Abstract
Bipolar disorder (BD) is associated with abnormalities of the ventral anterior cingulate cortex (vACC) and its connection sites, including the amygdala, which are key components of a corticolimbic neural system that subserves emotional regulation. Decreased functional connectivity from the vACC to the amygdala in healthy individuals is associated with the short “s” allele—as opposed to the long “l” allele—of a well-known serotonin transporter promoter polymorphism (5-HTTLPR, locus SLC6A4), as are features of BD. This study tests the hypothesis that the s allele influences dysfunction in the vACC-amygdala neural system in BD. Thirty euthymic individuals with BD (20 s carriers, 10 ll) and 48 healthy comparison (HC) participants (34 s, 14 ll) participated in an event-related functional magnetic resonance imaging scan while processing fearful, happy, or neutral faces. During fear and happy face processing, vACC activation was significantly lower in the BD compared to the HC group, and in s carriers compared to ll individuals within both the HC and BD groups, such that BD s carriers exhibited the greatest magnitude of vACC dysfunction. No significant differences were detected in amygdala activation. The findings suggest that the 5-HTTLPR s allele may contribute to a trait-related, genetically-derived, neurobiological subgroup within BD characterized by prominent vACC dysfunction. Future treatment may be optimized for this BD subgroup by targeting the serotonergic system and the vACC.
Keywords: bipolar disorder, magnetic resonance imaging, serotonin, serotonin transporter promoter polymorphism, gyrus cinguli, genetic polymorphism
INTRODUCTION
Emotional dysregulation is a cardinal feature of bipolar disorder (BD). The ventral anterior cingulate cortex (vACC) and the amygdala are highly interconnected structures that serve as essential nodes in emotional processing (Bush et al, 2000; Devinsky et al; 1995; Etkin et al, 2006; Maren and Quirk, 2004; Paus, 2001; Pezawas et al, 2005; Rosenkranz et al, 2003; Whalen et al, 1998a; Vogt et al, 1992). Neuroimaging studies of BD implicate aberrancies in vACC structure (Drevets et al, 1997; Lyoo et al, 2004; Sassi et al, 2004) and function (Blumberg et al, 2005; Drevets et al, 1997; Gruber et al, 2004; Kruger et al, 2002; Lawrence et al, 2004) including abnormalities in response to faces depicting positive, negative, or neutral emotional expressions (Blumberg et al, 2005; Lawrence et al, 2004). Heightened amygdala responses to faces have also been reported in BD (Altshuler et al, 2005; Blumberg et al, 2005; Chen et al, 2006; Ketter et al, 2001; Lawrence et al, 2004; Pavuluri et al, 2007; Rich et al, 2006; Yurgelun-Todd et al, 2000).
BD is a highly heritable disorder (Bertelsen et al, 1977; McGuffin et al, 2003; Smoller and Finn, 2003), and although several genetic loci have been linked to or associated with BD, many of these findings have not been consistently replicated (DePaulo, 2004). This suggests that BD may best be appreciated as a spectrum of illnesses consisting of several overlapping but possibly differentiable entities, with different sets of genes predisposing to particular phenotypes within this spectrum (Kelsoe, 2003). To understand this phenotypic heterogeneity in the context of a genetically complex background, researchers have begun to focus on endophenotypes within BD and on elucidating their underlying genes (Hasler et al, 2006).
The short“s” allele, as opposed to the long “l” allele, of a functional polymorphism (5-HTTLPR) within the promoter region of the serotonin transporter protein gene (locus SLC6A4) has been associated with younger age of BD onset (Bellivier et al, 2002), differences in mood-state cycling (Cusin et al, 2001; Rousseva et al, 2003), psychotic symptoms (Ho et al, 2000; Ospina-Duque et al, 2000), and violent suicidal behavior (Bellivier et al, 2000). These findings in BD suggest that variation at this locus may be associated with phenotypic features of the disorder. In addition, Pezawas and colleagues, using structural and functional magnetic resonance imaging (fMRI), found that healthy individuals carrying the s allele had reduced gray matter volume in the ACC as well as impaired structural and functional connectivity from the vACC to the amygdala (Pezawas et al, 2005). Given that the vACC contains the richest concentration of serotonergic neurons of any cortical structure (Varnas et al, 2004), it is not surprising that the vACC is particularly influenced by 5-HTTLPR. In BD, abnormalities in 5-HTT binding in the vACC were reported in a positron emission tomography study (Cannon et al., 2006). Also, Hariri and colleagues found that healthy s carriers had greater amygdala activation to negatively-valenced stimuli compared to ll homozygotes (Hariri et al, 2002), a finding replicated in other healthy samples (Bertolino et al, 2005; Canli et al, 2005; Heinz et al, 2005) and among individuals with mood and anxiety disorders (Dannlowski et al, 2007; Domschke et al, 2005; Furmark et al, 2004). Taken together, these findings suggest that 5-HTTLPR variation might contribute to abnormalities in vACC and amygdala response to emotional stimuli in BD.
The aim of the current study was to investigate whether 5-HTTLPR variation influences vACC and amygdala dysfunction in BD. This was measured by blood oxygen level-dependent (BOLD) response to emotional face stimuli that were found previously to differentially activate this neural system in a BD compared to a healthy comparison (HC) group (Blumberg et al, 2005). Euthymic BD individuals were studied to assess effects associated with the BD trait. It was hypothesized that in the vACC, BOLD response to face stimuli would be decreased in BD participants compared to HC individuals, and decreased amongst s allele carriers compared to ll homozygotes within each diagnostic group, such that the BD s carriers would have the greatest magnitude of dysfunction. It was similarly hypothesized that in the amygdala, BOLD response to faces would be increased in BD participants compared to HC individuals, and increased amongst s allele carriers compared to ll homozygotes within each diagnostic group. As differing effects within this vACC-amygdala neural system in association with specific facial emotions have previously been described (Blumberg et al., 2005; Lawrence et al., 2004), these effects were also explored in this study.
METHODS
Participants
The HC group included 48 participants, 58% of whom were female, with mean age and standard deviation of 28.2 ± 10.0 years, while the BD group included 30 participants, 67% female, age of 31.9 ± 13.5 yrs. Sixty-five percent of the HC group were European American (EA), 19% were African American, 6% were Asian American, and 10% were of other ethnicity, while 70% of the BD group were EA, 27% were African American, and one individual (3%) was of other ethnicity (see Table 1 for further details). BD participants were recruited from the Veterans Affairs Connecticut Healthcare System in West Haven, CT, other medical centers affiliated with the Yale University School of Medicine, and from surrounding communities. HC participants were recruited from surrounding communities. Exclusion criteria for both groups included: history of neurological disease, loss of consciousness for five minutes, any medical condition that may potentially affect neurovascular function including hypertension, or current substance abuse or dependence. Presence or absence of psychiatric illness was assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0 (First et al, 1995) which also was used to determine if BD participants were euthymic at scanning. In addition, scores on the Young Mania Rating Scale [YMRS (Young et al., 1978)] and the 29-item Hamilton Depression Rating Scale [HDRS (Williams et al. 1994)] were obtained from each participant except for one who did not complete these assessments. Exclusion criteria for the HC group included personal history of DSM-IV Axis I diagnosis or first-degree family member history of such illnesses as assessed by the Family History Screen for Epidemiologic Studies [FHE (Lish et al, 1995)]. Written informed consent was obtained prior to participation in accordance and with approval of the human investigation committees of the Yale School of Medicine and the Veterans Affairs Connecticut Healthcare System.
Table 1.
Features of the Bipolar Disorder and Healthy Comparison Groups
| HC ll n = 14 | HC s car n = 34 | BD ll n = 10 | BD s car n = 20 | Test for Significance | |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 6 (43) | 22 (65) | 7 (70) | 13 (65) | χ2 = 2.58, p = 0.46a |
| Male | 8 (57) | 12 (35) | 3 (30) | 7 (35) | |
| Age, in yrs | 26.1 ± 9.3 | 29.1 ± 10.3 | 36.9 ± 16.9 | 29.4 ± 11.0 | F = 1.86, p = 0.14b |
| Ethnicity | |||||
| European American | 7 (50) | 24 (71) | 5 (50) | 16 (80) | |
| African American | 5 (36) | 4 (12) | 5 (50) | 3 (15) | |
| Asian American | 1 (7) | 2 (6) | 0 | 0 | |
| Other | 1 (7) | 4 (12) | 0 | 1 (5) | |
| YRMS | 0.3 ± 0.6 | 0.2 ± 0.6 | 2.2 ± 2.8 | 2.0 ± 3.3 | |
| HDRS | 1.1 ± 3.2 | 0.6 ± 1.3 | 11.1 ± 10.8 | 7.8 ± 9.9 | |
| Rapid Cycling Subtype | - | - | 4 (40) | 10 (50) | p = 0.71c |
| Past Psychosis | - | - | 1 (10) | 8 (40) | p = 0.20c |
| On Medication at Scan | - | - | 8 (80) | 18 (90) | p = 0.58c |
| Lithium | - | - | 1 (10) | 9 (45) | p = 0.10c |
| Anticonvulsant | - | - | 5 (50) | 13 (65) | p = 0.46c |
| Atypical Antipsychotic | - | - | 3 (30) | 10 (50) | p = 0.44c |
| Antidepressant | - | - | 4 (40) | 11 (55) | p = 0.70c |
| Benzodiazepine | - | - | 2 (20) | 1 (5) | p = 0.25c |
| History of Psychiatric Hospitalization | - | - | 7 (70) | 15 (75) | p = 1.00c |
| History of Suicide Attempt | - | - | 3 (30) | 3 (15) | p = 0.37c |
| History of Alcohol Abuse/Dependence | - | - | 4 (40) | 5 (25) | p = 0.43c |
| History of Substance Abuse/Dependence | - | - | 5 (50) | 3 (15) | p = 0.078c |
| Reaction Time (msecs) | |||||
| Fear | 812 ± 182 | 868 ± 172 | 927 ± 112 | 836 ± 137 | F = 1.16, p = 0.33b |
| Happy | 787 ± 153 | 844 ± 159 | 911 ± 97.6 | 816 ± 109 | F = 1.72, p = 0.17b |
| Neutral | 786 ± 157 | 847 ± 161 | 920 ± 131 | 825 ± 145 | F = 1.53, p = 0.21b |
| Response Accuracy (%) | |||||
| Fear | 95.4 ± 10.6 | 96.6 ± 6.3 | 94.2 ± 9.5 | 98.5 ± 3.2 | F = 0.97, p = 0.41b |
| Happy | 97.5 ± 3.9 | 96.1 ± 6.3 | 96.0 ± 5.7 | 97.9 ± 4.7 | F = 0.59, p = 0.63b |
| Neutral | 95.2 ± 10.3 | 96.3 ± 5.9 | 96.5 ± 6.3 | 98.1 ± 4.0 | F = 0.60, p = 0.62b |
BD, Bipolar Disorder; HC, Healthy Control
YMRS, Young Mania Rating Scale; HDRS, 29-item Hamilton Rating Scale for Depression
Values as whole number and (percentage), except in case of continuous variable, given as mean ± standard deviation
Chi-square test
Analysis of Variance (ANOVA)
Fisher’s exact test
Fourteen (47%) of the BD participants met criteria for rapid-cycling and nine (30%) had a history of psychosis, but none were psychotic at time of scan. Four (13%) were off medications at time of scan, all for greater than one year prior to scan, with the remaining participants taking psychotropic medications at time of scan, including lithium (33%, 10 of 30), anticonvulsants (60%, 18), atypical antipsychotics (43%, 13), antidepressants (50%, 15) and benzodiazepines (10%, 3). Ten (33%) BD participants had a prior history of a substance related disorders. This included nine (30%) BD participants with a history of alcohol abuse or dependence, and eight (27%) with history of substance abuse or dependence; in all instances, individuals had been in remission for greater than six months at time of scan. The majority of BD subjects (80%) reported experiencing significant mood symptoms in childhood or adolescence, and all were symptomatic by young adulthood. Twenty-two (73%) of the BD participants had been hospitalized for psychiatric treatment at least once, and six (20%) had a history of suicide attempt.
Genotyping
Ten milliliter blood samples were drawn from participants for DNA extraction, which was accomplished by standard methods. Polymerase chain reaction (PCR) amplification was completed using an MJR tetrad cycler using methods and PCR primers previously described and designed by Gelernter and colleagues (Gelernter et al, 1997). Since the s allele has been reported to be dominant in transcriptional activity versus the ll genotype (Heils et al, 1996), genotyping led to the creation of two genotype comparison groups, ll individuals and s carriers (ls or ss).
MRI Data Acquisition and Emotional Face Paradigm
Participants were scanned using a 3-Tesla Siemens Trio MR scanner (Siemens, Erlangen, Germany) at the Yale Magnetic Resonance Research Center in New Haven, CT. FMRI data were acquired with a single-shot echo planar imaging (EPI) sequence in alignment with the anterior commissure-posterior commissure (AC-PC) plane to create 32 three-mm thick slices without gap with the following parameters: TR = 2000 ms, TE = 25 ms, matrix = 64 x 64, FOV = 240 × 240 mm2, and flip angle = 80°.
During the functional runs, an event-related emotional face task was completed by each participant. Faces from the Ekman series (Ekman and Friesen, 1979) depicting expressions of fear, happiness, or neutrality were shown to the participants via the PsyScope software package (Cohen et al, 1993) on a computer attached to a projector. Participants were asked to make a male-female discrimination via a two-response button box that they were oriented to prior to scanning. This task was chosen to induce implicit rather than explicit processing of the emotional face as the former has been found to activate the emotional processing neural circuit more readily (Whalen et al, 1998b). Each face was presented for two seconds and separated by four, eight, or twelve second intervals during which time a cross-hair fixation point was displayed. Face stimuli included images of ten actors (five of each sex), with each individual exhibiting all three of the expressions for a total of 30 faces per run. Ordering of face stimuli was varied systematically to control for sequential dependencies and counterbalanced for facial expression, sex, the identity of the face, and the length of the interval between stimuli. Each run lasted 4 minutes and 50 seconds and data was compiled and averaged over four runs.
fMRI Data Processing
Raw data pre-processing was completed using SPM99 software (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm). Two images at the beginning of each fMRI run were discarded to account for the approach of the hemodynamic response to steady-state. The functional scans were realigned to the first volume to correct for inter-scan motion. The functional data were then spatially normalized to a standard EPI template from the Montreal Neurological Institute (MNI) with resampling to 4 mm3 voxels, and spatially smoothed with a 12 mm full width at half maximum (FWHM) Gaussian kernel.
SPM99 was also used for the model specification and estimation. At the individual subject level, event-related response amplitudes were estimated using the general linear model (Friston et al, 1995) for each of the three event types: fearful, happy, and neutral expression. This created statistical images of the BOLD signal change associated with each of the three emotional face types versus the baseline fixation cross-hair control for each individual subject.
Region of Interest (ROI) Definition
The vACC region of interest (ROI) was defined by the WFU toolbox (Wake Forest University PickAtlas Region of Interest Toolbox; http://www.fmri.wfubmc.edu/download.htm) predefined anatomic label excluding the dorsal component (dorsal to the plane at z = 0), creating a region that was 5496 mm3 (86 voxels) in volume with center at MNI coordinates of x = 0 mm, y = 36 mm, z = −4 mm. This division approximates the region of the ACC most associated with emotional processing (Bush et al, 2000; Devinsky et al, 1995; Vogt et al, 1992). Bilateral amygdala ROIs were similarly defined by the WFU toolbox, each 1920 mm3 in volume (30 voxels). The mean percent signal change was extracted from the ROI using the MarsBar ROI toolbox (http://marsbar.sourceforge.net) from the BOLD contrast images for each of the three emotional stimulus types for each participant.
Statistical Analysis
Statistical procedures were performed in SAS software, version 9.1 (SAS Institute Inc., Cary, NC) using a linear mixed-model analysis, in which the mean signal change in the vACC ROI for each subject was the dependent measure. Diagnosis and genotype were included as between-subjects factors, while the three emotion types were included as within-subject factors. A second mixed-model analysis was done using the mean signal change in the amygdala ROIs with an additional left versus right hemisphere within-subject factor. Age and sex were entered as covariates in both initial models and were removed if they were not found to have significant effect. All two- and three-way interactions were tested. The correlation structure of the data was modeled by an unstructured variance-covariance matrix for observations on the three emotions. The latter variance-covariance structure was the best fitting according to the Akaike Information Criterion. Least square means (ls means) and standard errors (SE) were calculated in the mixed-model to allow interpretation of significant interactions. Planned post-hoc analyses were performed within the main effect analysis for genotype and diagnosis to allow for evaluation of the potential differential effects of the varying emotional face types. SPM99 was used to afford visual localization of significant difference clusters from the above analyses.
Post-hoc exploratory analyses were performed for potential main effects of clinical variables among BD participants on mean signal change values in the vACC and amygdala ROIs. Clinical factors examined included presence or absence of rapid cycling, history of psychosis, history of alcohol or substance abuse or dependence, history of psychiatric hospitalization, history of suicide attempt, and presence or absence of medication at time of scanning for specific medication subclasses.
A pertinent consideration in this study was the relative ethnic heterogeneity within the overall participant sample as there is evidence of variability in the frequency of the l and s alleles across ethnic groups (Gelernter et al, 1997), including EA and African American populations. Thus, it was important to attempt to disentangle, as much as possible, the effect of a specific allele versus a specific ethnicity. To address this, t-tests were done comparing activation in the vACC and amygdala between the EA and African American participants within each gene-by-diagnosis subgroup, and secondary within-population EA only subgroup analyses were done to verify that similar effects could be observed in a more homogeneous population (there were too few participants of the other population groups to allow for statistically meaningful analyses).
Whole Brain Analyses
In order to explore group differences not hypothesized a priori, SPM99 was used to perform whole brain voxel-based t-test comparisons between genotype and between diagnosis groups for each emotional face type. Findings were considered significant at a threshold of p<0.05, corrected for false discovery rate [FDR (Genovese et al., 2002)].
RESULTS
Participants
The HC and BD groups did not differ statistically in age (p = 0.18) or sex distribution (p = 0.39). Genotyping at the SLC6A4 locus led to four diagnosis-genotype groups: 14 HC ll participants, 34 HC s carriers (29 ls and 5 ss), 10 BD ll, and 20 BD s carriers (17 ls and 3 ss). The frequency of the l allele in this sample was 60.3%, and the frequency of the s allele was 39.7%. Clinical characteristics for these groups are summarized in Table 1. There was no significant difference in the distribution of EA versus non-EA participants across the groups (χ2 = 4.84, p = 0.18). The BD group had significantly higher YMRS and HDRS scores than the HC group (p < 0.001 for each). There was no significant difference in scores between the HC ll and s carriers or between the BD ll and s carriers.
The magnitude of movement during scanning was well within standards in the literature (Caparelli et al, 2003) and the groups did not have significantly different physical head motion in terms of translational (HC ll mean translation 0.62 ± standard deviation 0.27 mm, HC s car 0.56 ± 0.25 mm, BD ll 0.61 ± 0.20 mm, and BD s car 0.71 ± 0.28 mm; F = 1.57, p = 0.20) or rotational movement (HC ll having 0.51 ± 0.34 degrees, HC s car 0.45 ± 0.27 degrees, BD ll 0.50 ± 0.23 degrees, and BD s car 0.55 ± 0.44 degrees; F = 0.45, p = 0.72).
Accuracy and button box reaction time averages were calculated for each participant in response to the different face types and this data is also shown in Table 1. There were no significant differences in mean reaction time or accuracy between the four diagnosis by genotype groups.
Ventral Anterior Cingulate Cortex
Age and sex were initially entered as covariates but were removed from the model as they did not have a significant effect on the results. There was a significant effect for genotype [F(1,74) = 12.78, p = 0.0006]. The interaction between genotype and emotional face type was not significant [F(2,74) = 1.89, p = 0.16] and our planned post-hoc analyses supported a genotype main effect that was present amongst ll individuals compared to s allele carriers across emotional face types: response to fearful [F(1,74) = 15.2, p = 0.0002), happy [F(1,74) = 5.25, p = 0.025], and neutral faces [F(1,74) = 6.55, p = 0.013]. There was also a significant main effect of diagnosis [F(1,74) = 6.58, p = 0.012]. Here results of our post-hoc analyses for emotional face type showed that, although the interaction between diagnosis and emotional face type was not significant [F(2, 74) = 1.89, p = 0.47], activation increases in the HC group compared to the BD group were significant in response to fearful [F(1,74) = 7.85, p = 0.007] and happy faces [F(1,74) = 4.8, p = 0.032] but not in response to neutral faces [F(1,74) = 1.75, p = 0.19]. Figure 1 displays a graph depicting the ls mean signal change in each diagnosis by genotype group in response to the face stimuli in the vACC and Figure 2 shows BOLD contrast maps from SPM99 demonstrating the localization of clusters of difference within the vACC ROI for the significant comparisons from the mixed model. There were no significant interactions between genotype, diagnosis, or emotion type within the vACC mixed model.
Figure 1. BOLD Response in vACC to Faces.
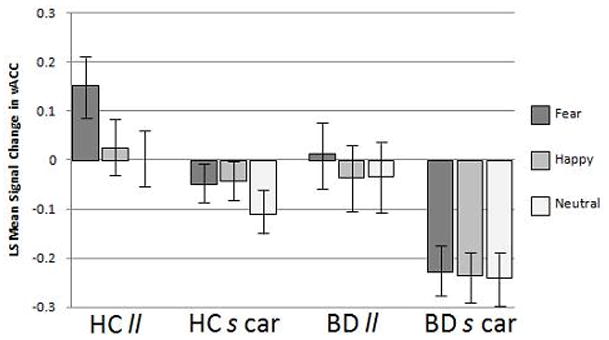
The graph displays the BOLD signal change to faces depicting fearful, happy and neutral expressions (ls mean ± standard error) by group [healthy comparison (HC) and bipolar disorder (BD)] and genotype [homozygous for the 5-HTTLPR “l” allele (ll) and s carriers heterozygous and homozygous for the “s” allele (s car)] for the ventral anterior cingulate cortex (vACC) demonstrating the lowest signal in BD s carriers.
Figure 2. Localization of BOLD Response Differences in vACC to Faces Depicting Fearful Expressions.
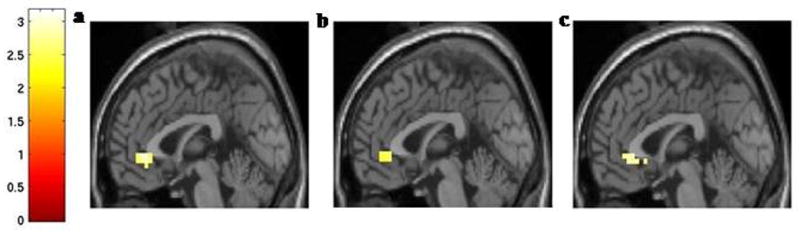
The mid-sagittal images (x = 0 mm) show the vACC region where activation to fear faces was decreased in (a) the BD group compared to the HC group, (b) the HC s carrier group compared to the HC ll group, and (c) the BD s carriers compared to the BD ll group (all at p < 0.01, uncorrected) displayed on an average T1 structural MRI template.
Post-hoc analyses revealed that, among the BD participants, no significant effects on vACC response to any of the face types were detected in relation to clinical factors including presence of rapid cycling, history of psychosis, history of alcohol abuse/dependence, history of substance abuse/dependence, history of psychiatric hospitalization, history of suicide attempt, or medication subclass including lithium, anticonvulsants, antipsychotics, antidepressants, and benzodiazepines.
Amygdala
Age and sex were removed from the model as they did not have a significant effect on results. There was a significant main effect of hemisphere as left amygdala activation was higher compared to right amygdala activation to all face types [F(1,370) = 10.2, p = 0.0015]. There was also a significant main effect of emotion as activation to fearful faces was greater than activation to both happy and neutral faces [F(2,370) = 9.25, p = 0.0001]. Graphs depicting ls mean signal change in the left and right amygdala in response to face stimuli are shown in Figure 3. There were no significant main or interaction effects of genotype, diagnosis, emotion type, or hemispheric laterality within the amygdala mixed model.
Figure 3. BOLD Response in Amygdala to Faces.
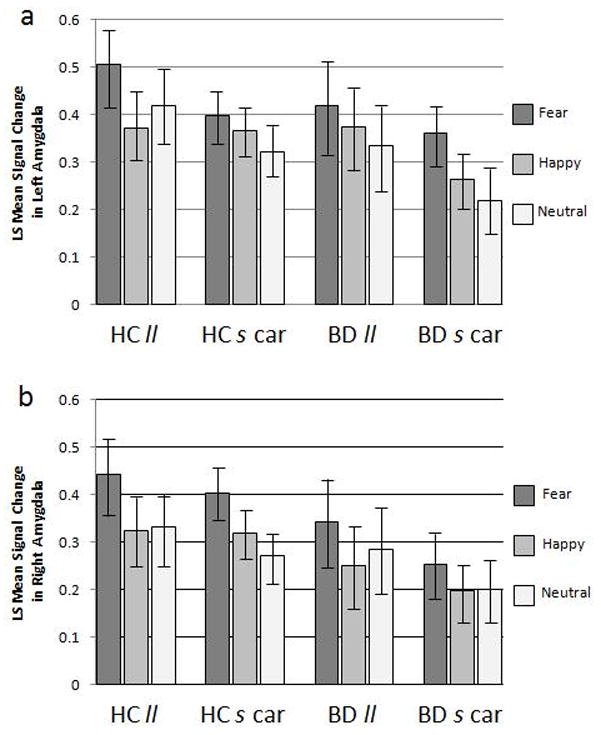
The graphs display the BOLD signal change to faces depicting fearful, happy and neutral expressions (ls mean ± standard error) by group [healthy comparison (HC) and bipolar disorder (BD)] and genotype [homozygous for the 5-HTTLPR “l” allele (ll) and s carriers heterozygous and homozygous for the “s” allele (s car)] for the (a) left amygdala and (b) right amygdala.
As in the vACC, amongst the BD participants, none of the clinical factors discussed above were found to have a significant effect on response in the amygdala to any of the emotional face types on either the left or right side.
Ethnicity Analyses
There were no statistically significant differences between EA and African American participants in response in the vACC or amygdala ROIs within any of the diagnosis-by-genotype subgroups (all p-values > 0.05). Consistent with the findings in the overall sample, in the EA only subgroup analyses for the vACC ROI, there was a significant main effect for genotype [F(1,48) = 8.03, p = 0.007], derived from an overall greater activation amongst ll individuals compared to s allele carriers in response to fear [F(1,48) = 11.61, p = 0.001] and neutral faces [F(1,48) = 6.28, p = 0.016]. There was no significant main effect for diagnosis, but the directionality of difference was the same as above.
Whole Brain Analyses
In the exploratory whole brain diagnosis comparisons, in response to fearful faces, an additional region of higher activation in the HC group than the BD was detected in the right parahippocampal gyrus (BA 37). There were no other significant regions for which activation was significantly greater between diagnosis groups or genotype within each diagnostic group in response to fearful, happy, or neutral faces.
DISCUSSION
In this study, fMRI analyses demonstrated decreased response in the vACC among BD participants compared to the HC group in response to fearful faces and happy faces. This reduction in vACC response during emotion processing in BD is consistent with previous reports (Blumberg et al, 2005; Kruger et al, 2002). As this sample included euthymic-only BD participants, the findings suggest that deficits in vACC response to emotionally-valenced face stimuli may be trait-features of the disorder.
In the same vACC region, response was also decreased amongst carriers of the s allele of the 5-HTTLPR polymorphism in both the HC and BD groups compared to the ll individuals within the respective diagnostic groups, with the greatest magnitude of dysfunction seen in the BD s carriers. While previous studies in healthy individuals reported an association of the s allele with disruption of functional connectivity between the vACC and the amygdala (Pezawas et al, 2005), the current study suggests it influences functioning within the vACC. This may be the result of a direct effect within the VACC. It is also possible that it is the result of modulation by other structures, such as the parahippocampal gyrus detected in exploratory analyses. In addition, while function in the vACC was found to be impaired in BD participants as a group overall, those carrying the s alleles exhibited the greatest magnitude of impairment in this region. While the interaction of diagnosis and genotype was not significant, and the results of greater vACC dysfunction in BD participants who were also s carriers could represent independent and addictive effects, the subgroup of BD s carriers may represent a biologically salient subtype of BD that might most benefit from treatments targeting serotonergic transmission or treatments that target the vACC.
While the vACC differences were consistent with our initial hypotheses, the lack of amygdala differences was not expected given reports of increased amygdala activation to emotional stimuli in BD participants (Altshuler et al, 2005; Blumberg et al, 2005; Chen et al, 2006; Drevets et al, 2002; Ketter et al, 2001; Lawrence et al, 2004; Pavuluri et al, 2007; Rich et al, 2006; Yurgelun-Todd et al, 2000), as well as in HC s carriers compared to HC ll individuals (Bertolino et al, 2005; Canli et al, 2005; Hariri et al, 2002; Heinz et al, 2005). A recent meta-analysis did note that there are unpublished data of samples in which those carrying the s allele did not have greater amygdala activation compared to ll individuals (Munafo et al, 2008). Many of the published reports in this meta-analysis involved subtraction of the response to a neutral condition from an emotional stimulus experimental condition (Munafo et al, 2008); in the current study the fixation crosshair was used as the baseline as amygdala response to neutral faces has been suggested to be heightened in BD and other disorders of emotional regulation (Donegan et al, 2003); significant amygdala activation to neutral face stimuli was also detected in the data presented herein. The level of amygdala signal change suggests that the emotional face task was effective in eliciting amygdala response across the entire sample, but perhaps so robustly as to disallow detection of significant group differences that might be detected with paradigms including faces depicting milder emotional expressions (Lawrence et al, 2004). Given the vACC differences, and the known functional and structural connectivity between the vACC and amygdala, the dissociation between effects in vACC and amygdala also raises the possibility that there is a defect, perhaps, in vACC-amygdala communication that is most prominent in the BD s carriers that will be of interest to pursue in future work.
The lack of a between-diagnosis difference in amygdala response may also have been due to the fact that the majority (86.7%) of the BD participants were on medications, which has been suggested to blunt amygdala activation in BD individuals compared to participants not taking medications (Blumberg et al, 2005). Although there was no significant difference in amygdala activation overall between the unmedicated and medicated BD participants perhaps due to the small number of unmedicated participants in this sample, the direction of the difference is consistent with this blunting, with greater activation in the unmedicated BD group compared to the medicated group (percent signal change across all face emotion types 0.41 versus 0.25 respectively), and three of these four participants having mean BOLD response in the upper third of the total distribution. Also, previous reports of increased amygdala BOLD activation in BD adults included individuals in non-euthymic mood states (Altshuler et al, 2005; Chen et al, 2006; Lawrence et al, 2004; Yurgelun-Todd et al, 2000), whereas the current study included only individuals who were euthymic at time of scan, raising the possibility that dysfunction in the amygdala is more pronounced during acute mood episodes of BD.
Analyses were done to attempt to disentangle the potential effect of ethnicity given that there is known variability in allelic frequency amongst different ethnic groups (Gelernter et al, 1997; Lotrich et al, 2003). There was no significant difference in ethnic distribution between the groups and there were no significant differences found in vACC or amygdala response to the emotional face stimuli between the EA and African American participants within each of the four diagnosis-by-genotype subgroup. In addition, in analyses for the EA only group, the direction of the effects of genotype and group in the vACC were the same as in the overall sample; however, the effects only reached significance for the effect of genotype for the fear faces, likely owing to the decreased power caused by the smaller number of EA only subjects. Sample sizes for the other ethnic groups did not permit meaningful analyses with those subjects only, and thus replication in larger, more homogeneous samples will be important.
We did not detect significant influences on neural circuitry functioning of clinical factors such as presence of rapid cycling, history of psychosis, history of substance or alcohol abuse/dependence, onset of mood symptoms in adolescence/young adulthood, history of psychiatric hospitalization, history of suicide attempt, or medication subclasses. However, it was unlikely that we had sufficient power to detect such effects. Continued study of larger samples will hopefully allow characterization of a “gene expression” to “neural circuit dysfunction” to “clinical phenotype” mechanistic bridge, and further characterize the BD s carrier subgroup. As one potential example, we did note that the majority (88.9%) of the BD participants with a history of psychosis were s carriers, consistent with prior epidemiological studies associating the s allele with psychosis in BD (Ho et al, 2000; Ospina-Duque et al, 2000), suggesting that future studies of 5-HTTLPR variation, vACC neural system functioning and psychosis may further clarify this potential relationship. In addition, reports of interactions between stress and other genetic variants such as those in brain-derived neurotrophic growth factor with 5-HTTLPR variation (Kaufman et al, 2006; Pezawas et al, 2008) suggest that future work investigating these interactions may help to elucidate how variations in several genes and environmental factors may converge to lead to the development of a BD phenotype.
Whole brain analyses were conducted to explore for regions in the brain besides the vACC and amygdala for which BOLD response to the emotional face task differed across diagnosis and genotype groups. The only significant finding was a decrease in activation in the BD group compared to the HC group in response to fearful faces in the right parahippocampal gyrus. This area is considered part of the limbic association cortex, which also includes the vACC (Ebert and Ebmeier, 1996), and has been implicated in the processing of social and emotional stimuli (Rich et al., 2008). In addition, Lawrence and colleagues found that parahippocampal gyrus response was decreased in adults with BD in response to emotional face stimuli (2004), and Rich and colleagues have reported similar functional deficits in pediatric BD participants (2008).
In the future, studies of children and adolescents with BD, and family members at high risk for BD, may allow examination of groups less likely to be affected by medications and may reveal how the 5-HTTLPR influences neurodevelopmental changes over time. Positron emission tomography studies of healthy adults (Parsey et al, 2006) and postmortem histological studies of suicide victims have not shown differences in serotonin transporter distribution or concentration in ventral frontal regions amongst individuals carrying the s allele compared to ll individuals (Mann et al, 2000). These findings suggest that variation in 5-HTTLPR may result in vACC dysfunction in adulthood due to neurodevelopmental effects rather than acute effects on 5-HTT distribution. This is particularly relevant to BD, as ACC structural differences have not been consistently found in pediatric samples (Blumberg et al, 2006; Chang et al, 2005; Kaur et al, 2005; Sanches et al, 2005; Wilke et al, 2004), again suggesting that this dysfunction may progress over the course of adolescence as the vACC continues to mature (Blumberg, 2007).
In conclusion, the present work provides evidence for an association of the s allele of the 5-HTTLPR with deficits in the response of the vACC to emotional stimuli, a region that also demonstrated dysfunction in a sample of euthymic individuals with BD, with dysfunction greatest in those in the BD group carrying the s allele. In combination, these findings suggest that pronounced vACC dysfunction may be a heritable neurobiological trait, which underlies a distinct subtype within the more heterogeneous BD clinical phenotype. Evidence is emerging that this polymorphism may influence response to psychotropic medications, with s allele homozygotes having less favorable response to selective serotonin reuptake inhibitors (Smits et al, 2004) but a greater benefit with lithium compared to ll homozygotes (Stamm et al, 2007). Together, with continued work on the effects of neural circuitry, further investigation of treatment response related to 5-HTTLPR variation may in the future direct treatments to those most likely to benefit from serotonergic treatments and those that target the vACC (Mayberg et al, 2005).
Acknowledgments
This article is dedicated to Ms. Kathleen Colonese who was devoted to helping those suffering from psychiatric illnesses and whose kindness to colleagues and participants alike touched us all.
We give thanks to all of the participants in this study, and hope that this work may one day contribute to helping those living with bipolar disorder. We would like to thank the nurses of the VA CT Healthcare System Biostudies Unit Ms. Angelina M. Genovese RNC, MBA, Ms. Elizabeth O’Donnell RN, and Ms. Brenda Breault RN, BSN. We would also like to thank Ms. Ann Marie Lacobelle, MS for her assistance in genotyping. We want to acknowledge the help and support of all members of the Mood Disorders Laboratory at Yale including Ms. Erin Edmiston, Ms. Linda Spencer, Ms. Allison McDonough, Ms. Gina Williams, and Mr. Thomas Flanagan. We also appreciate the efforts of the staff at the Yale Magnetic Resonance Research Center including Ms. Cheryl Lacadie, Ms. Karen Martin, Ms. Terry Hickey, and Ms. Hedy Sarofin.
This work was supported by grants from the Howard Hughes Medical Institute (MPS), the Department of Veterans Affairs Research Career Development (HPB), Merit Review (HPB and JG) and Research Enhancement Award Program (REAP)(HPB and JG) Awards, the National Institute of Mental Health R01MH69747 (HPB), R01MH070902 (HPB), K24 15105 (JG), T32MH14276 (LGC and JHK), CTSA Grant Number UL1 RR0249139 from the NIH National Center for Research Resources (NCRR), the National Alliance for Research on Schizophrenia and Depression (Great Neck, NY) (HPB, JHK), The Attias Family Foundation (HPB), Marcia Simon Kaplan (JHK), The Ethel F. Donaghue Women’s Investigator Program at Yale (New Haven, CT) (HPB) and the Klingenstein Foundation (JHK).
Dr. Gelernter has received financial support or compensation for the following: related to consultation for Columbia University, the Thailand Center for Excellence for Life Sciences (TCELS), the University of CT Health Center, and NIH; related to grant reviews for the National Institutes of Health; and related to academic lectures and editorial functions in various scientific venues (including the ACNP).
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare that except for income received from primary employers no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service aside from those listed below and there are no personal financial holdings that could be perceived as constituting a potential conflict of interests.
References
- Altshuler L, Bookheimer S, Proenza MA, Townsend J, Sabb F, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–3. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Szoke A, Henry C, Lacoste J, Bottos C, et al. Possible association between serotonin transporter gene polymorphism and violent suicidal behavior in mood disorders. Biol Psychiatry. 2000;48:319–322. doi: 10.1016/s0006-3223(00)00891-x. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Leroux M, Henry C, Rayah F, Rouillon F, et al. Serotonin transporter gene polymorphism influences age at onset in patients with bipolar affective disorder. Neuroscience Letters. 2002;334:17–20. doi: 10.1016/s0304-3940(02)01029-7. [DOI] [PubMed] [Google Scholar]
- Bertelsen A, Harvald B, Hauge M. A Danish twin study of manic-depressive disorders. Br J Psychiatry. 1977;130:330–351. doi: 10.1192/bjp.130.4.330. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, et al. Variation of human amygdala response during threatening stimuli as a function of 5-HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Krystal JH, Bansal R, Martin A, Dziura J, et al. Age, rapid-cycling, and pharmacotherapy effects on ventral prefrontal cortex in bipolar disorder: A cross-sectional study. Biol Psychiatry. 2006;59:611–618. doi: 10.1016/j.biopsych.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Blumberg HP. Dimensions in the development of bipolar disorder. Biol Psychiatry. 2007;62:104–106. doi: 10.1016/j.biopsych.2007.04.035. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, et al. Beyond affect: A role for genetic variation of the serotonin transporter in neural activation during a cognitive attention risk. Proc Natl Acad Sci. 2005;102:12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon D, Ichise M, Fromm S, Nugent A, Rollis D, et al. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Caparelli EC, Tomasi D, Arnold S, Chang L, Ernst T. k-Space based summary motion detection for functional magnetic resonance imaging. NeuroImage. 2003;20:1411–1418. doi: 10.1016/S1053-8119(03)00339-2. [DOI] [PubMed] [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, Simeonova DI, Barnes P, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biol Psychiatry. 2005;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Cohen J, MacWhinney B, Flatt M, Provost J. PsyScope: An interactive graphical system for designing and controlling experiments in the psychology laboratory using Macintosh computers. Behavior Methods, Research, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Cusin C, Serretti A, Lattuada E, Lilli R, Lorenzi C, et al. Influence of 5-HTTLPR and TPH variants on illness time course in mood disorders. J Psychiatric Res. 2001;35:217–223. doi: 10.1016/s0022-3956(01)00026-7. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, et al. Serotonergic genes modulate amygdala activity in major depression. Genes, Brain, and Behavior. 2007;6:672–676. doi: 10.1111/j.1601-183X.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- DePaulo JR. Genetics of bipolar disorder: Where do we stand? Am J Psychiatry. 2004;161:595–597. doi: 10.1176/appi.ajp.161.4.595. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Domschke K, Braun M, Ohrmann P, Suslow T, Kugel H, et al. Association of the functional-1019C/G 5-HT1A polymorphism with prefrontal cortex and amygdala activation measured with 3 T fMRI in panic disorder. International Journal of Neuropsychopharmacology. 2005;9:1–7. doi: 10.1017/S1461145705005869. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, et al. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- Ebert D, Ebmeier KP. The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol Psychiatry. 1996;39:1044–1050. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Consulting Psychologists; Palo Alto, CA: 1979. [Google Scholar]
- Etkin A, Egner T, Peraza DM. Resolving emotional conflict: A role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;6:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I & II Disorders (Version 2.0) New York State Psychiatric Institute; New York: 1995. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, et al. Statistical Parametric Mapping: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Furmark T, Tillfors M, Garpenstrand H, Marteinsdottir I, Langstrom B, et al. Serotonin transporter polymorphism related to amygdala excitability and symptom severity in patients with social phobia. Neuroscience Letters. 2004;362:189–192. doi: 10.1016/j.neulet.2004.02.070. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Human Genetics. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols TE. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:772–786. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Ho LW, Furlong RA, Rubinsztein JS, Walsh C, Paykel ES, et al. Genetic associations with clinical characteristics in bipolar affective disorder and recurrent unipolar depressive disorder. Am J Med Genetics. 2000;96:36–42. [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, et al. Brain-derived neurotrophic factor–5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kaur S, Sassi RB, Axelson D, Nicoletti M, Brambilla P, et al. Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry. 2005;162:1637–1643. doi: 10.1176/appi.ajp.162.9.1637. [DOI] [PubMed] [Google Scholar]
- Kelsoe JR. Arguments for the genetic basis of the bipolar spectrum. J Affective Disorders. 2003;73:183–197. doi: 10.1016/s0165-0327(02)00323-3. [DOI] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psych. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Kruger S, Goldapple K, Kennedy S, Houle S, Mayberg H. Regional cerebral blood flow in bipolar disorder measured with PET: trait effects at rest and after mood induction. Bipolar Disorders. 2002;4:88. [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Lish JD, Weissman MM, Adams PB, Hoven CW, Bird H. Family psychiatric screening instrument for epidemiologic studies: pilot testing and validation. Psychiatry Research. 1995;57:169–180. doi: 10.1016/0165-1781(95)02632-7. [DOI] [PubMed] [Google Scholar]
- Lotrich FE, Pollock BG, Ferrell RE. Serotonin transporter promoter polymorphism in African Americans: Allele frequencies and implications for treatment. Am J PharmacoGenomics. 2003;3:145–147. doi: 10.2165/00129785-200303020-00007. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AJ, Demopulos CM, Parow AM. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang Y, Underwood MD, Kassir SA, Oppenheim S, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely H, Seminowicz D, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, et al. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Brown SM, Hariri AR. Serotonin Transporter (5-HTTLPR) Genotype and amygdala activation: A meta-analysis. Biol Psychiatry. 2008;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ospina-Duque J, Duque C, Carmona-Carvajal L, Ortiz-Barrientos D, Soto I, et al. An association study of bipolar mood disorder (type I) with the 5-HTTLPR serotonin transporter polymorphism in a human population isolate from Colombia. Neurosci Letters. 2000;292:199–202. doi: 10.1016/s0304-3940(00)01464-6. [DOI] [PubMed] [Google Scholar]
- Parsey RV, Hastings RS, Oquendo MA, Hu X, Goldman D, et al. Effect of a triallelic functional polymorphism of the serotonin-transporter-linked promoter region on expression of serotonin transporter in the human brain. Am J Psychiatry. 2006;163:48–51. doi: 10.1176/appi.ajp.163.1.48. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: Where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O’Connor MM, Harrael E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interaction: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Goldman AL, Verchinski BA, Chen G, et al. Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.32. (electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- Rich BA, Vinton DT, Roberson-Nay R, Hommer RE, Berghorst LH, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol Psychiatry. 2008;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditional stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseva A, Henry C, van den Bulke D, Fournier G, Laplanche J-L, et al. Antidepressant-induced mania, rapid cycling and the serotonin transporter gene polymorphism. Pharmacogenomics J. 2003;3:101–104. doi: 10.1038/sj.tpj.6500156. [DOI] [PubMed] [Google Scholar]
- Sanches M, Sassi RB, Axelson D, Nicoletti M, Brambilla P, et al. Subgenual prefrontal cortex of child and adolescent bipolar patients: A morphometric magnetic resonance imaging study. Psychiatry Res. 2005;138:43–49. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Smits KM, Smits LJM, Schouten JSAG, Stelma FF, Nelemans P, et al. Influence of SERTPR and STin2 in the serotonin transporter gene on the effect of SSRIs in depression: a systematic review. Molecular Psychiatry. 2004;9:433–441. doi: 10.1038/sj.mp.4001488. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Finn CT. Family, twin, and adoption studies of bipolar disorder. Am Journal Medical Genetics (Semin Med Genet) 2003;123C:48–58. doi: 10.1002/ajmg.c.20013. [DOI] [PubMed] [Google Scholar]
- Stamm TJ, Adli M, Kirchheiner J, Smolka MN, Kaiser R, et al. Serotonin transporter gene and response to lithium augmentation in depression. Psychiatric Genetics. 2007;18:92–97. doi: 10.1097/YPG.0b013e3282f08a19. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerey SC, et al. The emotional counting stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998a;44:1219–1228. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, et al. Masked presentations of emotional face expressions modulate amygdala activity without explicit knowledge. J Neurosci. 1998b;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Kowatch RA, Delbello MP, Mills NP, Holland SK. Voxel-based morphometry in adolescents with bipolar disorder: first results. Psychiatry Res. 2004;131:57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Williams JBW, Link MJ, Rosenthal NE, Amira L, Terman M. Structured interview guide for the Hamilton depression rating scale: seasonal affective disorder version (SIGH-SAD) New York, NY: New York State Psychiatric Institute; 1994. [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: The anterior executive and posterior evaluative Regions. Cerebral Cortex. 1992;2:435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004;22:246–260. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, et al. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]


