Abstract
Active gibberellin (GA1) is an important mediator of thermoperiodic growth in pea. Plants grown under lower day than night temperature (negative DIF) elongate less and have reduced levels of GA1 compared with plants grown at higher day than night temperature (positive DIF). By comparing the wild type (WT) and the elongated DELLA mutant la crys, this study has examined the effect of impaired GA signalling on thermoperiodic growth, photosynthesis, and respiration in pea. In the WT a negative DIF treatment reduced stem mass ratio and increased both root mass ratio and leaf mass ratio (dry weight of specific tissue related to total plant dry weight). Leaf, root and stem mass ratios of la crys were not affected by DIF. Under negative DIF, specific leaf area (projected leaf area per unit leaf dry mass), biomass, and chlorophyll content of WT and la crys plants were reduced. Young, expanding leaves of plants grown under negative DIF had reduced leaf area-based photosynthetic capacity. However, the highest photosynthetic electron transport rate was found in fully expanded leaves of WT plants grown under negative DIF. Negative DIF increased night respiration and was similar for both genotypes. It is concluded that GA signalling is not a major determinant of leaf area-based photosynthesis or respiration and that reduced dry weight of plants grown under negative DIF is caused by a GA-mediated reduction of photosynthetic stem and leaf tissue, reduced photosynthesis of young, expanding leaves, and reduced growth caused by low temperature in the photoperiod.
Keywords: DIF, gibberellins, photosynthesis, Pisum sativum, plant morphology, respiration, stem elongation, thermoperiodism
Introduction
Thermoperiodism was defined by Went (1944) as ‘all effects of a temperature differential between light and dark periods on responses of the plants, whether they be flowering, fruiting or growth’. In general, when plants are grown at the same average diurnal temperature, plants grown under a negative temperature difference [negative DIF; day temperature (DT) < night temperature (NT)] elongate less than those grown under positive DIF (DT > NT). The DIF concept was introduced by Erwin et al. (1989) and has since become widely used for growth control in greenhouse production of ornamental plants. A negative DIF treatment often results in reduced total plant dry weight as compared with a positive DIF (Heuvelink, 1989; Myster et al., 1997; Xiong et al., 2002).
Gibberellins (GAs) are hormones contributing to the control of growth and development of plants throughout their life cycle and have been known for decades for their strong growth-promoting effect on stems. In pea (Pisum sativum), Campanula isophylla, and Dendranthema grandiflorum, regulation of GA metabolism has been hypothesized to mediate the effects of diurnal temperature alternations on growth and morphology (Jensen et al., 1996; Nishijima et al., 1997; Grindal et al., 1998; Stavang et al., 2005). Thus, reduced stem elongation and leaf area in plants grown under negative DIF is caused by reduced amounts of active GA. However, it has also been shown that in Lilium longiflorum and cucumber, a negative DIF treatment reduces net photosynthetic rate and chlorophyll content compared with a positive DIF treatment (Berghage et al., 1990; Agrawal et al., 1993). Is GA also regulating photosynthesis? Recently, it has been shown that increased or decreased GA levels in transgenic Populus and tobacco plants improved or reduced growth and biomass production, respectively (Eriksson et al., 2000; Biemelt et al., 2004). Also, transgenic citrus with elevated levels of GAs are reported to have increased growth and photosynthesis (Huerta et al., 2008).
In an early GA application experiment, spraying red clover with GA3 increased photosynthesis and Rubisco activity, and a regulatory effect of the hormone on photosynthetic activity was suggested (Treharne and Stoddart, 1968). A positive effect of GA application on photosynthesis has also been found in other similar studies (Khan, 1996; Hayat et al., 2001; Yuan and Xu, 2001). However, there are also published reports where applied GA stimulated growth, but decreased the rate of photosynthesis (Dijkstra et al., 1990). Thetford et al. (1995) found that application of inhibitors of GA biosynthesis stimulated photosynthesis, while reducing elongation growth. There are also reports showing that application of GA biosynthesis inhibitors reduced both growth and photosynthetic rate (Bode and Wild, 1984; Heide et al., 1985). Furthermore, Cramer et al. (1995) found no difference in photosynthetic rate between the wild type (WT) and a low GA mutant of tomato (Solanum lycopersicum, formerly Lycopersicon esculentum). Thus, the studies published so far are contradictory and do not show a clear relationship between GA levels and rates of photosynthesis, respiration, and growth (Nagel and Lambers, 2002).
Although application of GAs and GA biosynthetic inhibitors often has been used as a fruitful approach in studies of GA physiology, differences in uptake and access to target tissue of the applied GA in different species, tissues, and experiments cannot be excluded. Thus, to study the effect of GAs on photosynthetic and respiratory capacity, using plants with different endogenous GA content or different response to GAs is preferable to application experiments. In WT pea, detailed analyses of GA content in different tissues have shown that the levels of active GA1 are down-regulated under negative DIF as compared with positive DIF (Grindal et al., 1998; Stavang et al., 2005). Furthermore, the la crys mutant responds poorly to DIF treatments (Grindal et al., 1998). This elongated pea mutant does not respond to applied GAs or GA biosynthesis inhibitors, but shows a saturated GA response independently of growth conditions (Potts et al., 1985; Ingram and Reid, 1987). This mutant has now been identified as a double DELLA mutant (Weston et al., 2008). Mutations in LA and CRYs in pea result in non-functional DELLA proteins, and thus the growth inhibitory effect of these proteins is not present in the la crys mutant. Since stem elongation in the la crys mutant is similar to that of the WT given a saturating dose of active GA, it is suggested by Weston et al. (2008) that LA and CRY are the only DELLA-encoding genes involved in shoot growth in pea. Therefore, any regulation of GA levels occurring in la crys should have no effect in downstream signalling in the GA response pathway mediated by these DELLAs.
By exposing the WT and the DELLA mutant la crys to positive and negative DIF treatments, this study aimed to separate thermoperiodic growth mediated by GA regulation from thermoperiodic growth independent of GA signalling through these DELLAs in pea.
Materials and methods
Plant material and experimental conditions
Seed of P. sativum L. WT line 107 (cv. Torsdag) or the slender saturated GA response mutant la crys, which is mutated in the LA and CRYS genes encoding DELLA proteins (Potts et al., 1985; Ingram and Reid, 1987; Weston et al., 2008), were sown in an 11 cm pot containing fertilized peat (Floralux, Nittedal Torvindustrier, Nittedal, Norway) and grown under controlled environmental conditions (Conviron growth chambers, Controlled Environments Ltd, Winnipeg, Canada). The humidity was adjusted to give 0.47±0.03 kPa water vapour deficit. The daily light period was 12 h (light period, 09:00–21:00; dark period, 21:00–09:00) with an irradiance of 160±10 μmol m −2 s−1 at 400–700 nm [F96T12/CW/1500 fluorescent tubes (General Electric, Fairfield, CT, USA) enriched with light from incandescent lamps (OSRAM, Munich, Germany)]. The red/far red ratio was 1.7±0.1. The seedlings were watered daily with a complete nutrient solution of EC=1.5 mS cm−1. The temperature was kept at 17 °C until the hypocotyls had straightened (6 d), then the plants were transferred to two separate growth chambers with different combinations of day (DT) and night temperature (NT), both at a daily average temperature of 17 °C. The effect of a DT/NT of 13 °C/21 °C treatment (negative DIF) was compared with a 21 °C/13 °C treatment (positive DIF). The DIF treatments started on day 6 when the light was turned on. Plant height was recorded every third or fourth day. Light level at the top of the plants was maintained at 160 μmol m −2 s−1during the experimental period. For chlorophyll measurements, some plants were held at constant temperatures of 21 °C and 13 °C.
Leaf chlorophyll fluorescence recording, chlorophyll estimation, leaf area, and dry weight measurements
On day 12 after the start of the DIF treatments, relative chlorophyll content was estimated with a Hansatech CL-01-chlorophyll content meter (Hansatech Instruments, King's Lynn, Norfolk, UK) in leaf 1–5 or 6 counted from the bottom of WT and la crys plants. Ten plants per genotype were harvested 14 d after the start of the DIF treatments. Plants were divided into three fractions: leaves, roots, and stem tissue (stem tissue included stem, tendrils, and petioles and is henceforth referred to as stem). Leaf area was determined with a Li-Cor LI-3100 area meter (Li-Cor Biosciences, Lincoln, NE, USA). The root was rinsed with water before drying, and dry masses were determined after drying in an oven at 70 °C for 5 d.
Leaf chlorophyll fluorescence was recorded with a portable, modulated fluorometer (PAM-2000, Walz, Effeltrich, Germany). Chlorophyll fluorescence parameters were measured on seven plants per treatment, and on each plant measurements were performed both on a young expanding (leaf 5) and on a fully expanded leaf (leaf 3). The measurements were performed on dark-adapted leaves in the morning before the lights were turned on on day 13. The following protocol was used for the quenching analysis: first F0 and Fm were determined for dark-adapted leaves using a saturating pulse of 0.6 s duration. Then, after 10 s, actinic irradiance (210 μmol m−2 s−1 from internal red diodes in the fluorometer) was turned on. Steady-state chlorophyll fluorescence (Ft) was reached after 5 min, and Fm′ was determined with a new saturating pulse. Finally, the actinic light was turned off and F0′ was determined after far-red irradiation for 3 s. The following parameters were determined: (i) maximal photochemical quantum yield of photosystem II [PSII; Fv/Fm=(Fm–F0)/Fm]; (ii) irradiance-adapted quantum yield of PSII [ΦPSII=Fv′/Fm′=(Fm′–Ft)/Fm′] (Genty et al., 1989); (iii) non-photochemical quenching, NPQ=(Fm–Fm′)/Fm′ (Bilger and Björkman, 1990); (iv) electron transport rate, ETR=ΦPSII×PDF×0.5 (Genty et al., 1989); and (v) proportion of open PSII, Qp=(Fm′–Ft)/(Fm′–F0′) (e.g. Maxwell and Johnson, 2000).
Stem elongation rate recordings
After 10 d of growth of the WT and the la crys mutant at 17 °C, negative or positive DIF treatments were introduced. For comparison, a subset of plants was also kept at 17 °C (zero DIF) or moved to constant 13 °C and constant 21 °C. The stem elongation rate was then continuously measured every tenth second for 3 d according to Torre and Moe (1998) by an angular Displacement Transducer, series 604 (Trans-Tec. Ellington, CT, USA) connected to a data logger, type CR10-AM416 (Campbell Scientific Inc., Shepshed, UK). The water vapour deficit could not be precisely controlled in these chambers and relative humidity varied from 45% to 65%.
GA measurements
After 12 d of DIF treatment, leaf 3 and 5 counted from the bottom of WT and la crys mutant plants were harvested in liquid nitrogen. For each DIF treatment, genotype, and leaf number, four samples consisting of leaves from 20 plants were extracted and analysed for their GA contents according to Stavang et al. (2007) and Olsen and Junttila (2002). This included extraction in cold methanol, addition of [17, 17-2H]GA19, [17, 17-2H]GA20, [17, 17-2H]GA29, [17, 17-2H]GA1, and [17, 17-2H]GA8 (LN Mander, Australian National University, Canberra, Australia), partition against ethyl acetate, use of QAE Sephadex A25 (Pharmacia, Uppsala, Sweden) anion-exchange columns combined with 0.5 g Sep-Pak Vac C18 cartridges (Varian, Harbor City, CA, USA), methylation, and purification on 0.1 g bond elute aminopropyl cartridges (Varian), followed by reverse-phase HPLC. Combined HPLC fractions were trimethylsilylated and subjected to combined gas chromatography–mass spectrometry in the selected ion monitoring mode. For each GA, two characteristic ions and their deuterated analogues were recorded.
Gas exchange measurements
All gas exchange measurements were performed with a portable leaf cuvette system [CIRAS-2 portable photosynthesis system attached to a Parkinson leaf cuvette (PLC6), PP systems, Hitchin, Hertfordshire, UK]. The CIRAS-2 Remote Control Software (PP systems) was used to program the parameters of the analyses and to collect and store data. In addition to showing recordings of CO2 assimilation (Pnet), cuvette temperature, and light, this software automatically calculates internal CO2 concentrations (Ci) and stomatal conductance (gS). All gas exchange measurements were performed 11–13 d after the start of the DIF treatments. Night respiration (Rnight) measurements were made on leaf pair 3, 4, and 5 (counted from the soil). Leaf 3 was fully expanded, while leaf 4 and leaf 5 (the youngest) were still expanding. Rnight measurements were performed from 23:00 to 07:30, and results were averaged. Net photosynthesis (Pnet) and day respiration (Rday) were measured from 10:00 to 20:00 on day 12, and averages of the measurements are presented. Pnet and Rnight measurements were done at ambient leaf temperature and for 6–7 plants in each case (in the light period leaf temperatures were ∼2 °C above air temperature). Pnet was measured at a photon flux density of 160 μmol m −2 s−1.
In preliminary studies, light response curves were made to establish which photon flux densities were saturating. It was then found that a photon flux density of 1000 μmol m−2 s−1 was saturating under both positive and negative DIF. This photon flux density gave only slightly (but not statistically significantly, t-test; P ≥0.05) higher CO2 assimilation than both 600 μmol m−2 s−1 and 800 μmol m−2 s−1 under negative DIF. CO2 response curves were recorded at saturating light conditions (1000 μmol m−2 s−1) at day 12 (leaf 5) and day 13 (leaf 3), following the recommendations by Long and Bernacchi (2003). Intercellular CO2 response curves (A/Ci curves) were analysed as follows (n=7). First, estimates of maximum carboxylation velocity of Rubisco, Vcmax, and day respiration rate, Rday, were obtained by fitting the following equation to the rates of CO2 assimilation, A, at low intercellular CO2 partial pressures (von Caemmerer and Farquhar, 1981):
| (1) |
Ci is the intercellular partial pressure of CO2 (here assumed to be equal to that at the sites of carboxylation), Γ* is the CO2 photocompensation point in the absence of non-photorespiratory CO2 evolution, Rday, Kc and Ko are the Michaelis–Menten constants of Rubisco for CO2 and O2, respectively, and O is the oxygen concentration. The kinetic constants for Rubisco were assumed to be equal to those determined for tobacco (von Caemmerer et al., 1994), namely 36.9 μbar for Γ* and 730 μbar for K′ at 25 °C. When calculating Vcmax and Jmax, parameter values were adjusted using the Arrhenius equation and activation energies given by de Pury and Farquhar (1997). Having derived the best fit for the lower range of Ci, the estimate of maximum electron transport rate contributing to RuBP regeneration, Jmax, was then obtained by fitting the following equation to the rates of CO2 assimilation at high intercellular CO2 partial pressures (von Caemmerer and Farquhar, 1981):
| (2) |
The relative effect of stomatal limitation on photosynthesis (S%) was estimated by the following equation (Farquhar and Sharkey, 1982):
| (3) |
where ACe represents the net photosynthesis at an ambient external CO2 concentration of 350 ppm and ACi represents the photosynthesis rate if there were no stomatal limitation to A, e.g. Ci=Ce (Farquhar and Sharkey, 1982).
Statistical analyses
The effects of the two experimental factors, DIF treatment and genotype, on measured growth parameters were analysed using a general linear model (GLM) approach in the Minitab statistical software (Minitab 15.1, Minitab Inc., PA, USA). The model used was: mean=DIF treatment+genotype+replicate+DIF treatment×genotype+replicate×DIF treatment+DIF treatment×genotype+replicate×DIF treatment×genotype. For analysis of leaf chlorophyll fluorescence, chlorophyll level, Asat, Vcmax, Jcmax, and S%, the model was: mean=DIF treatment+genotype+leaf number+DIF treatment×leaf number+DIF treatment×leaf number+genotype×leaf number+DIF treatment×genotype×leaf number. For these analyses, values from two replicate experiments were pooled. In the analysis of GA content, each leaf was analysed separately and the model was: mean=DIF treatment+genotype+DIF treatment×genotype. Tukey's test was used for testing for differences between means.
Results
Temperature, light, darkness, and gibberellin interact to determine stem elongation rate
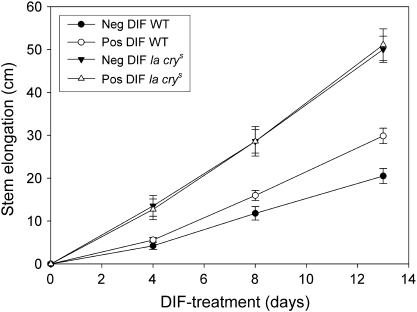
It has been shown earlier that the level of active GA1 in stem and leaf tissue of pea is down-regulated under negative DIF as compared with positive DIF (Grindal et al., 1998; Stavang et al., 2005). To investigate in more detail the influence of GAs in thermoperiodic control of stem elongation, WT pea and the saturated GA response DELLA mutant la crys (Weston et al., 2008) were both grown under negative and positive DIF for subsequent analysis of stem elongation. Negative DIF reduced the height of WT plants by >30% compared with positive DIF, whereas height of the la crys mutant was not affected by the DIF treatments within the experimental period (Fig. 1). After 13 d the la crys mutant was on average 65% and 100% higher than the WT at positive and negative DIF, respectively.
Fig. 1.
Effect of 12 d of different (DIF) day and night temperatures (DT/NT) on stem elongation of the la crys mutant and WT of pea. Seedlings were grown for 6 d at a constant temperature of 17 °C prior to the start of the DIF treatments. The temperature regimes were negative DIF (13 °C/21 °C) and positive DIF (21 °C/13 °C). Results are the mean ±SD of 10 plants.
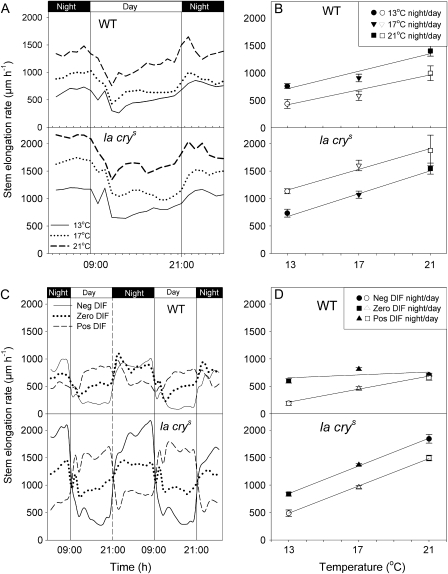
By using triangular transducer equipment it was possible to examine the influence of growth temperature on the stem elongation rate in more detail. At constant temperatures, (13 °C, 17 °C, and 21 °C), the rate of stem elongation was lower in the light period than in the dark for both genotypes (Fig. 2A), and the diurnal stem elongation rate was affected similarly by night and day cycling in the WT and la crys. From the results presented in Fig. 2A, the average stem elongation rate during the day and night for each temperature was calculated separately (Fig. 2B). For both WT and la crys plants, a linear correlation between stem elongation rate and DT and NT was observed (Fig. 2B). To study the effects of DIF on stem elongation rate, an experiment with negative, zero, and positive DIF (with all plants having the same average daily temperature) was conducted. In contrast to the stem elongation rate under constant temperature (zero DIF), the stem elongation rate under positive DIF was higher during the light period than during the night for both genotypes (Fig. 2C). Under negative DIF, the stem elongation rate of the WT was strongly inhibited during the daytime, and, importantly, the WT did not compensate for the reduced stem elongation rate during the day the following night. However, the la crys plants grown under negative DIF compensated for the reduced growth in the daytime under negative DIF by having a very high stem elongation rate the following night(s) (Figs 1, 2C). When averages of stem elongation rate in the day or night were plotted against temperature, a linear correlation was observed between stem elongation rate and DT, but not for NT in the WT. In la crys plants a linear correlation was found for both DT and NT (Fig. 2D). Since negative DIF reduces GA1 in WT plants (Grindal et al., 1998; Stavang et al., 2005), and la crys plants show a GA-saturated response, this response of the WT to negative DIF indicates that GA regulation is important for mediating a DIF response and that the stem elongation rate is determined by ambient growth temperature, light/darkness, and GA levels/GA response in an interacting manner. In conclusion, DELLAs, light, and low temperatures inhibit stem elongation, and an intact GA signalling pathway is needed to mediate the DIF effect on stem elongation.
Fig. 2.
Stem elongation rate recordings of plants grown under different temperature regimes. (A) Diurnal growth rhythms of the la crys mutant and WT pea as affected by different constant growth temperatures (13 °C/13 °C, 17 °C/17 °C, and 21 °C/21 °C DT/NT). (B) Average stem elongation rate ±SE in the light and dark of WT and la crys plants grown under constant temperatures (calculated from A) plotted against growth temperature. A regression line is fitted to the dark and light mean recordings. (C) Diurnal growth rhythms of the la crys mutant and WT pea as affected by different day and night temperature (DT/NT) combinations. The temperature regimes were negative DIF (13 °C/21 °C DT/NT), zero DIF (17 °C/17 °C), and positive DIF (21 °C/13 °C). (D) Average stem elongation rate ±SE in the light and dark of WT and la crys plants grown under negative, zero, and positive DIF (calculated from C) plotted against growth temperature. A regression line is fitted to the dark and light mean recordings. In all experiments seedlings were grown for 10 d at a constant temperature of 17 °C prior to the start of treatments. Stem elongation rate recordings show days 11–13. Results are the average of eight individual plants.
Thermoperiodic regulation of GA levels in leaves of the WT and the DELLA mutant la crys
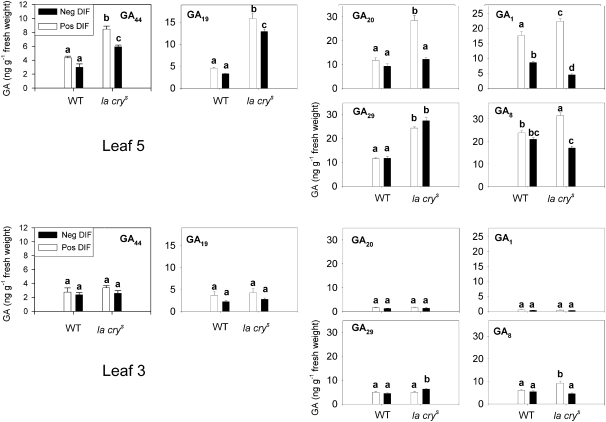
To study effects of GAs on the photosynthetic and respiratory capacity of pea, it was relevant to measure GA1 levels in the leaves in parallel, and therefore GA measurements were also included in this study. It turned out that both genotypes responded to a negative DIF treatment by down-regulating the level of GA1. The GA1 content of young leaves (leaf 5) of WT and la crys plants grown under negative DIF was reduced by 51% and 80%, respectively (Fig. 3). There was no statistically significant effect of genotype, but DIF treatment (P <0.01) and the interaction term DIF×genotype (P <0.01) were both highly significant. Thus, the data suggest that the mutant is more sensitive to the temperature regimes with respect to regulation of the GA1 level. However, due to lack of functional DELLA proteins resulting in a GA-saturated response, there was no effect of DIF on stem elongation in la crys within the time frame of the experiment (Figs 1, 2). Irrespective of this, the la crys mutant generally followed the pattern of the WT regarding the effect of DIF on the levels of other GAs, even though the levels of GA44, GA29, and GA19 were higher in the mutant. However, the level of GA20 was almost a 3-fold higher in expanding leaves of la crys plants grown at positive DIF than under negative DIF, whereas in the WT there was no significant difference between the DIF treatments (Fig. 3). In fully expanded leaves (leaf 3) GA1 levels were low in both genotypes. This might at least partly be due to high inactivation rates, since GA1/GA8 and GA20/GA29 ratios were increased as compared with those in younger leaves (see also Ross et al., 2003). Also, there were no significant differences between WT and la crys plants or between DIF treatments in the expanded leaves.
Fig. 3.
GA levels in young expanding (leaf 5) and expanded (leaf 3) leaves of WT and the la crys mutant of pea seedlings after 12 d of growth under negative (13 °C/21 °C DT/NT) or positive DIF (21 °C/13 °C DT/NT). Presented are the mean of 3–4 independent samples, where each sample contained leaves from 20 plants, ±SE. Different letters denote significantly different values.
Non-functional DELLA proteins restrain changes in dry matter allocation in response to alternating diurnal temperatures
To study the effects of the DIF treatments on growth and biomass allocation in WT and la crys plants, the dry weights of several tissues were measured at the end of the experimental period. The la crys seeds were heavier than WT seeds [la crys, 0.331±0.015 (mean±SE) g seed−1, n=10; and WT, 0.247±0.007 g seed−1, n=10]. Total dry weight accumulation (dry weight of shoot and root tissue–seed weight) of WT plants grown under negative DIF was reduced from 0.317 g plant−1 to 0.215 g plant−1 as compared with plants grown at positive DIF (P <0.01). Stem was the tissue most affected by negative DIF in the WT, with a reduction in dry weight of 34% (P <0.01). Negative DIF reduced leaf dry weight by 16% (P <0.01) (Table 1). Leaf area was also significantly reduced under negative DIF (9%; P=0.05), but less than dry weight. Accordingly specific leaf area (SLA; projected leaf area per unit leaf dry mass) increased under negative DIF, from 615 cm2 g−1 to 670 cm2 g−1 (P <0.01), indicating that the leaves got thinner or became less dense under negative DIF. Root dry weight was reduced by 5% in the WT (not significant). In la crys, dry weight accumulation was reduced from 0.203 g plant−1 to 0.121 g plant−1 (P <0.01). In contrast to the WT where stem was the tissue most affected by negative DIF, the reduction of dry weight in la crys was quite similar for all tissue types, ∼14–17%. The roots of the la crys mutant developed poorly as compared with the WT, and even with the remnants of the seeds included, the dry weight of the roots of la crys was significantly lower than that of the WT (on average 27% less root dry weight, P <0.01; Table 1). la crys had 6% lower leaf area than the WT (P <0.01). However, the mutant was similar to the WT regarding SLA, and negative DIF increased SLA by ∼10% in both genotypes. Thus, with no significant difference between the genotypes regarding SLA, this indicates that GA signalling through DELLAs does not affect SLA.
Table 1.
Dry weight (g) parameters, leaf area (cm2), and specific leaf area (cm2 g−1) of WT and la crys mutant pea seedlings after 20 d of growth, of which the last 14 d were under negative (13 °C/21 °C DT/NT) and positive DIF (21 °/13 °C DT/NT)
| Treatment and genotype | Leaf dry weight (g) | Stem dry weight (g) | Root dry weight (g) | Leaf area (cm−2) | Specific leaf area (cm−2 g−1) |
| Positive DIF WT | 0.229±0.012 | 0.165±0.009 | 0.170±0.008 | 139±5 | 615±21 |
| Negative DIF WT | 0.191±0.011 | 0.109±0.006 | 0.161±0.009 | 127±5 | 670±21 |
| Positive DIF la crys | 0.184±0.008 | 0.221±0.006 | 0.129±0.009 | 114±4 | 624±12 |
| Negative DIF la crys | 0.159±0.008 | 0.182±0.003 | 0.111±0.007 | 110±5 | 696±17 |
| Statistical analysis (ANOVA: GLM) | |||||
| DIF treatment | 0.000 | 0.000 | 0.001 | 0.02 | 0.000 |
| Genotype | 0.000 | 0.000 | 0.000 | 0.000 | NS |
| Treatment×genotype | NS | NS | NS | NS | NS |
Presented are means ±SE with n=19–20. Data on significant effects of replicates are not shown.
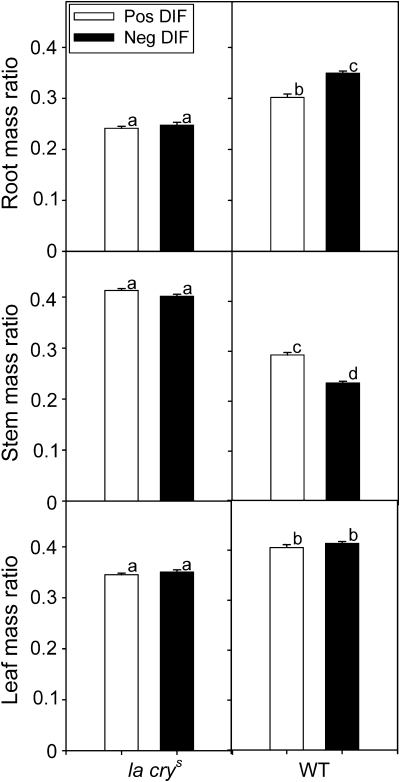
In order to get a picture of how a negative DIF treatment affected plant architecture, stem mass ratio (SMR; stem mass per unit plant mass), root mass ratio (RMR; root mass per unit plant mass), and leaf mass ratio (LMR; leaf mass per unit plant mass) were calculated. On average, the la crys mutant had a significantly higher SMR, and lower RMR and LMR than the WT under both positive and negative DIF (P <0.01; Fig. 4), reflecting the higher amount of stem tissue, and less root and leaf tissue in the mutant as compared with the WT (Table 1). In the WT, a negative DIF treatment resulted in a reduction of SMR from 0.29 to 0.24 (P <0.01) and an increase in the RMR from 0.30 to 0.34 (P <0.01). There was also a less pronounced effect of DIF on LMR in the WT, with a reduction from 0.421 to 0.405 (P=0.01). In contrast to the WT, la crys did not show any changes in RMR, LMR, or SMR. Taken together, these data indicate that DIF regulation of GA levels contributes to mediate these changes in dry matter allocation, while a saturated GA response to a large extent prevents these changes (Figs 3, 4, and Table 1).
Fig. 4.
Morphological responses to negative and positive DIF. Leaf mass ratio is the leaf mass per unit plant mass in WT and la crys mutant pea seedlings after 20 d of growth. Root mass ratio is the root mass per unit plant mass. Stem mass ratio is the shoot mass per unit plant mass. DIF treatments were applied for the last 14 d. Positive DIF was 21 °C/13 °C. Negative DIF was 13 °C/21 °C. Results are the mean ±SE of 20 plants. Different letters denote significantly different values.
There was a tendency for la crys plants to develop more leaves than the WT in the time frame of the experiment. After 12 d of DIF treatment (and 18 d of total growth), WT plants had unfolded leaf 5 while la crys plants were unfolding leaf 6. When grown for 1 month, la crys developed 15.5 leaf pairs as compared with 12.6 leaf pairs for the WT (G Grindal Patil, unpublished results), demonstrating that DELLA proteins affect the leaf unfolding rate.
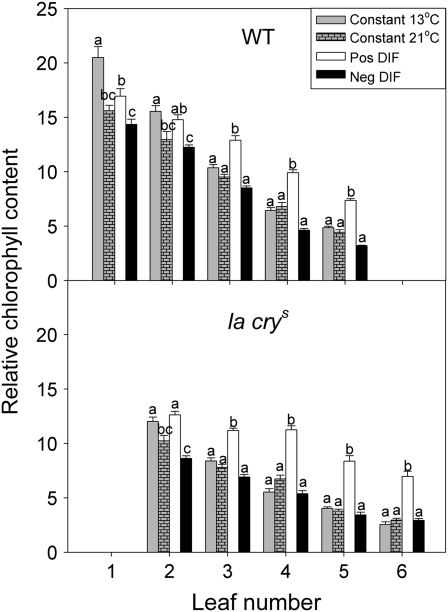
Negative DIF reduces chlorophyll content in both the WT and the DELLA mutant
Plants grown at negative DIF are reported to be paler green due to lower chlorophyll content than plants grown at positive DIF (Moe and Heins, 2000), and this was also found in our experiment (Fig. 5). In the youngest leaves there was more than a 50% reduction of relative chlorophyll content in both genotypes in plants grown at negative DIF compared with plants grown at positive DIF, while the reduction in older leaves was less pronounced. Furthermore, in young leaves of la crys and the WT there were no or only small differences in relative chlorophyll content, while the oldest leaves of the WT contained more chlorophyll than those of la crys. In order to try to separate the effects of different day and night temperatures from the DIF effect, plants grown at constant 13 C or 21 °C were included in the experiment. WT plants grown under constant temperatures contained intermediate levels of chlorophyll as compared with plants grown under positive and negative DIF, but clearly age was a factor as the oldest leaves of plants grown under constant 13 °C had the highest chlorophyll content (Fig. 5). We analysed the genotypes separately and, for both genotypes, the main factors and the interaction factor were significant (temperature treatment, P <0.0001; leaf number, P <0.0001; temperature treatment and leaf, P <0.01 for both genotypes).
Fig. 5.
Effect of constant day and night temperatures (13 °C/13 °C and 21 °C/21 °C DT/NT) and of positive and negative DIF (21 °C/13 °C and 13 °C/21 °C DT/NT) on chlorophyll content of leaves of WT and the la crys mutant of pea. Leaf number 1, which represents the oldest leaf, the epicotyledons, were too small to be measured in la crys. Results are the mean ±SE of 10 plants. Different letters denote a significant difference.
Negative DIF reduces Fv/Fm and electron transport rate in young, expanding leaves
In order to study in more detail the effect of a negative DIF treatment and impaired GA signalling on photochemistry of the leaves, a complete chlorophyll fluorescence quenching analysis by the saturation pulse method was performed in dark-adapted leaves (Table 2). A GLM analysis revealed that negative DIF slightly reduced Fv/Fm, especially in expanding leaves [significant interaction between leaf and DIF treatment (P <0.01)]. For the expanding leaves, Fv/Fm was slightly higher in the mutant leaves than in the WT, whereas there was almost no difference for the expanded leaves [significant interaction between leaf and genotype (P <0.01)]. However, as mentioned above, there was a slight difference in developmental stage between the mutant and WT, and this might have had an effect on the measurements. The ETR at 210 μmol m−2 s−1 was higher in expanded leaves than in young leaves. This effect was greater for the WT than for la crys [significant interaction between leaf and genotype (P <0.01)]. ETR was also higher at positive DIF for young leaves, whereas there was no difference for expanded leaves [significant interaction between leaf and DIF treatment (P <0.01)]. The expanding leaves had higher NPQ than the fully expanded leaves (P=0.02). This higher capacity to dissipate excess energy may be an acclimation to higher irradiance because they are closer to the fluorescent tubes, or it may be a result of a lower photosynthetic capacity in the youngest leaves (Fig. 6). There were only small differences in the proportion of open PSII centres (Qp) (Table 2).
Table 2.
Chlorophyll fluorescence parameters of young expanding and fully expanded leaves of WT and la crys mutant of 18-day-old pea seedlings grown under negative (13 °C/21 °C DT/NT) and positive DIF (21 °C/13 °C DT/NT)
| Treatment/leaf/genotype | Fv/Fm | ETR (μmol e– m−2 s−1) | NPQ | Qp |
| Positive DIF/leaf 5/WT | 0.801±0.003 | 53.0±2.2 | 1.16±0.13 | 0.807±0.010 |
| Negative DIF/leaf 5/WT | 0.776±0.002 | 50.4±1.4 | 1.00±0.06 | 0.789±0.011 |
| Positive DIF/leaf 3/WT | 0.829±0.001 | 58.2±0.8 | 0.87±0.03 | 0.789±0.010 |
| Negative DIF/leaf 3/WT | 0.820±0.002 | 62.2±1.0 | 0.66±0.03 | 0.826±0.090 |
| Positive DIF/leaf 5/la crys | 0.821±0.001 | 60.5±0.4 | 0.86±0.98 | 0.832±0.002 |
| Negative DIF/leaf 5/la crys | 0.795±0.006 | 53.1±1.3 | 0.98±0.03 | 0.787±0.010 |
| Positive DIF/leaf 3/la crys | 0.834±0.001 | 58.1±1.5 | 1.06±0.08 | 0.792±0.011 |
| Negative DIF/leaf 3/la crys | 0.827±0.001 | 57.2±0.9 | 0.96±0.05 | 0.797±0.008 |
| Statistical analysis (ANOVA: GLM) | ||||
| DIF treatment | 0.000 | NS | NS | NS |
| Genotype | 0.000 | NS | NS | NS |
| Leaf | 0.000 | 0.000 | 0.017 | NS |
| DIF×genotype | NS | 0.010 | 0.039 | 0.027 |
| DIF×leaf | 0.000 | 0.001 | NS | 0.000 |
| Genotype×leaf | 0.001 | 0.000 | 0.000 | NS |
| Genotype×leaf×DIF treatment | NS | NS | NS | NS |
Fv/Fm represent maximal dark-adapted PSII efficiency, ETR is the electron transport rate through PSII at 210 μmol m−2 s−1 irradiance, NPQ is non-photochemical quenching, and Qp is the proportion of open PSII centres. Presented are means ±SE with n=7.
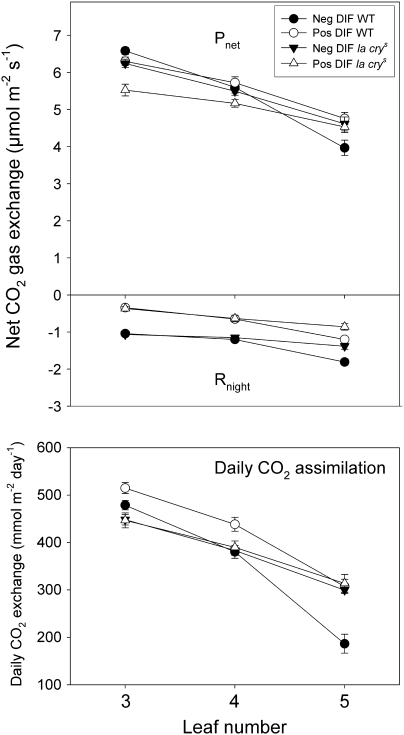
Fig. 6.
Gas exchange under ambient growth temperatures in leaves of WT and the la crys mutant of pea grown under positive and negative DIF. The upper diagram is night respiration (Rnight), the middle diagram is net photosynthesis (Pnet), and the lowest diagram is diurnal net assimilation of CO2 (diurnal CO2 assimilation= Pnet–Rnight). The temperature regimes were: negative DIF 13 °C/21 °C day temperature/night temperature and positive DIF 21 °C/13 °C. Each value is the mean ±SE of six measurements. The measurements were done at ambient leaf temperature (15 °C under negative DIF and 23 °C under positive DIF). Measurements were done on days 12–13 after the start of the DIF treatments.
Impaired DELLA signalling does not affect photosynthetic capacity or respiration
To try to explain the observed growth/biomass responses of pea plants to the DIF treatments, and to evaluate the role of GA signalling through DELLA on photosynthetic capacity, net CO2 gas exchange was measured in young, expanding (leaf 5 and 4) and fully expanded leaves (leaf 3), during a 24 h night/day cycle using a portable leaf cuvette gas exchange system set to ambient growth conditions.
Younger leaves had significantly higher night respiration (Rnight) than older leaves in both genotypes, and plants grown at negative DIF had higher Rnight than plants grown at positive DIF (Fig. 6). The effect of DIF on Rnight was most pronounced in expanded leaves (leaf 3) where the relative difference was 3-fold, with Rnight values of ∼0.35 μmol m−2 s−1 and 1.05 μmol m−2 s−1 under negative and positive DIF, respectively, for both genotypes. In expanding leaves of WT plants (leaf 5), Rnight was 1.2 μmol m−2 s−1 and 1.8 μmol m−2 s−1 under negative DIF and positive DIF, respectively. Corresponding Rnight values for la crys were 0.9 μmol m−2 s−1 and 1.4 μmol m−2 s−1. There was no significant difference in Rnight between leaves of WT and la crys, except for the youngest leaf (leaf 5). The difference in CO2 exchange for these young leaves is most probably due to the fact that leaf number 5 in la crys had developed further than leaf number 5 of the WT, and probably releases less CO2 related to growth respiration. The temperature response Rnight was also tested in leaves from plants grown under negative and positive DIF, and, as expected, Rnight increased almost 2-fold from 13 °C to 21 °C (Supplementary Fig. S1 aavaailable at JXB online). Thus, temperature and developmental state determines Rnight, while a saturated GA response has no effect on Rnight.
Net photosynthesis (Pnet) in the daytime was measured at ambient leaf temperature (2 °C higher than air temperature). In both genotypes, young leaves had significantly lower Pnet than older leaves, and thus followed the opposite pattern to Rnight (Fig. 6). Pnet of the WT was similar at negative and positive DIF for leaf 3 and 4, but was reduced in leaf 5 at negative DIF. la crys behaved differently from the WT, with less Pnet under positive than negative DIF for leaf 3, but with similar Pnet under negative and positive DIF in leaf 4 and 5. Differences in Pnet between leaves could not be explained by differences in gs (data not shown).
Daily CO2 assimilation (Pnet–Rnight) was higher in expanded leaves than in young, expanding leaves of both genotypes (Fig. 6). In the WT, daily CO2 assimilation was significantly higher in leaves grown at positive DIF compared with negative DIF. The difference was most pronounced in the youngest leaves. A negative DIF treatment reduced diurnal CO2 assimilation by 39% in leaf 5, 13% in leaf 4, and 8% in leaf 3. In contrast to the WT, there was no difference in diurnal net CO2 assimilation of the la crys mutant grown at positive DIF or negative DIF.
In order to get an indication of the effect of the DIF treatments on true photosynthesis (P; P=Pnet+Rday), the rate of day respiration (Rday) was measured using two different methods. The first method involved reducing the light intensity from ambient light level to a photon flux density of 1–2 μmol photons m−2 s−1 PAR for 2 min and then recording Rday. The second method involved fitting Equation 1 (see Materials and methods) for the net CO2 exchange versus internal CO2 partial pressure over the low partial pressure range according to von Caemmerer and Farquhar (1981). The absolute values of Rday depended on which method was used (data not shown). Despite variation in absolute values in the data sets, both methods significantly indicated that Rday was 1.2–2.0 times higher in leaves at positive DIF compared with corresponding leaves at negative DIF in both genotypes. Thus an attempt to calculate P revealed a significantly higher true photosynthesis in plants grown at positive DIF, with the largest difference in expanding leaves (data not shown). This suggests that 8 °C lower day temperature under negative DIF might reduce photosynthetic performance. This is further supported by the finding that at saturating light and CO2 conditions, Pnet is positively correlated with temperature and increases by almost 1.5-fold from 15 °C to 23 °C (Supplementary Fig. S2 at JXB online).
Respiration in leaves (Rday or Rnight) correlated with growth (as measured by stem elongation rate; Fig. 2C) and ambient temperature. It was also found that there was a strong positive correlation between temperature and Rnight (Supplementary Fig. S2 at JXB online). The response of Rnight to a temperature change was rapid and occurred within a few minutes (data not shown). Stavang et al. (2007) showed that stem elongation responds within minutes to a drop in temperature, and thus growth rate, temperature, and respiration are closely correlated.
Initial studies on the WT indicated the following: (i) as maximum photosynthetic performance is temperature dependent (Supplementary Fig. S2 at JXB online), it is crucial to measure photosynthetic performance at the same measurement temperature; and (ii) young expanding leaves of plants grown under negative DIF performed less well than corresponding leaves developing under positive DIF under saturated light and CO2 conditions (Supplementary Fig. S3). Therefore, further measurements were done at the same measurement temperature (23 °C) on fully expanded (leaf 3) and young, expanding leaves (leaf 5).
To study further the kinetics of photosynthetic capacity of both genotypes and to compare leaves developed under negative or positive DIF conditions, CO2 response curves (10–1000 ppb) at saturating light conditions (1000 μmol m−2 s−1) were recorded for expanded (leaf 3) and young, expanding leaves (leaf 5) at the same measurement temperature (23 °C). From the A/Ci curves, the maximum carboxylation velocity of Rubisco (Vcmax) and the maximum electron rate contributing to RuBP regeneration (Jmax) were estimated as described by von Caemmerer and Farquhar (1981).
Expanding leaves had lower Vcmax values than fully expanded leaves (Table 3). Negative DIF reduced Vcmax in both expanding and fully expanded leaves, but the reduction was more pronounced in expanding leaves (significant interaction for DIF×leaf number; P=0.04). However, this difference was significant only for young leaves of la crys. There was no effect of genotype on Vcmax. Whether the leaves were expanded or not accounted for most of the variation regarding Jmax, with expanding leaves having lower values than expanded leaves. A negative DIF treatment reduced the Jmax of expanding leaves even more in both genotypes (significant interaction between DIF treatment and leaf number). There was quite a good correlation between electron transport measured with chlorophyll fluorescence (ETR: Table 2) and Jmax estimated from gas analysis measurements (r2=0.72). With la crys being GA saturated, it appears that GA signalling through DELLA cannot be the cause of the reduction of Jmax and Vcmax observed in expanding leaves under negative DIF.
Table 3.
Photosynthetic parameters of leaves of 18-day-old WT and la crys mutant pea seedlings grown under negative (DT13 °C/NT21 °C) and positive DIF (DT13 °C/NT21 °C)
| Treatment/leaf/genotype | Vcmax (μmol CO2 m−2 s−1) | Jmax (μmol e– m−2 s−1) | S% | Asat (μmol CO2 m−2 s−1) |
| Positive DIF/leaf 5/WT | 56.3±1.3 | 102.4±1.5 | 30.2±2.0 | 20.5±0.3 |
| Negative DIF/leaf 5/WT | 49.9±2.8 | 87.0±2.9 | 29.3±2.5 | 16.6±0.6 |
| Positive DIF/leaf 3/WT | 74.9±2.3 | 125.0±2.6 | 29.4±1.0 | 25.3±0.6 |
| Negative DIF/leaf 3/WT | 72.5±1.6 | 129.8±1.3 | 38.3±1.9 | 26.0±0.3 |
| Positive DIF/leaf 5/la crys | 64.5±2.3 | 108.5±2.1 | 31.3±1.8 | 20.9±0.6 |
| Negative DIF/leaf 5/la crys | 46.3±2.1 | 86.1±2.8 | 28.2±2.5 | 17.4±0.7 |
| Positive DIF/leaf 3/la crys | 70.5±3.1 | 113.0±3.0 | 34.5±2.1 | 21.7±0.5 |
| Negative DIF/leaf 3/la crys | 63.4±3.3 | 118.3±3.0 | 41.1±3.0 | 24.4±0.6 |
| Statistical analysis (ANOVA: GLM) | ||||
| DIF | 0.000 | 0.000 | 0.04 | 0.03 |
| Genotype | NS | 0.01 | NS | 0.03 |
| Leaf | 0.000 | 0.000 | 0.000 | 0.000 |
| DIF×genotype | 0.03 | NS | NS | NS |
| DIF×leaf | 0.04 | 0.000 | 0.005 | 0.000 |
| Genotype×leaf | 0.02 | 0.000 | NS | 0.000 |
| DIF×genotype×leaf | NS | NS | NS | NS |
Leaf 3 represents a fully expanded leaf and leaf 5 had just been unfolded and was expanding. Maximum carboxylation velocity of Rubisco, Vcmax, maximum electron transport rate contributing to RuBP regeneration, Jmax, and relative effect of stomatal limitation of photosynthesis, S%, were calculated from A/Ci curves performed at 23 °C, and as described by von Caemmerer and Farquhar (1981) and Farquhar and Sharkey (1982). Asat is maximum CO2 assimilation at an irradiance of 1000 μmol m−2 s−1 and CO2 of 1000 ppb. Presented are means ±SE with n=6–7.
Calculations of stomatal limitation from the CO2 response curves revealed that the WT and la crys behaved similarly regarding S% (Table 3). Under positive DIF there were no significant differences between expanding and fully expanded leaves regarding S% in either the WT or la crys. However, under negative DIF, S% was higher in expanded leaves of both genotypes [even though the difference was not statistically significant for the WT (P=0.10)]. Asat values were also calculated from the CO2 response curves, and the highest numbers were found in expanded leaves under negative DIF in both the WT and la crys, even though stomatal limitation was higher in these leaves. In expanding leaves Asat was significantly reduced as compared with expanded leaves. Furthermore, Asat of young expanding leaves grown under negative DIF was significantly lower than Asat of expanding leaves in plants grown under positive DIF.
In conclusion, negative DIF decreased the photosynthetic capacity of young expanding pea leaves. However, based on the observation that la crys behaves as the WT regarding respiration and photosynthetic capacity of pea, GA signalling through DELLA appears to have no or only a minor role in determining these capacities.
Discussion
WT pea plants grown under negative DIF contain less active GA1 in developing stem and leaf tissue than WT plants grown at positive DIF (Fig. 3; Grindal et al., 1998; Stavang et al., 2005). There are reports indicating that a negative DIF treatment reduces photosynthesis (Berghage et al., 1990; Agrawal et al., 1993) and also recent studies report a potential connection between GA levels and photosynthesis (Biemelt et al., 2004; Huerta et al., 2008). The purpose of this study was to separate thermoperiodic growth responses mediated by regulation of GA levels from those independent of GA signalling through DELLAs, and thereby also to investigate a potential connection between GA, growth, and photosynthetic and respiratory capacity in pea.
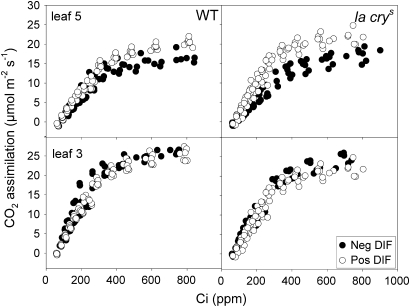
Expanding leaves of WT pea grown under negative DIF had a significantly lower photosynthetic capacity measured as both net CO2 uptake (Fig. 7) and ETR (Table 2) than expanding leaves of plants grown under positive DIF, where GA1 levels were 60% higher (Fig. 3). Thus, at first glance there appeared to be a correlation between GA1 levels and photosynthetic capacity. This correlation was still valid in fully expanded leaves; there was no significant difference in GA1 levels between DIF treatments and no significant difference regarding photosynthetic capacity, even though expanded leaves of plants grown under negative DIF tended to have a slightly higher photosynthetic capacity than expanded leaves of plants grown under positive DIF (ETR, Table 2, Fig. 7; and Jmax and Vcmax, Table 3). However, a correlation between photosynthetic performance and GA1 signalling through DELLAs falls apart when the double DELLA mutant la crys is taken into consideration. Being almost twice as tall as the WT and growing as if saturated with GA independently of temperature treatment (Fig. 1) and GA1 levels (Fig. 3), no effect was found of non-functional DELLAs on photosynthetic parameters such as ETR (Table 2) and Asat, Jmax, and Vcmax (Fig 7, Table 3). Furthermore, la crys was affected by the DIF treatments in a similar way to the WT regarding photosynthetic capacity, indicating that regulation of these processes is independent of GA signalling through DELLAs. Based on these observations, it is unlikely that GA is a main determinant of a plant's photosynthetic capacity on a leaf area basis, as could be expected from earlier results in GA application studies (Khan, 1996; Hayat et al., 2001; Yuan and Xu, 2001). Thus, the present results are in line with work performed on tomato, where it was concluded that the rate of photosynthesis per unit leaf area was similar for GA-deficient plants and WT plants (Nagel and Lambers, 2002). Interestingly, in transgenic tobacco (Nicotiana tabacum), light-saturated photosynthesis per leaf area was actually higher in transgenic plants with low GA content as compared with the WT and plants with a high content of GA (Biemelt et al., 2004). This is comparable with the finding that application of paclobutrazol, a GA biosynthesis inhibitor, to Catharanthus roseus plants enhanced photosynthesis (Jaleel et al., 2007). The existence of GA signalling pathways other than through the DELLA pathway cannot yet be excluded (see for instance Maymon et al., 2009) but, based on the published and contradictory reports, it seems unlikely that there exists a simple control of photosynthetic capacity by GAs. Furthermore, no effect of a saturated GA signalling response on respiration was found, and Rnight of the DELLA mutant la crys was identical to that of the WT. In addition Rnight of the mutant and WT was affected similarly by different night temperatures (Fig. 6).
Fig. 7.
CO2 assimilation/internal CO2 (A/Ci) curves of plants of WT and the la crys mutant of pea, grown under positive and negative DIF. Before the measurements the plants were grown at negative DIF (13 °C/21 °C DT/NT) and positive DIF (21 °C/13 °C) for 12 d. The measurements were performed at a leaf temperature of 23 °C for both DIF treatments, and 6–7 measurements are plotted for each leaf and DIF treatment.
The present study, as well as earlier studies, has shown that a negative DIF treatment reduces the levels of chlorophyll as compared with a positive DIF treatment (Fig. 4; Erwin and Heins, 1995; Grindal, 1997; Vaagen et al., 2003). The negative DIF treatment reduced chlorophyll content in both genotypes and in all leaves of the pea plants. It is reported that chlorophyll content increases from a negative to a positive DIF (Erwin and Heinz, 1995). This observation supports a very simple hypothesis; low temperature in DT inhibits production of chlorophyll, while high NT stimulates degradation processes of chlorophyll. Thus, such a shift in equilibrium could explain the connection between DIF and chlorophyll content of plants grown under different DIF regimes. If this is the case, plants grown at constant temperatures should contain intermediate amounts of chlorophyll. The data from this experiment partly support such a hypothesis. However, it is also possible that a negative DIF temperature regime (the opposite of common temperature oscillations in nature) might disturb or delay the synchronizing of chlorophyll- and/or chlorophyll-binding protein (CAB) production, resulting in reduced chlorophyll accumulation under negative DIF. It has been shown that temperature alterations influence the oscillation patterns of CAB mRNAs (Piechulla and Riesselmann, 1990). The chlorophyll content was very low in the shoot tip (Fig. 5), and it could be that a reduced amount of chlorophyll under negative DIF is part of the reason for reduced ETR and Jmax. However, a negative DIF treatment only slightly reduced the maximal quantum efficiency (Fv/Fm) of PSII (Table 2). Also, even though the amount of chlorophyll was reduced in expanded leaves of WT plants grown under negative DIF, these leaves tended to have a higher photosynthetic capacity than corresponding expanded leaves of WT plants grown under positive DIF (Fig. 7, Tables 2, 3). Thus, at least at this stage of development, a lower chlorophyll level has no effect on photosynthetic capacity or quantum efficiency of PSII. A change from negative to positive DIF makes the leaves green within a few day/night cycles (Moe and Heins, 2000), and chlorosis as a result of negative DIF does not represent a practical problem in commercial pot and bedding plant production.
The reason for reduced dry weight accumulation in WT plants grown at negative DIF in this experiment does seem to be partly an effect of reduced area of photosynthetic tissue (leaf and stem), especially leaf area (Table 2), and partly an effect of ambient temperature on plant photosynthesis. The reduction of leaf area under negative DIF in the WT is probably related to the down-regulation of GA1 levels, as there was no significant reduction of leaf area in la crys. The negative DIF treatment in our study resulted in 8 °C cooler temperatures in the photoperiod and 8 °C higher temperatures in the dark period as compared with the positive DIF temperature regime. This resulted in significantly higher Rnight under negative DIF (Fig. 6). The temperature responsiveness of Rnight in leaves from plants grown under negative and positive DIF was tested and, as expected, Rnight increased with temperature (Supplementary Fig. S1 at JXB online). Photosynthesis is temperature dependent as well (Hikosaka et al., 2006), and the temperature response of maximum photosynthetic capacity from 13 °C to 21 °C demonstrated clearly that maximum photosynthetic capacity increased with temperature (Supplementary Fig. S2). However, this does not need to be the case at ambient CO2 concentrations and light levels where photorespiration will also influence the temperature response of photosynthesis.
Pnet was quite similar in WT plants under positive and negative DIF. However, attempts to measure Rday indicated that Rday was affected by temperature similarly to Rnight. Consequently, since Rday is lower under negative DIF, so also is true photosynthesis. True photosynthesis thus depends on the magnitude of Rday, and different methods gave different answers, but this suggest that true photosynthesis is higher under positive DIF. Probably, part of the Rday and Rnight is related to growth, and thus a correlation with growth as measured by stem elongation and temperature was expected. Indeed, plants under positive DIF grow more during daytime than plants under negative DIF (Fig. 2). Rnight subtracted from Pnet also resulted in a reduced average CO2 accumulation on a daily basis under negative DIF (Fig. 6).
Dry weight accumulation in the la crys mutant was only half of that of the WT (Table 1). One of the main reasons for this is probably that the two first leaf pairs developed were very small, often malformed, and most probably did not contribute much to a positive net CO2 assimilation on a whole-plant basis. Therefore, growth during the first 8–10 d for the mutant probably occurred very much at the expense of stored reserves in the seed. The third, fourth, fifth, and sixth leaf pair developed properly, but total leaf area (Table 1) was lower in la crys despite more rapid leaf unfolding. Due to less root development in the la crys mutant, one should expect that it had less root respiration than the WT, and that further growth might improve dry weight accumulation compared with the WT, if there were free access to nutrients. Higher specific respiratory costs for stem growth in la crys could be expected if the stem biomass of the mutants contained a larger fraction of compounds such as lipids or lignin, for which biosynthesis is highly energy demanding, compared with the WT. If so, higher energy costs for stem growth could contribute to the low dry weight accumulation in the la crys mutant in this study. Supporting such an idea is the finding that tobacco plants overexpressing a GA biosynthesis gene increased their content of lignin in stem tissue (Biemelt et al., 2004). There was no difference in diurnal net assimilation rate between negative and positive DIF treatments in the la crys mutant (Fig. 4), and in this regard it differed from the WT.
In the present study, reduced levels of active GA in expanding shoot tissue in the WT caused by a negative DIF treatment reduced dry matter accumulation in stem tissue more than in root and leaves (Table 1). As a consequence, the SMR decreased while the RMR increased (Table 2). DIF did not affect RMR or LMR and only slightly reduced SMR in the la crys mutant. The root growth of la crys was limited as compared with the WT, and the shoot/root ratio was significantly higher. A reduction in the relative growth rate of GA-deficient tomato plants was mainly caused by reduced investments of carbon in stem and leaf tissues, and higher allocation of carbon to root growth and root respiration (Nagel et al., 2001). Thus, it appears that regulation of active GA in shoot tissue plays a major role in controlling root growth by controlling how much of the totally assimilated carbon in the shoot should be allocated to growth of stem tissue. Growth of root tissue in transgenic Populus with high GA content was reduced compared with the WT, and this caused problems with rooting of the transplants (Eriksson et al., 2000). However, root growth improved at later stages.
Stem elongation is largely controlled by GAs, and there is a log linear relationship between stem elongation and the amount of GA1 (Ross et al., 1989; Weller et al., 1994; Grindal et al., 1998). Our study shows that la crys grown at negative DIF can compensate for reduced stem elongation rate in the daytime in the night, and thus DIF did not affect stem elongation of the mutant within the experimental period (Figs 1, 2C). However, the WT did not compensate for reduced stem growth in the daytime during the night when grown at negative DIF, and this is probably due to a direct effect of a down-regulation of GA1 levels in developing tissue. A saturated GA response in la crys thus makes the mutant unable to respond to DIF treatments, at least in a short-term experiment, and further supports the conclusions that GA is an important mediator of thermoperiodic stem elongation (Grindal et al., 1998; Stavang et al., 2005). The inhibition of the stem elongation rate by light (Fig. 2) in both the WT and la crys is likely to be mediated by phyB (Weller et al., 1994), phytochrome-interacting factors (Nozue et al., 2007), and circadian clock regulation (Michael et al., 2008).
Even if the DELLA proteins are non-functional in la crys, GA1 levels drop in response to a negative DIF treatment as they do in the WT (Fig 3), indicating that the DELLAs are not involved in the temperature regulation of GA1 levels by feedback mechanisms. High levels of the GA precursors GA44, GA20, and GA19, as well as high levels of the inactivate GA29, might indicate that GA 3-oxidation is inhibited in la crys (Fig. 3). This is probably a sign of feedback regulation of GA biosynthesis, and Weston et al. (2008) showed that PsGA20ox1, PsGA3ox1, and PsGA3ox2 were down-regulated and that PsGA2ox1 and PsGA2ox2 were up-regulated in the whole shoot tissue of the la crys mutant. These expression patterns indicate that GA1 levels are low in la crys. However, the measurements of GA1 in leaves revealed that there is no difference between la crys and the WT (Fig. 3). This apparent discrepancy might be explained by tissue-specific GA regulation in pea. Such an explanation is supported by the fact that even though it is GA saturated, la crys has smaller leaves than the WT.
A negative DIF treatment reduced the level of GA19 and increased the level of GA29 in la crys. Also the ratio of GA1/GA8 increased, indicating that temperature-mediated GA inactivation resulting from increased transcript levels of GA2ox1 and GA2ox2 observed in WT pea under negative DIF also is functional in la crys (Stavang et al., 2005). This regulation of GA1, however, is of no use for the mutant, as a typical DIF response on stem elongation is absent within the time frame of the experiment. Given that GAs act through DELLAs only, this suggests that GA is not involved in determining the photosynthetic and respiratory capacity of pea, but affects plant architecture only. However, the role of GA in leaf development is not as clear as in the stem, and other pathways may exist. In an interesting study by Huerta et al. (2008), a transgenic line of citrus with elevated levels of GAs was found to have a higher photosynthetic capacity, stomatal conductance, and expression of genes related to photosynthesis than control plants. However, when GA was applied in a short-term experiment, no such positive effect on genes related to photosynthesis was observed, indicating that structural and architectural changes (more light entering into the canopy, etc.) mediated by GAs indirectly can result in increased photosynthetic performance and growth. Support for such a hypothesis is the finding that tall transgenic Populus with elevated GA levels had a higher RGR than control plants (Eriksson et al., 2000).
Thermoperiodic growth involves regulation of GA1 levels by GA 2-oxidation in pea (Grindal et al., 1998; Stavang et al., 2005), but other plant hormones might contribute as well. For instance, high temperature-stimulated hypocotyl elongation in Arabidopsis seedlings is under hormonal control and involves GA, auxin, and brassinosteroids as well as the PIF4 protein that interacts with phytochrome (Stavang et al., 2009). Whether similar mechanisms are acting in pea remains to be investigated.
The present study shows that the effect of negative DIF on plant biomass production in WT pea is a result of the net effect of reduced growth and photosynthesis during daytime, increased respiration during the night and altered plant morphology. A saturated GA response in the la crys mutant neither resulted in increased photosynthetic capacity, as measured on a leaf area basis, nor increased dry weight accumulation. Instead the saturated GA response mutant allocated more dry matter to stem tissue and less to root and leaf tissue. Furthermore, it did not respond to DIF with changes in biomass allocations or changes in stem elongation as did the WT. In conclusion, higher GA levels or a saturated GA response do not appear to be major determinants of photosynthetic capacity on a leaf area basis in pea, and the primary effect of different GA levels appears to be a change in plant morphology.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Responsiveness of Rnight to temperature of young expanding (leaf 5) and fully expanded (leaf 3) leaves of plants grown under negative or positive temperature. The 95% confidence intervals were calculated by Sigmaplot 11.0.
Figure S2. Responsiveness of light- and CO2-saturated photosynthesis of young expanding (leaf 5) and fully expanded (leaf 3) leaves of plants grown under negative or positive temperature. The 95% confidence intervals were calculated by Sigmaplot 11.0.
Figure S3. Light- and CO2-saturated photosynthesis of young (leaf 4–5) and fully expanded (leaf 2–3) leaves of WT pea measured under ambient growth temperatures in plants grown under negative (13 °C/21 °C DT/NT) and positive DIF (21 °C/13 °C DT/NT).
Supplementary Material
Acknowledgments
We wish to thank the reviewers for constructive comments that have improved the manuscript. We wish to thank Marit Siira at the Norwegian University of Life Sciences, and Rigmor Reiersen and Bente Lindgård at the University of Tromsø for skilful technical assistance. Thanks also to Professor John Ross for providing us with seeds for the la crys mutant. This study has been carried out with financial support from the Norwegian Research Council (project nos 140322/110 and 167890/110) and the Norwegian University of Life Sciences.
References
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006;311:91–94. doi: 10.1126/science.1118642. [DOI] [PubMed] [Google Scholar]
- Agrawal M, Krizek DT, Agrawal SB, Kramer GF, Lee EH, Mirecki RM, Rowland RA. Influence of inverse day–night temperature on ozone sensitivity and selected morphological and physiological-responses of cucumber. Journal of the American Society for Horticultural Science. 1993;118:649–654. [Google Scholar]
- Berghage RD, Flore JA, Heins RD, Erwin JE. The relationship between day and night temperature influences photosynthesis but not light compensation point or flower longevity of easter lily, Lilium longiflorum Thunb. Acta Horticulturae. 1990;272:91–95. [Google Scholar]
- Biemelt S, Tschiersch H, Sonneweld U. Impact of altered gibberellin metabolism on biomass accumulation, lignin biosynthesis and photosynthesis in transgenic tobacco plants. Plant Physiology. 2004;135:1–12. doi: 10.1104/pp.103.036988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilger W, Björkman O. Role of xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynthesis Research. 1990;25:173–185. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- Bode J, Wild A. The influence of (2-chloroethyl)trimethylammoniumchloride (CCC) on growth and photosynthetic metabolism of young wheat plants (Triticum aestivum L.) Journal of Plant Physiology. 1984;116:435–446. doi: 10.1016/S0176-1617(84)80135-2. [DOI] [PubMed] [Google Scholar]
- Cramer MD, Nagel OW, Lips SH, Lambers H. Reduction, assimilation and transport of N in normal and gibberellin-deficient tomato plants. Physiologia Plantarum. 1995;95:347–354. [Google Scholar]
- de Pury DGG, Farquhar GD. Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant, Cell and Environment. 1997;20:537–557. [Google Scholar]
- Dijkstra P, Terreegen H, Kuiper PJC. Relation between relative growth-rate, endogenous gibberellins, and the response to applied gibberellic-acid for Plantago major. Physiologia Plantarum. 1990;79:629–634. doi: 10.1111/j.1399-3054.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Eriksson ME, Israelsson M, Olsson O, Moritz T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nature Biotechnology. 2000;18:784–788. doi: 10.1038/77355. [DOI] [PubMed] [Google Scholar]
- Erwin JE, Heins RD. Thermomorphogenic responses in stem and leaf development. HortScience. 1995;30:940–949. [Google Scholar]
- Erwin JE, Heins RD, Karlsson MG. Thermomorphogenic responses in stem and leaf development. HortScience. 1989;30:940–949. [Google Scholar]
- Farquhar GD, Sharkey TD. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology. 1982;33:317–345. [Google Scholar]
- Genty B, Briantais JM, Baker NR. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta. 1989;990:87–92. [Google Scholar]
- Grindal G. Vol. 27. Norway: Agricultural University of Norway; 1997. Thermoperiodic stem elongation in Pisum sativum L. and Cucumis sativus L.: the role of gibberellins and phytochrome. Doctor Scientiarum Theses 1997. [Google Scholar]
- Grindal G, Ernstsen A, Reid JB, Junttila O, Lindgard B, Moe R. Endogenous gibberellin A(1) levels control thermoperiodic stem elongation in Pisum sativum. Physiologia Plantarum. 1998;102:523–531. [Google Scholar]
- Hayat S, Ahmad A, Mobin M, Fariduddin Q, Azam ZM. Carbonic anhydrase, photosynthesis, and seed yield in mustard plants treated with phytohormones. Photosynthetica. 2001;39:111–114. [Google Scholar]
- Heide OM, Bush MG, Evans LT. Interaction of photoperiod and gibberellin on growth and photosynthesis of high-latitude Poa pratensis. Physiologia Plantarum. 1985;65:135–145. [Google Scholar]
- Heuvelink E. Influence of day and night temperature on the growth of young tomato plants. Scientia Horticulturae. 1989;38:11–22. [Google Scholar]
- Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onada Y. Temperature acclimation of photosynthesis; mechanisms involved in the changes in temperature dependence of photosynthetic rate. Journal of Experimental Botany. 2006;57:291–302. doi: 10.1093/jxb/erj049. [DOI] [PubMed] [Google Scholar]
- Huerta L, Forment J, Gadea J, Fagoaga C, Peña L, Perez-Amador MA, Garcia-Martinez JL. Gene expression analysis in citrus reveals the role of gibberellins on photosynthesis and stress. Plant, Cell and Environment. 2008;31:1620–1633. doi: 10.1111/j.1365-3040.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- Ingram TJ, Reid JB. Internode length in Pisum—biochemical expression of the Le and Na mutations in the slender phenotype. Journal of Plant Growth Regulation. 1987;5:235–243. [Google Scholar]
- Jaleel CA, Manivannen P, Sankar B, Kishorekumar A, Sankari S, Panneerselvam R. Paclobutrazol enhances photosynthesis and ajmalicine production in Catharanthus roseus. Process Biochemistry. 2007;42:1566–1570. [Google Scholar]
- Jensen E, Eilertsen S, Ernsten A, Juntilla O, Moe R. Thermoperiodic control of stem elongation and endogenous gibberellins in Campanula isophylla. Journal of Plant Growth Regulation. 1996;15:167–171. [Google Scholar]
- Khan NA. Effect of gibberellic acid on carbonic anhydrase, photosynthesis, growth and yield of mustard. Biologia Plantarum. 1996;38:145–147. [Google Scholar]
- Long SP, Bernacchi CJ. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. Journal of Experimental Botany. 2003;54:2393–2401. doi: 10.1093/jxb/erg262. [DOI] [PubMed] [Google Scholar]
- Maxwell K, Johnson GN. Chlorophyll fluorescence: a practical guide. Journal of Experimental Botany. 2000;51:659–658. doi: 10.1093/jxb/51.345.659. [DOI] [PubMed] [Google Scholar]
- Michael TP, Breton G, Hazen SP, Priest H, Mockler TC, Kay SA, Chory J. A morning-specific phytohormone gene expression program underlying rhythmic plant growth. PLoS Biology. 2008 doi: 10.1371/journal.pbio.0060225. 6, e225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moe R, Heins RD. Thermo- and photomorphogenesis in plants. In: Stroemme E, editor. Advances in floriculture research. Report no. 6/2000: Agricultural University of Norway; 2000. pp. 52–64. [Google Scholar]
- Myster J, Junttila O, Lindgaard B, Moe R. Temperature alternations and the influence of gibberellins and indoleacetic acid on elongation growth and flowering of Begonia×hiemalis Fotsch. Plant Growth Regulation. 1997;21:135–144. [Google Scholar]
- Nagel OW, Konings H, Lambers H. Growth rate and biomass partitioning of wild type and low-gibberellin tomato (Solanum lycopersicum) plants growing at a high and low nitrogen supply. Physiologia Plantarum. 2001;111:33–39. [Google Scholar]
- Nagel OW, Lambers H. Changes in the acquisition and partitioning of carbon and nitrogen in the gibberellin-deficient mutants A70 and W335 of tomato (Solanum lycopersicum L.) Plant, Cell and Environment. 2002;25:883–891. [Google Scholar]
- Nishijima T, Nonaka M, Koshioka M, Ikeda H, Douzono M, Yamazaki H, Mander LN. Role of gibberellins in the thermoperiodic regulation of stem elongation in Dendranthema grandiflorum Tsvelev. Bioscience Biotechnology and Biochemistry. 1997;61:1362–1366. [Google Scholar]
- Nozue K, Covington MF, Duek PD, Lorrain S, Frankhauser C, Harmer SL, Maloof JN. Rhytmic growth explained by coincidence between internal and external cues. Nature. 2007;448:358–361. doi: 10.1038/nature05946. [DOI] [PubMed] [Google Scholar]
- Olsen JE, Junttila O. Far-red end of day treatment restores WT like length, cell number and gibberellin content in phytochrome A overexpressing hybrid aspen. Physiologia Plantarum. 2002;115:448–457. doi: 10.1034/j.1399-3054.2002.1150315.x. [DOI] [PubMed] [Google Scholar]
- Piechulla B, Riesselmann S. Effect of temperature alterations on the diurnal expression pattern of the chlorophyll-A/B binding-proteins in tomato seedlings. Plant Physiology. 1990;94:1903–1906. doi: 10.1104/pp.94.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts WC, Reid JB, Murfet IC. Internode length in Pisum. Gibberellins and the slender phenotype. Physiologia Plantarum. 1985;63:357–364. [Google Scholar]
- Ross JJ, Davidson SE, Wolbang CM, Bayly-Stark E, Smith JJ, Reid JB. Developmental regulation of the gibberellin pathway in pea shoots. Functional Plant Biology. 2003;30:83–89. doi: 10.1071/FP02108. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Reid JB, Gaskin P, Macmillan J. Internode length in Pisum—estimation of GA1 levels in genotypes Le, le and led. Physiologia Plantarum. 1989;76:173–176. [Google Scholar]
- Stavang J, Gallego-Bartolome J, Yoshida S, Asami T, Olsen JE, Garcia-Martinez JL, Alabadi D, Blazquez M. Hormonal regulation of temperature-induced growth in Arabidopsis. The Plant Journal. 2009;60:598–601. doi: 10.1111/j.1365-313X.2009.03983.x. [DOI] [PubMed] [Google Scholar]
- Stavang JA, Junttila O, Moe R, Olsen JE. Differential temperature regulation of GA metabolism in light and darkness. Journal of Experimental Botany. 2007;58:3061–3069. doi: 10.1093/jxb/erm163. [DOI] [PubMed] [Google Scholar]
- Stavang JA, Lindgaard B, Erntsen A, Lid SE, Moe R, Olsen JE. Thermoperiodic stem elongation involves transcriptional regulation of GA deactivation in pea. Plant Physiology. 2005;138:2344–2353. doi: 10.1104/pp.105.063149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thetford M, Warren SL, Blazich FA, Thomas JF. Response of Forsythia-x intermedia ‘Spectabilis’ to uniconazole 2. Leaf and stem anatomy, chlorophyll, and photosynthesis. Journal of the American Society for Horticultural Science. 1995;120:983–988. [Google Scholar]
- Torre S, Moe R. Temperature, DIF and photoperiod effects on the rhythm and rate of stem elongation in Campanula isophylla Moretti. Scientia Horticulturae. 1998;72:123–133. [Google Scholar]
- Treharne KJ, Stoddart JL. Effects of gibberellin on photosynthesis in red clover (Trifolium pratense L) Nature. 1968;220:457–458. doi: 10.1038/220457a0. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Evans JR, Hudson GS, Andrews TJ. The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in-vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta. 1994;195:88–97. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas-exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Vaagen IM, Moe R, Ronglan E. Diurnal temperature alternations (DIF/drop) affect chlorophyll content and chlorophyll a/chlorophyll b ratio in Melissa officinalis L. and Ocimum basilicum L., but not in Viola×wittrockiana Gams. Scientia Horticulturae. 2003;97:153–162. [Google Scholar]
- Weller JL, Ross JJ, Reid JB. Gibberellins and phytochrome regulation of stem elongation in pea. Planta. 1994;192:489–496. [Google Scholar]
- Went FW. Plant growth under controlled conditions. II. Thermoperiodicity in growth and fruiting of tomato. American Journal of Botany. 1944;31:135–150. [Google Scholar]
- Weston DE, Elliott RC, Lester DR, Rameau C, Reid JB, Murfet IC, Ross JJ. The pea DELLA proteins LA and CRY are important regulators of gibberellin synthesis and root growth. Plant Physiology. 2008;147:199–205. doi: 10.1104/pp.108.115808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JQ, Patil GG, Moe R. Effect of DIF and end-of-day light quality on stem elongation in Cucumis sativus. Scientia Horticulturae. 2002;94:219–229. [Google Scholar]
- Yuan L, Xu DQ. Stimulation effect of gibberellic acid short-term treatment on leaf photosynthesis related to the increase in Rubisco content in broad bean and soybean. Photosynthesis Research. 2001;68:39–47. doi: 10.1023/A:1011894912421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.