Abstract
The non-protein amino acid beta-aminobutyric acid (BABA) enhances Arabidopsis resistance to microbial pathogens and abiotic stresses through potentiation of the Arabidopsis defence responses. In this study, it is shown that BABA induces the stress-induced morphogenic response (SIMR). SIMR is observed in plants exposed to sub-lethal stress conditions. Anthocyanin, a known modulator of stress signalling, was also found to accumulate in BABA-treated Arabidopsis. These data and a previous microarray study indicate that BABA induces a stress response in Arabidopsis. High concentrations of amino acids, except for L-glutamine, cause a general amino acid stress inhibition. General amino acid inhibition is prevented by the addition of L-glutamine. L-Glutamine was found to inhibit the BABA-mediated SIMR and anthocyanin accumulation, suggesting that the non-protein amino acid BABA causes a general amino acid stress inhibition in Arabidopsis. L-Glutamine also blocked BABA-induced resistance to heat stress and to the virulent bacterial pathogen Pseudomonas syringae pv. tomato DC3000. During bacterial infection, priming of the salicylic acid-dependent defence marker PR1 was abolished by L-glutamine treatment. These results indicate that L-glutamine removal of the BABA-mediated stress response is concomitant with L-glutamine inhibition of BABA priming and BABA-induced resistance.
Keywords: Acquired thermotolerance, Arabidopsis, beta-aminobutyric acid, priming, Pseudomonas syringae, stress, stress imprinting, stress-induced morphogenic response
Introduction
Throughout evolution, plants have developed numerous defence mechanisms manifested through altered physiology to endure environmental abiotic stress and to combat challenges arising from biotic stress. Abiotic stresses include drought, excess water, salinity, heat, cold, wounding, and exposure to chemical stress (Shao et al., 2006; Wu et al., 2007).
A common theme underlying responses to a range of biotic and abiotic stresses is the phenomenon of priming. Priming refers to a phenomenon where plants are sensitized to stress (Conrath et al., 2002; Prime-A-Plant Group, 2006; Van der Ent et al., 2009). Typically primed plants display either faster and/or stronger, activation of the various defence responses that are induced following attack by microbial pathogens, or in response to abiotic stresses (Conrath et al., 2002; Prime-A-Plant Group, 2006; Beckers et al., 2009). Priming is observed in plants and also in animals (Pham et al., 2007; Beckers et al., 2009; Jung et al., 2009). Priming provides low-cost protection in relatively high disease pressure conditions (van Hulten et al., 2006).
The non-protein amino acid beta amino-butyric acid (BABA) increases Arabidopsis resistance to different, unrelated stresses such as microbial pathogens, salt, drought, and heat shock (Zimmerli et al., 2000, 2001, 2008; Ton and Mauch-Mani, 2004; Jakab et al., 2005; Ton et al., 2005). BABA primes Arabidopsis plants to respond quicker and stronger to biotic and abiotic stresses (Conrath et al., 2002; Prime-A-Plant Group, 2006). Typically, following infection by Pseudomonas syringae pv. tomato DC3000, the salicylic acid (SA)-dependent defence marker PATHOGENESIS-RELATED gene 1 (PR1) is induced earlier and stronger in BABA-treated Arabidopsis (Zimmerli et al., 2000; Ton et al., 2005). Plant responses to different stresses are controlled at the molecular level by changes in gene expression and many genes are involved in such stress responses (Kreps et al., 2002; Tardif et al., 2007). A recent microarray study revealed that BABA enhances mRNA accumulation of abscisic acid (ABA) and ethylene early signalling intermediates (Zimmerli et al., 2008). ABA and ethylene are two plant hormones involved in the Arabidopsis stress response (Xiong et al., 2002; van Loon et al., 2006). These observations, and the fact that many stress-responsive genes were found to be up-regulated by BABA, suggests that this chemical activates a stress response in Arabidopsis (Zimmerli et al., 2008).
Previous plant exposure to stress can modify the plant response to a subsequent, different stress (Bruce et al., 2007). Higher plants are capable of demonstrating some stress ‘memory’, or stress imprinting (Bruce et al., 2007). Stress imprinting is usually defined as genetic or biochemical modifications induced by a first stress exposure that lead to enhanced resistance to subsequent stress. Preliminary stress exposure is indeed known to boost the stress tolerance of the plant through induction of acclimation responses (Bruce et al., 2007). Tolerance can be linked to an array of morphological, physiological, and biochemical responses that decrease stress exposure damage or facilitate repair of damaged systems (Potters et al., 2007).
Exposure of plants to a mild chronic stress can cause the induction of a specific, stress-induced morphogenic response (SIMR) (Potters et al., 2007). These responses are characterized by a blockage of cell division in the main meristematic tissues, an inhibition of elongation, and a redirected outgrowth of lateral organs (Potters et al., 2009). The SIMR is part of a general acclimation strategy, whereby plants do redirect their growth when exposed to stress. These stress responses are also characterized by the presence of antioxidants that prevent damage caused by reactive oxygen species, and the accumulation of foliar anthocyanin that acts as modulators of stress signals (Steyn et al., 2002; Gould and Lister, 2006).
The objective of this work was to understand better how the priming agent BABA potentiates the Arabidopsis defence responses. The data presented here provide evidence that BABA induces the SIMR in Arabidopsis. In addition, this work demonstrates for the first time that BABA-mediated SIMR, priming and resistance to stress can be inhibited by L-glutamine. This observation suggests that the non-protein amino acid BABA primes by inducing a general amino acid inhibition stress response in Arabidopsis. The relationship between BABA-mediated stress imprinting and priming is discussed.
Materials and methods
Biological materials and growth conditions
Arabidopsis thaliana (L. Heyhn.) Columbia (Col-0) were grown in commercial potting soil/perlite (3:2 v/v) at 22/18 °C day/night temperatures with 9 h light per 24 h for the indicated time. The proCYCB1;1:GUS transgenic line was kindly provided by Negi et al. (2008). For in vitro culture, seeds were first surface-sterilized with commercial bleach diluted to 1:10, washed with deionized distilled water three times, suspended in 0.15% agar (Bioman Scientific Co., Ltd., Taiwan) and stored at 4 °C in the dark. After 2–3 d of stratification, seeds were sown on sterilized half-strength MS medium [1/2 Murashige and Skoog salt (Sigma, USA), 1.5% agar (Bioman Scientific Co., Ltd, Taiwan), pH 5.7] and cultivated as indicated. Strain DC3000 of Pst was cultivated at 28 °C, 340 rpm in King's B medium (Bioman Scientific Co. Ltd., Taiwan) containing rifampicin (100 mg l−1) for selection.
Chemical treatment
BABA (Sigma, USA) and L-glutamine (Sigma, USA) were dissolved in water and pots were soil drenched 48 h before pathogen inoculation. Pots were syringe infiltrated with a 4-fold concentrated BABA or L-glutamine solution. To get the final indicated concentration, a volume of solution of one-quarter of the final volume of the pot was used. The BABA and L-glutamine concentrations used are indicated in the figure legends.
Root growth assay
In vitro Arabidopsis seedlings were grown vertically under 16 h light conditions at 22 °C on half-strength MS medium supplemented with the indicated concentration of BABA (Sigma, USA) or L-glutamine (Sigma, USA). Root length was recorded on 12-d-old seedlings and relative root growth rates were evaluated by comparison with the water control.
Lateral root quantification
Lateral root number evaluation was performed on 15-d-old in vitro seedlings grown on half strength MS medium containing BABA (Sigma, USA), L-glutamine (Sigma, USA), or both chemicals at the indicated concentration. The number of lateral roots on the primary root was determined with a dissecting microscope (Negi et al., 2008).
Measurement of anthocyanin content
Measurement of anthocyanin content was performed according to Mita et al. (1997). Approximately 100 mg of 15-d-old seedlings grown in pots were collected in Eppendorf tubes, flash-frozen in liquid N2 and ground into powder. One ml of 1% (v/v) hydrochloric acid in methanol was added to each sample tube and the tubes were vigorously vortexed. After 1 d of incubation at 4 °C, the mixture was centrifuged at 13 000 rpm for 15 min and absorbance of the supernatant was measured at 530 nm and 657 nm. The formula [A530–(1/4)×A657] was used to determine anthocyanin concentration. One anthocyanin unit is equivalent to one absorbance unit [A530–(1/4×A657)] in 1 ml of extraction solution.
Heat-shock treatment
Heat-shock treatments were performed according to Zimmerli et al. (2008). One to two-hundred surface-sterilized seeds were plated in rows on sterilized half-strength MS medium and grown horizontally under continuous light conditions. Arabidopsis-acquired thermotolerance was evaluated by moving 12-d-old-plantlets in vitro grown at 22 °C to 38 °C for 45 min. The chamber containing the plants was then allowed to heat up for 10 min to reach 45 °C. The plants were then kept at 45 °C for an additional 80 min. All heat-shock treatments were performed in the dark. After heat-shock treatment, the plants were returned to 22 °C in continuous light, and the evaluation of viability was assessed 4 d later. Seedlings were also photographed after 4 d. Plants were considered as survivors if no necrosis was visible on true leaves when observed at ×100 magnification with a stereo microscope.
Measurement of leaf weight
Forty-eight hours after chemical treatment, rosettes from 4-week-old pot-grown Arabidopsis were excised for fresh weight analysis.
Pseudomonas syringae bioassays
For bacterial inoculation, cells were collected by centrifugation, resuspended in 10 mM MgSO4.7H2O at A600=0.2, corresponding to a concentration of 108 cfu ml−1. Three-week-old plants were dipped in a solution of 2×107 cfu ml−1 bacteria containing the surfactant, Silwet L-77 (Bioman Scientific Co., Ltd., Taiwan) at a concentration of 0.01% of the volume of buffer. Plants inoculated with Pst DC3000 were then kept in 100% relative humidity during the first 24 h post-inoculation. For colony-forming units (cfu) determination, infected tissues were collected 48 h post-inoculation. After collection and weight evaluation, leaves were washed twice with sterile water and homogenized in 10 Mm MgSO4.7H2O. Quantification was done by plating appropriate dilutions on King's B agar containing rifampicin (100 mg −1; Bioman Scientific Co., Ltd., Taiwan) using sterilized microbeads (Boeco, Germany). All plates were cultivated in the dark at 28 °C and colonies formed were quantified after 48 h.
qRT-PCR
For each sample, leaves from three pots containing 20–30 3-week-old Arabidopsis were harvested at the indicated time points, flash-frozen in liquid N2, and kept at –80 °C. Total RNA was extracted and purified using the RNeasy Plant Mini Kit (Qiagen, Germany) with additional DNA clean-up using the RNase-Free DNase Set (Qiagen, Germany). Complementary DNA was synthesized from 2 μg of total RNA using oligo(dT) primers and the reverse transcriptase from the M-MLV kit (Invitrogen, USA). SYBR Green PCR Master Mix (Bio-Rad, USA) and the iCycler Sequence Detection System (iQ5 Real-Time PCR Detection System, Bio-Rad, USA) was employed for quantitative real-time PCR (qRT-PCR) analysis. The thermal cycling program was composed of an initial 3 min at 95 °C, followed by 40 cycles of 95 °C for 30 s, 54 °C for 35 s, 72 °C for 35 s. Melting curve was run from 55 °C to 95 °C with 10 s time intervals to ensure the specificity of the product. Data were analysed using Bio-Rad iQ5 software (version 2.0). EF-1-ALPHA (At5g60390), was used as the reference gene for normalization of gene expression levels in all samples. For amplification, primer sequences were AAAACTTAGCCTGGGGTAGCGG (forward) and CCACCATTGTTACACCTCACTTTG (reverse) for PR1 (AT2G14610), AAGCGTCTCATGATGTACC (forward) and ACTGAAAAGAGCCTGACC (reverse) for CHS (AT5G13930), ATGGTTAGTCAGAAAGAGACC (forward) and TAAAGTGAGTAGCGTCTTGG (reverse) for DFR (AT5G42800), and TGAGCACGCTCTTCTTGCTTTCA (forward) and GGTGGTGGCATCCATCTTGTTACA (reverse) for EF-1-ALPHA.
Gus staining
For GUS expression analysis, 4-d-old seedlings were transferred to GUS staining buffer (100 mM sodium phosphate, pH 7.0, 10 mM EDTA, 0.5 mM K4Fe(CN)6, 0.5 mM K3Fe(CN)6, and 0.1% Triton X-100) containing 1 mM 5-bromo-4-chloro-3-indolyl β-D-GlcUA (X-gluc) and incubated at 37 °C for the specified time.
Results
BABA induces a SIMR in Arabidopsis
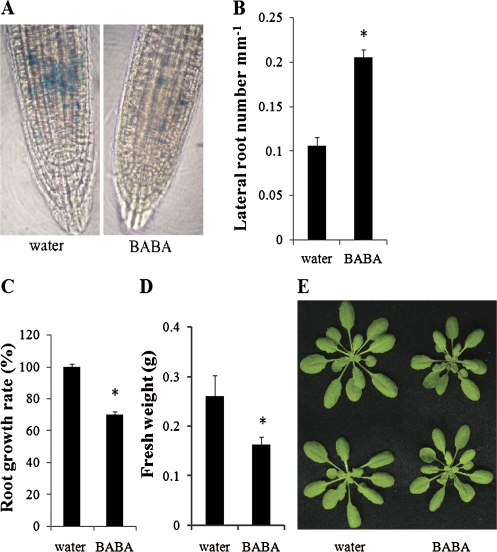
A recent study suggests that BABA induces a stress response in Arabidopsis (Zimmerli et al., 2008). Plants exposed to sub-lethal stresses exhibit the SIMR (Potters et al., 2007, 2009). Since BABA is known to inhibit root growth (Zimmerli et al., 2008), the possibility that BABA induces a SIMR in Arabidopsis was further investigated by testing whether BABA-mediated root growth inhibition is correlated with reduced cell division in the meristematic root tissue. The mitotic activity of the root meristem was evaluated by analysing the promoter activity of the mitotic cyclin CYCB1;1 (DiDonato et al., 2004; Negi et al., 2008). BABA reduced cell cycle activity of the root meristem, as monitored by the proCYCB1;1:GUS reporter activity (DiDonato et al., 2004; Negi et al., 2008) (Fig. 1A). This observation suggests that BABA reduced cell division in the root meristematic zone. BABA also increased lateral organs by increasing lateral root density (Fig. 1B). As already observed (Zimmerli et al., 2008), BABA inhibited root growth (Fig. 1C). In addition, BABA-treated plants were found to be smaller with a reduced fresh weight as compared to the wild type (Fig. 1D, E). Together these data suggest that BABA acts as a stress agent that provokes a SIMR in Arabidopsis.
Fig. 1.
BABA provokes a stress-induced morphogenic response in Arabidopsis. (A) BABA affects cell cycle activity of the root meristem. Cell cycle activity was evaluated by measuring primary root tip proCYCB1;1:GUS reporter activity (Negi et al., 2008). Five-d-old seedlings were vertically grown in the presence of water or 500 μM BABA and stained for 2 h for GUS activity. (B) Effects of BABA on lateral root density. Lateral root density was evaluated on 15-d-old in vitro plants vertically grown on half-strength MS medium supplemented with 75 μM BABA or water (control). The data are means ±SE (n >30). (C) Comparison of primary root length of 15-d-old Arabidopsis seedlings vertically grown on half-strength MS medium containing 75 μM BABA with the water control. (D, E) BABA inhibits Arabidopsis growth. Fresh weight (D) and size (E) were evaluated 3 d after water (control) or 300 μM BABA treatments. Error bars are SD. All experiments were repeated at least three times. Representative results are shown. (B, C, D) The asterisk indicates significant difference (P <0.01, Student's t-Test).
BABA induces accumulation of anthocyanin
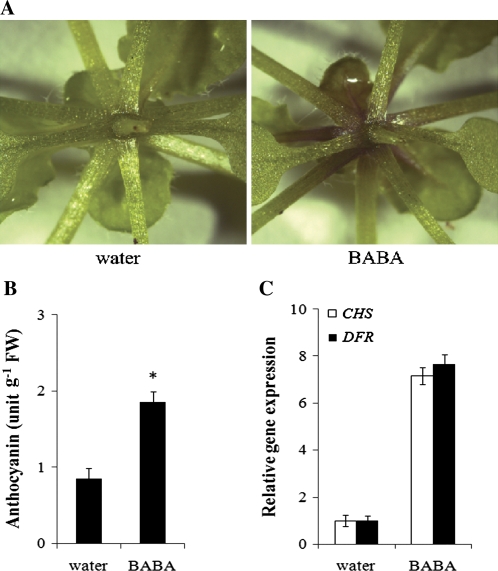
Foliar anthocyanin acts as modulators of stress signals (Steyn et al., 2002; Gould and Lister, 2006). BABA-treated Arabidopsis accumulated more anthocyanin in the petioles of 4-week-old Arabidopsis compared to the non-treated control (Fig. 2A). In addition, the total anthocyanin content in 5-week-old BABA-treated Arabidopsis was significantly higher than the water control (Fig. 2B). The anthocyanin pathway in Arabidopsis is catalysed by several important regulatory enzymes, including chalcone synthase (CHS) and dihydroflavonol-4-reductase (DFR) (Lillo et al., 2008). qRT-PCR analyses revealed that the expression levels of these genes were elevated in BABA-treated plants (Fig. 2C). These results indicate that BABA may stimulate anthocyanin biosynthesis by regulating the expression of CHS and DFR.
Fig. 2.
BABA induces accumulation of anthocyanin. Foliar anthocyanin accumulation was observed in 4-week-old (A) or in 5-week-old (B) Arabidopsis. The asterisk indicates significant difference (P <0.01, Student's t-Test). (C) Effect of BABA on the transcript levels of CHS and DFR genes in Arabidopsis. qRT-PCR relative expression levels were monitored in 3-week-old Arabidopsis. EF-1-ALPHA was used as an internal standard control. Expression levels of BABA-treated Arabidopsis were compared to water-treated controls (defined value of 1). Error bars are SD (n=3 technical replicates). For all experiments, Arabidopsis were treated with water (control) or 200 μM BABA and samples were collected 48 h later. Experiments were repeated twice with similar results.
L-Glutamine reduces the BABA-mediated SIMR
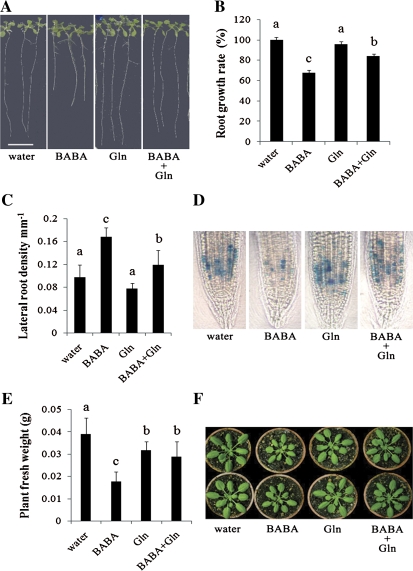
All amino acids, except for L-glutamine, cause the so-called general amino acid inhibition (Bonner et al., 1996; Bonner and Jensen, 1997). The molecular basis for this phenomenon is not clear, but it is prevented by L-glutamine (Bonner et al., 1996; Bonner and Jensen, 1997). Since the chemical BABA is a non-protein amino acid, it may, like natural amino acids, induce the general amino acid inhibition response. To confirm this hypothesis, inhibition of BABA-mediated SIMR by L-glutamine was evaluated. L-Glutamine was found to reduce BABA-mediated root growth inhibition and the BABA-mediated increase in lateral root density (Fig. 3A, B, C). In addition, BABA reduction of CYCB1:1 promoter activity as demonstrated by proCYCB1;1:GUS staining was partially abolished by L-glutamine (Fig. 3D). L-Glutamine treatment also partially restored a normal vegetative growth pattern in Arabidopsis treated with BABA (Fig. 3E, F). Together these data indicate that L-glutamine can largely rescue the BABA-mediated SIMR. These results also suggest that BABA may cause a general amino acid inhibition in Arabidopsis.
Fig. 3.
L-Glutamine partially rescues the BABA-mediated stress-induced morphogenic response. (A, B) Comparison of primary root length (A) and growth rate (B) of 15-d-old Arabidopsis seedlings grown on half-strength MS medium containing 75 μM BABA, 75 μM L-glutamine (Gln) or both L-glutamine and BABA (BABA+Gln). Scale bar=1 cm. (C) Lateral root density. Treatments were performed as in (A). The data are means ±SE (n >30). (D) L-Glutamine removes BABA inhibition on cell cycle activity. Cell cycle activity was evaluated by measuring primary root tip proCYCB1;1:GUS reporter activity (Negi et al., 2008). Five-day-old seedlings were grown in the presence of 500 μM BABA, 500 μM L-glutamine (Gln) or BABA and L-glutamine together (BABA+Gln) and stained for 2 h for GUS activity. (E, F) Fresh weight (E) and plant size (F) 3 d after treatment with 300 μM BABA, 10 mM L-glutamine (Gln) or both BABA and L-glutamine (BABA+Gln). Error bars are SD (n >30). Experiments were repeated three times. Representative results are shown. (B, C, D) Means with different letters are significantly different (P <0.05) based on a Least Significant Different (LSD) test.
L-Glutamine inhibits BABA-mediated accumulation of anthocyanin
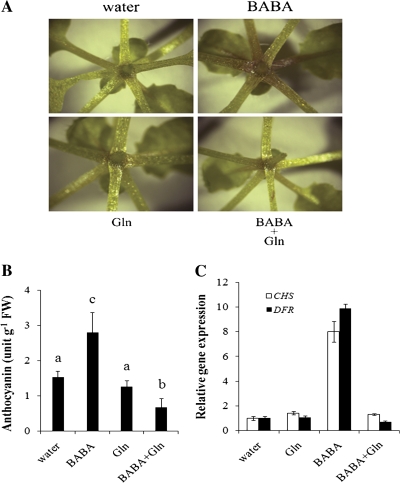
Arabidopsis plants showed increased accumulation of anthocyanin after treatment with BABA (Figs 2A, B, 4A). To test whether L-glutamine can also rescue this phenotype, anthocyanin concentration was analysed in plants treated with both BABA and L-glutamine. Similar to the effect of L-glutamine on the BABA-mediated SIMR, L-glutamine reduced the BABA-mediated increase of anthocyanin accumulation (Fig. 4A, B). L-Glutamine also inhibited the accumulation of CHS and DFR transcripts of the anthocyanin biosynthetic pathway in BABA-treated Arabidopsis (Fig. 4C). Together these data shows that L-glutamine treatment restores a normal level of anthocyanin in BABA-treated plants.
Fig. 4.
L-Glutamine inhibits BABA mediated anthocyanin accumulation. (A) Foliar anthocyanin accumulation in 3-week-old Arabidopsis. (B) Evaluation of anthocyanin content in 5-week-old Arabidopsis. Means with different letters are significantly different (P <0.05, LSD test). (C) BABA-mediated up-regulation of CHS and DFR mRNAs is inhibited by L-glutamine. qRT-PCR relative expression levels were monitored in 3-week-old Arabidopsis. EF-1-ALPHA was used as an internal standard control. Expression levels were compared to water-treated controls (defined value of 1). Error bars are SD (n=3 technical replicates). For all experiments, Arabidopsis samples were collected 48 h post-treatment with 250 μM BABA, 10 mM L-glutamine (Gln), or both BABA and L-glutamine (BABA+Gln). Experiments were repeated twice with similar results.
BABA-enhanced Arabidopsis thermotolerance is altered by L-glutamine
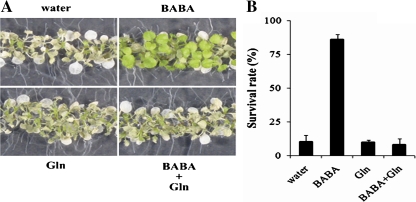
L-Glutamine treatment was found to inhibit the BABA-mediated SIMR and anthocyanin accumulation in Arabidopsis. To test further whether L-glutamine can counteract the BABA effect, its impact on BABA-mediated Arabidopsis acquired thermotolerance was analysed (Zimmerli et al., 2008). Ten-day-old Arabidopsis seedlings were heat acclimated at 38 °C for 45 min and then heat shocked at 45 °C for 90 min (Zimmerli et al., 2008). As preliminary experiments observed (Zimmerli et al., 2008), most of the BABA-treated Arabidopsis survived this heat shock treatment, while the water-treated controls did not (Fig. 5A, B). L-Glutamine did not affect the level of heat resistance, but when added with BABA, L-glutamine dramatically reduced BABA-induced heat protection (Fig. 5A, B). These results indicate that L-glutamine inhibits BABA-mediated Arabidopsis acquired thermotolerance and further support the idea that L-glutamine counteracts the BABA effect in Arabidopsis.
Fig. 5.
BABA-enhanced Arabidopsis acquired thermotolerance is inhibited by l-glutamine. (A, B) Symptoms (A) and survival rate (B) of 2-week-old heat-shocked Arabidopsis seedlings (see details in the Materials and methods) pretreated with 500 μM BABA, 500 μM l-glutamine (Gln) or both BABA and l-glutamine (BABA+Gln). Heat-induced and damaged cells in (A) were evaluated 4 d after the heat shock treatment. Survival rates represent the mean percentage survivors and SD (n >10 plates consisting of approximately 150 seedlings per plate). Experiments were repeated three times. Representative results are shown. (This figure is available in colour at JXB online.)
L-Glutamine inhibits BABA-induced resistance to Pst DC3000
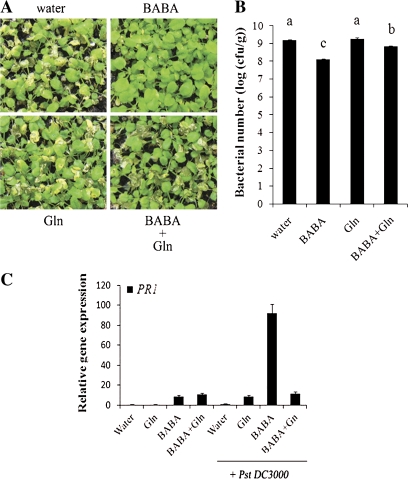
BABA enhances Arabidopsis resistance to biotic stresses (Conrath et al., 2002; Prime-A-Plant Group, 2006). The effect of L-glutamine on BABA-induced resistance was further evaluated on the symptoms of Arabidopsis infected with the virulent bacterial pathogen Pst DC3000 3 d post-inoculation (dpi). In addition, bacterial titres were also evaluated at 2 dpi. L-Glutamine reduced BABA-induced resistance at the symptom and titre levels (Fig. 6A, B). These data indicate that L-glutamine treatment partially counteracts BABA-induced resistance against virulent bacteria.
Fig. 6.
L-Glutamine inhibits BABA-induced resistance to bacteria and priming. (A) Disease symptoms were evaluated 3 dpi with the virulent bacterial pathogen Pst DC3000. (B) Bacterial growths were evaluated 48 h post-bacterial dip inoculation. Means with different letters are significantly different (P <0.05, LSD test). (C) BABA priming of PR1 expression is inhibited by L-glutamine. qRT-PCR relative expression levels at 18 h post-bacterial inoculation. EF-1-ALPHA was used as an internal standard control. Expression levels were compared to water-treated controls (no bacterial inoculation, defined value of 1). For all experiments, three-week-old Arabidopsis were treated 48 h before Pst DC3000 inoculation with 150 μM BABA, 10 mM L-glutamine (Gln) or both BABA and L-glutamine (BABA+Gln). Error bars are SD (n=3 technical replicates). Experiments were repeated three times. Representative results are shown.
BABA-induced priming of PR1 expression is inhibited by L-glutamine
BABA enhances Arabidopsis resistance to Pst DC3000 through priming of the SA defence signalling (Zimmerli et al., 2000; Ton et al., 2005). Since L-glutamine treatment inhibits BABA-induced resistance to Pst DC3000 (Fig. 6A, B), the possibility that L-glutamine also blocks BABA-mediated priming of the SA defence marker gene PR1 was tested. The expression level of PR1 was evaluated by qRT-PCR at 18 h post Pst DC3000 inoculation. As expected, BABA primed PR1 expression (Fig. 6C). Without bacterial infection, L-glutamine treatment alone or mixed with BABA did not affect PR1 expression levels. However, Arabidopsis treated with both L-glutamine and BABA did not demonstrate a primed PR1 expression after bacterial infection (Fig. 6C). L-Glutamine thus strongly reduced BABA priming of PR1. This observation suggests that L-glutamine -mediated inhibition of BABA-induced resistance against Pst DC3000 functions through a blockage of BABA priming.
Discussion
BABA induces a SIMR in Arabidopsis
In Arabidopsis, the chemical BABA has been shown to enhance disease resistance and to increase salt, drought, and thermotolerance (Zimmerli et al., 2000, 2001, 2008; Ton and Mauch-Mani, 2004; Jakab et al., 2005; Ton et al., 2005). BABA does not activate the defence response directly but rather sensitizes plants to respond more quickly and strongly to biotic and abiotic stresses. This process is referred to as priming (Conrath et al., 2002; Prime-A-Plant Group, 2006). The mechanisms underlying the BABA mode of action and, particularly, the priming phenomenon are still poorly understood. In addition, the metabolic pathways through which BABA mediates both abiotic and biotic stress resistance are still being elucidated. In the present study, it was shown that BABA induces a phenotypical response similar to the recently described SIMR (Potter et al., 2007, 2009). BABA-treated seedlings clearly demonstrated an inhibition of cell division in the meristematic root tissue (Fig. 1A) and a concomitant reduction in root growth is observed (Fig. 1C; Zimmerli et al., 2008). Secondly, BABA was found to increase lateral organs by increasing lateral root density (Fig. 1B). Furthermore BABA-treated Arabidopsis seedlings demonstrated a reduced vegetative growth and were found to be slightly stunted (Fig. 1D, E). All of these phenotypes are characteristic of the SIMR (Potter et al., 2007, 2009). Consequently, BABA may act as a stressing agent in Arabidopsis. This hypothesis has been further confirmed as BABA-treated Arabidopsis activate ABA and ethylene stress signalling concomitantly with an accumulation of stress-induced transcripts (Zimmerli et al., 2008). In addition, known modulators of stress signalling such as anthocyanin (Steyn et al., 2002; Gould and Lister, 2006) accumulated upon BABA treatment (Fig 2A, B). Taken together, it suggests that BABA provokes a mild chronic stress in Arabidopsis that would explain the observed SIMR.
What kind of mild chronic stress may BABA induce?
Since BABA is a non-protein amino acid and is not metabolized in planta (Zimmerli et al., 2000), it was speculated that this chemical provokes a general amino acid inhibition, as do amino acids when supplied to the plant at high concentration (Bonner et al., 1996).
The molecular basis for this phenomenon is still not clear, it is, however, known that exogenous application of amino acids to suspension culture of Nicotiana sylvestris cells is toxic and inhibits cell growth (Bonner et al., 1996; Bonner and Jensen, 1997). It has been shown that this ‘general amino-acid stress’ is prevented by the addition of L-glutamine (Bonner et al., 1996; Bonner and Jensen, 1997). The removal of BABA-induced stress effects in Arabidopsis by L-glutamine was thus tested. BABA-induced SIMR was found to be largely reduced by L-glutamine treatment and, concomitantly, BABA-induced resistance to heat shock and to virulent bacteria such as Pst DC3000 was also reduced by L-glutamine. These observations suggest that BABA induces a general amino acid stress inhibition. It is, however, still possible that L-glutamine and BABA may share a common transporter and L-glutamine compete for BABA transport. L-Glutamine may thus inhibit BABA translocation into the cell in the presence of excess of L-glutamine. Future work is needed to elucidate this point. Importantly, it is clear that L-glutamine attenuates the BABA effect in Arabidopsis. It is worthwhile realizing that both the BABA-mediated SIMR and the protective effects are affected by L-glutamine treatment.
Does BABA prime the Arabidopsis defence response by stress imprinting?
Priming behaviour is critical for adaptation to complex, ever-changing environmental conditions (Conrath et al., 2002; Prime-A-Plant Group, 2006; Beckers and Conrath, 2007; Bruce et al., 2007; Frost et al., 2008). Priming is usually defined as a sensitization to stress responsiveness. As a result, priming boosts the plant's defence response and primed plants are more resistance to biotic and abiotic stress (Conrath et al., 2002; Prime-A-Plant Group, 2006; Pham et al., 2007; Beckers et al., 2009; Jung et al., 2009). It was shown here that BABA acts as a chemical stress and therefore induces the SIMR in Arabidopsis. The removal of the SIMR by the addition of L-glutamine was found to be correlated with a loss of BABA-mediated priming and protection. This observation suggests that BABA primes the Arabidopsis defence response by stress imprinting. Preliminary stress exposure or stress imprinting is indeed known to induce priming and resistance in plants (Bruce et al., 2007; Galis et al., 2009). Typically, repeated exposure to stressful concentrations of the phytohormone ABA impaired the Arabidopsis stomatal response to light (Goh et al., 2003). Similarly, treatment with sub-lethal concentrations of paraquat correlates with greater oxidative resistance in plants (Ye and Gressel, 2000). Arabidopsis first exposed to osmotic stress demonstrate an altered Ca2+ response that leads to the acquisition of stress-tolerance (Knight et al., 1998). Although all these examples suggest that stress imprinting is caused by previous exposure to mild stress conditions, most of them relate to priming and protection after a second exposure to the same or to a very similar stress. Soil drench treatment with the chemical BABA primes appropriate defence mechanisms and provides long-term protection against biotrophic bacteria (Zimmerli et al., 2000; Ton et al., 2005; Goellner and Conrath, 2008), necrotrophic fungi (Zimmerli et al., 2001; Ton and Mauch-Mani, 2004; Flors et al., 2008), and abiotic stresses (Jakab et al., 2005). L-Glutamine was found to inhibit BABA-induced resistance to both heat shock and the bacterial pathogen Pst DC3000, two different type of stresses (i.e. abiotic and biotic). This suggests that this observed multifaceted BABA-mediated resistance is induced by a mild chronic stress imprinting. Together, these observations indicate that BABA-mediated stress imprinting may act at a convergent node that induces resistance to different, unrelated stresses. Determining the underlying mechanisms involved in BABA-mediated priming should thus uncover general, global components of the stress imprinting phenomenon.
Acknowledgments
We thank Dr GK Muday (Wake Forest University, Winston-Salem, NC, USA) for providing proCYCB1;1:GUS seeds. We also acknowledge W Schmidt and members of the Zimmerli laboratory for critical comments. We thank the Technology Commons (TechComm), College of Life Science, National Taiwan University for providing qRT-PCR equipment. We also thank YL Tzeng from TechComm for qRT-PCR training. This project was supported by the National Science Council of Taiwan grant 96-2628-B-002-112-MY3 and the National Taiwan University.
Glossary
Abbreviations
- ABA
abscisic acid
- BABA
beta-aminobutyric acid
- dpi
days post-inoculation
- PR1
PATHOGENESIS-RELATED gene 1
- qRT-PCR
quantitative real-time-PCR
- SA
salicylic acid
- SIMR
stress-induced morphogenic response
References
- Beckers GJ, Conrath U. Priming for stress resistance: from the lab to the field. Current Opinion in Plant Biology. 2007;10:425–431. doi: 10.1016/j.pbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, Conrath U. Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. The Plant Cell. 2009;21:944–953. doi: 10.1105/tpc.108.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner CA, Williams DS, Aldrich HC, Jensen RA. Antagonism by L-glutamine of toxicity and growth inhibition caused by other amino acids in cell cultures of Nicotiana sylvestris. Plant Science. 1996;113:43–58. [Google Scholar]
- Bonner CA, Jensen RA. Recognition of specific patterns of amino acid inhibition of growth in higher plants, uncomplicated by glutamine-reversible ‘general amino acid inhibition’. Plant Science. 1997;130:133–143. [Google Scholar]
- Bruce JA, Matthes MC, Napier JA, Pickett JA. Stressful ‘memories’ of plants: evidence and possible mechanisms. Plant Science. 2007;173:603–608. [Google Scholar]
- Conrath U, Pieterse CM, Mauch-Mani B. Priming in plant–pathogen interactions. Trends in Plant Science. 2002;7:210–216. doi: 10.1016/s1360-1385(02)02244-6. [DOI] [PubMed] [Google Scholar]
- DiDonato RJ, Arbuckle E, Buker S, Sheets J, Tobar J, Totong R, Grisafi P, Fink GR, Celenza JL. Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. The Plant Journal. 2004;37:340–353. doi: 10.1046/j.1365-313x.2003.01964.x. [DOI] [PubMed] [Google Scholar]
- Flors V, Ton J, van Doorn R, Jakab G, García-Agustín P, Mauch-Mani B. Interplay between JA, SA and ABA signalling during basal and induced resistance against Pseudomonas syringae and Alternaria brassicicola. The Plant Journal. 2008;54:81–92. doi: 10.1111/j.1365-313X.2007.03397.x. [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Carlson JE, Moraes CMD. Plant defense priming against herbivores: getting ready for a different battle. Plant Physiology. 2008;146:818–824. doi: 10.1104/pp.107.113027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gális I, Gaquerel E, Pandey SP, Baldwin IT. Molecular mechanisms underlying plant memory in JA-mediated defence responses. Plant, Cell and Environment. 2009;32:617–627. doi: 10.1111/j.1365-3040.2008.01862.x. [DOI] [PubMed] [Google Scholar]
- Goellner K, Conrath U. Priming: it's all the world to induced disease resistance. European Journal of Plant Pathology. 2008;121:233–242. [Google Scholar]
- Goh CH, Nam HG, Park YS. Stress memory in plants: a negative regulation of stomatal response and transient induction of rd22 gene to light in abscisic acid-entrained Arabidopsis plants. The Plant Journal. 2003;36:240–255. doi: 10.1046/j.1365-313x.2003.01872.x. [DOI] [PubMed] [Google Scholar]
- Gould KS, Lister C. Flavonoid functions in plants. In: Andersen ØM, Markham KR, editors. Flavonoids: chemistry, biochemistry, and applications. Boca Raton: CRC Press; 2006. pp. 397–441. [Google Scholar]
- Jakab G, Ton J, Flors V, Zimmerli L, Métraux JP, Mauch-Mani B. Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses. Plant Physiology. 2005;139:264–174. doi: 10.1104/pp.105.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HW, Tschaplinski TJ, Wang L, Glazebrook J, Greenberg JT. Priming in systemic plant immunity. Science. 2009;324:89–91. doi: 10.1126/science.1170025. [DOI] [PubMed] [Google Scholar]
- Knight H, Brandt S, Knight MR. A history of stress alters drought calcium signalling pathways in Arabidopsis. The Plant Journal. 1998;16:681–687. doi: 10.1046/j.1365-313x.1998.00332.x. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiology. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillo C, Lea US, Ruoff P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant, Cell and Environment. 2008;31:587–601. doi: 10.1111/j.1365-3040.2007.01748.x. [DOI] [PubMed] [Google Scholar]
- Mita S, Murano N, Akaike M, Nakamura K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for beta-amylase and on the accumulation of anthocyanin that are inducible by sugars. The Plant Journal. 1997;11:841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK. Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. The Plant Journal. 2008;55:175–187. doi: 10.1111/j.1365-313X.2008.03495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham LN, Dionne MS, Shirasu-Hiza M, Schneider DS. A specific primed immune response in Drosophila is dependent on phagocytes. PLoS Pathogens. 2007;3:e26. doi: 10.1371/journal.ppat.0030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA. Stress-induced morphogenic responses: growing out of trouble? Trends in Plant Science. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Jansen MA. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell and Environment. 2009;32:158–169. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- doi: 10.1094/MPMI-19-1062. Prime-A-Plant Group: Conrath U, Beckers GJ, Flors V, et al. 2006. Priming: getting ready for battle. Molecular Plant–Microbe Interactions 19, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Shao HB, Guo QJ, Chu LY, Zhao XN, Su ZL, Hu YC, Cheng JF. Understanding molecular mechanism of higher plant plasticity under abiotic stress. Colloids and Surfaces B: Biointerfaces. 2006;54:37–45. doi: 10.1016/j.colsurfb.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Steyn WJ, Wand SJE, Holcroft DM, Jacobs G. Anthocyanins in vegetative tissues: a proposed unified function in photoprotection. New Phytologist. 2002;155:349–361. doi: 10.1046/j.1469-8137.2002.00482.x. [DOI] [PubMed] [Google Scholar]
- Tardif G, Kane NA, Adam H, Labrie1 L, Major G, Gulick P, Sarhan F, Laliberté JF. Interaction network of proteins associated with abiotic stress response and development in wheat. Plant Molecular Biology. 2007;63:703–708. doi: 10.1007/s11103-006-9119-6. [DOI] [PubMed] [Google Scholar]
- Ton J, Mauch-Mani B. Beta-amino-butyric acid-induced resistance against necrotrophic pathogens is based on ABA-dependent priming for callose. The Plant Journal. 2004;38:119–130. doi: 10.1111/j.1365-313X.2004.02028.x. [DOI] [PubMed] [Google Scholar]
- Ton J, Jakab G, Toquin V, Flors V, Iavicoli A, Maeder MN, Métraux JP, Mauch-Mani B. Dissecting the beta-aminobutyric acid induced priming pathways in Arabidopsis. The Plant Cell. 2005;17:987–999. doi: 10.1105/tpc.104.029728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ent S, Van Hulten M, Pozo MJ, Czechowski T, Udvardi MK, Pieterse CM, Ton J. Priming of plant innate immunity by rhizobacteria and beta-aminobutyric acid: differences and similarities in regulation. New Phytologist. 2009;183:419–431. doi: 10.1111/j.1469-8137.2009.02851.x. [DOI] [PubMed] [Google Scholar]
- van Hulten M, Pelser M, van Loon LC, Pieterse CMJ, Ton J. Costs and benefits of priming for defense in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2006;103:5602–5607. doi: 10.1073/pnas.0510213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon LC, Geraats BP, Linthorst HJ. Ethylene as a modulator of disease resistance in plants. Trends in Plant Science. 2006;11:184–191. doi: 10.1016/j.tplants.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Wu L, Chen X, Ren H, Zhang Z, Zhang H, Wang J, Wang XC, Huang R. ERF protein JERF1 that transcriptionally modulates the expression of abscisic acid biosynthesis-related gene enhances the tolerance under salinity and cold in tobacco. Planta. 2007;226:815–825. doi: 10.1007/s00425-007-0528-9. [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK. Cell signaling during cold, drought, and salt stress. The Plant Cell. 2002;14:S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye B, Gressel J. Transient, oxidant-induced antioxidant transcript and enzyme levels correlate with greater oxidant-resistance in paraquat-resistant Conyza bonariensis. Planta. 2000;211:50–61. doi: 10.1007/s004250000257. [DOI] [PubMed] [Google Scholar]
- Zimmerli L, Jakab C, Metraux JP, Mauch-Mani B. Potentiation of pathogen-specific defense mechanisms in Arabidopsis by beta-aminobutyric acid. Proceedings of the National Academy of Sciences, USA. 2000;97:12920–12925. doi: 10.1073/pnas.230416897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L, Métraux JP, Mauch-Mani B. beta-Aminobutyric acid-induced protection of Arabidopsis against the necrotrophic fungus Botrytis cinerea. Plant Physiology. 2001;126:517–523. doi: 10.1104/pp.126.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli L, Hou BH, Tsai CH, Jakab G, Mauch-Mani B, Somerville S. The xenobiotic beta-aminobutyric acid enhances Arabidopsis thermotolerance. The Plant Journal. 2008;53:144–156. doi: 10.1111/j.1365-313X.2007.03343.x. [DOI] [PubMed] [Google Scholar]