone ionophore A23187-induced acrosome reaction, and their subsequent development into blastocysts. Zygote. 1996;4:93–99. doi: 10.1017/S0967199400002963. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Yanagimachi R. Intracytoplasmic sperm injection in the mouse. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 19.Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J Reprod Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza C, Carreras A, Moos J, Tesarik J. Distinction between true acrosome reaction and degenerative acrosome loss by a one-step staining method using Pisum sativum agglutinin. J Reprod Fertil. 1992;95:755–763. doi: 10.1530/jrf.0.0950755. [DOI] [PubMed] [Google Scholar]
- 21.Mikamo K, Kamiguchi Y. A new assessment system for chromosomal mutagenicity using oocytes and early zygotes of the Chinese hamster. In: Ishihara T, Sasaki MS, editors. Radiation-induced chromosome damage in man. New York: Alan R Liss; 1983. pp. 411–432. [Google Scholar]
- 22.Tateno H, Kimura Y, Yanagimachi R. Sonication per se is not as deleterious to sperm chromosomes as previously inferred. Biol Reprod. 2000;63:341–346. doi: 10.1095/biolreprod63.1.341. [DOI] [PubMed] [Google Scholar]
- 23.Tateno H. Possible causal factors of structural chromosome aberrations in intracytoplasmic sperm injection of the mouse. Reprod Med Biol. 2009;8:89–95. doi: 10.1007/s12522-009-0017-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross NL. Effect of methyl-β-cyclodextrin on the acrosomal responsiveness of human sperm. Mol Reprod Dev. 1999;53:92–98. doi: 10.1002/(SICI)1098-2795(199905)53:1<92::AID-MRD11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Maione B, Pittoggi C, Achene L, Lorenzini R, Spadafora C. Activation of endogenous nucleases in mature sperm cells upon interaction with exogenous DNA. DNA Cell Biol. 1997;16:1087–1097. doi: 10.1089/dna.1997.16.1087. [DOI] [PubMed] [Google Scholar]
- 26.Martin G, Sabido O, Durand P, Levy R. Phosphatidylserine externalization in human sperm induced by calcium ionophore A23187: relationship with apoptosis, membrane scrambling and the acrosome reaction. Hum Reprod. 2005;20:3459–3468. doi: 10.1093/humrep/dei245. [DOI] [PubMed] [Google Scholar]