Abstract
Trichomes are specialized epidermal structures that function as physical and chemical deterrents against arthropod herbivores. Aerial tissues of cultivated tomato (Solanum lycopersicum) are populated by several morphologically distinct trichome types, the most abundant of which is the type VI glandular trichome that produces various specialized metabolites. Here, the effect of the hairless (hl) mutation on trichome density and morphology, chemical composition, and resistance to a natural insect herbivore of tomato was investigated. The results show that the major effect of hl on pubescence results from structural distortion (bending and swelling) of all trichome types in aerial tissues. Leaf surface extracts and isolated type VI glands from hl plants contained wild-type levels of monoterpenes, glycoalkaloids, and acyl sugars, but were deficient in sesquiterpene and polyphenolic compounds implicated in anti-insect defence. No-choice bioassays showed that hl plants are compromised in resistance to the specialist herbivore Manduca sexta. These results establish a link between the morphology and chemical composition of glandular trichomes in cultivated tomato, and show that hl-mediated changes in these leaf surface traits correlate with decreased resistance to insect herbivory.
Keywords: Acyl sugar, herbivory, jasmonate, phenolics, plant defence, plant–insect interaction, secondary metabolite, terpene, tomato, trichome
Introduction
Trichomes are uni- or multicellular structures that originate from epidermal cells of above-ground plant tissues. These aerial appendages are broadly distributed in species throughout the plant kingdom, and vary considerably in their size, shape, and density. Trichomes have been implicated in protection against various biotic and abiotic stress conditions, including insect and pathogen attack, extreme temperature, and excessive light (Larkin et al., 2003; Martin and Glover, 2007). Trichomes can be classified morphologically as being either non-glandular or glandular. The latter structures are remarkable for their capacity to synthesize, store, and secrete large amounts of specialized metabolites, many of which are commercially important as pharmaceuticals, fragrances, food additives, and natural pesticides (Wagner, 1991; Duke et al., 2000; Wagner et al., 2004).
Cultivated tomato (Solanum lycopersicum) and its wild relatives produce several different types of trichomes on hypocotyls, stems, leaves, floral organs, and immature fruit. Luckwill's taxonomic survey of tomato species documented four morphologically distinct glandular trichomes: type I trichomes characterized by a multicellular base, a long (∼2 mm) multicellular stalk, and a small glandular tip; shorter (∼0.3 mm) type IV trichomes, which have a unicellular base, a multicellular stalk shorter than type I, and a small glandular tip; type VI trichomes containing a four-celled glandular head on a short (∼0.1 mm) multicellular stalk; and type VII trichomes consisting of a short (<0.05 mm) unicellular stalk and an irregularly shaped 4- to 8-celled gland (Luckwill, 1943). Cultivated and wild species of tomato also produce several non-glandular trichome types. According to Luckwill's classification scheme, type II and type III hairs are similar in length (0.2–1.0 mm) but differ by the presence of a multicellular and unicellular base, respectively, whereas type V trichomes are shorter (0.1–0.3 mm) and have a unicellular base. There is considerable diversity of trichome habit (e.g. type and density) and chemical composition within tomato species (Schilmiller et al., 2008). For example, acyl sugar-producing type IV glandular trichomes are highly abundant in the wild species S. pennellii but are reported to be absent in cultivated tomato (S. lycopersicum) (Luckwill, 1943; Antonious, 2001).
Numerous studies have shown that trichomes play an important role in resistance of tomato species to arthropod herbivores. Non-glandular trichomes may contribute to resistance by mechanically obstructing the movement of herbivores across the plant surface (Kennedy, 2003; Simmons et al., 2005). More importantly, resistance is also mediated by glandular trichome-borne metabolites that exert toxic effects on insect herbivores, or that physically entrap the insect upon rupture of the trichome gland. Among the broad classes of tomato leaf compounds known to deter insect herbivores are acyl sugars, methyl ketones, terpenes, and alkaloids. Acyl sugars exuded by type IV trichomes of S. pennellii confer resistance to numerous insect pests of tomato, including aphids (Macrosiphum euphorbiae and Myzus persicae), whitefly (Bemisia argentifolii), leaf miners (Liriomyza trifolii), tomato fruitworm (Helicoverpa zea), and beet armyworm (Spodoptera exigua) (Gentile and Stoner, 1968; Goffreda et al., 1990; Rodriguez et al., 1993; Juvik et al., 1994; Blauth et al., 1998; Hartman and St Clair, 1999). Type VI trichomes on certain accessions of S. habrochaites produce 2-tridecanone and other methyl ketones that are highly toxic to numerous arthropod pests of tomato (Williams et al., 1980; Kennedy, 2003). Other accessions of S. habrochaites are characterized by type VI glands that produce various sesquiterpenes, including zingiberene, that are acutely toxic to insect and arachnid herbivores (Carter et al., 1989; Eigenbrode et al., 1994; Maluf et al., 2001). Although the glycoalkaloid tomatine is also implicated as a foliar defence in tomato (Duffey and Stout, 1996; Kowalski et al., 2000; Kozukue et al., 2004; Enya et al., 2007), it is unclear whether tomatine and related compounds are produced in trichomes. The diversity of trichome chemistry within tomato species provides an attractive opportunity to study the chemical basis of plant–insect interactions in a genetically tractable and economically important host plant.
Our current understanding of the role of trichomes in the resistance of tomato to insect herbivores is based almost entirely on experiments performed with wild tomato species. The extent to which trichomes mediate arthropod resistance in cultivated tomato is largely unknown. Several features of cultivated tomato make it an attractive model system in which to investigate this question. As a host to >100 insect species that feed on roots, leaves, or fruit (Lange and Bronson, 1981), S. lycopersicum has most types of glandular and non-glandular trichomes but produces only trace amounts of various anti-insect compounds, including acyl sugars and methyl ketones (Kennedy, 2003). Biosynthetic pathways or regulatory genes involved in production of these specialized compounds in wild tomato species presumably were lost or down-regulated during the domestication of cultivated tomato. Nevertheless, extensive genetic analysis indicates that cultivated tomato possesses a panoply of inducible, jasmonate (JA)-regulated resistance traits that target a broad spectrum of arthropod herbivores (Howe et al., 1996; Thaler, 1999; C Li et al., 2002; Howe, 2004; Bostock, 2005; Howe and Jander, 2008). Among the many defence-related processes regulated by the JA pathway are the density and chemical composition of glandular trichomes (Li et al., 2004; Boughton et al., 2005; van Schie et al., 2007).
To study the role of trichomes in the resistance of cultivated tomato to insect herbivores, the effect of the recessive hairless (hl) mutation (Rick and Butler, 1956) on the morphology, density, and chemical composition of trichomes, including isolated type VI glands, was analysed. The results show that hl causes severe morphological distortion of all trichome types on aerial tissues. It is also shown that hl plants are deficient in the accumulation of several trichome-borne metabolites implicated in anti-insect defence, and that the mutant is compromised in resistance to a natural lepidopteran herbivore of tomato. These findings establish a link between the morphology and chemical composition of glandular trichomes in cultivated tomato, and show that hl-mediated changes in trichome traits correlate with decreased resistance to herbivory.
Materials and methods
Plant materials and growth conditions
Tomato (S. lycopersicum) cv Alisa Craig (accession number LA2838A) was used as the wild type (WT) for all experiments. Seeds for WT and hl (LA3556) were obtained from C.M. Rick Tomato Genetics Resource Center (University of California, Davis, CA, USA). Seedlings were grown in Jiffy peat pots (Hummert International, Earth City, MO, USA) in a growth chamber maintained under 17 h of light (265 mE m−2 s−1) at 27 °C and 7 h of dark at 18 °C and 60% humidity. Three- to four-week-old plants were sampled for morphological and secondary metabolite analysis.
Analysis of trichome density and morphology
A dissecting microscope (Leica MZ16, Wetzlar, Germany) equipped with KL 2500 LCD light sources (Schott, Jena, Germany) and a Leica DFC 290 camera (Leica, Wetzlar, Germany) was used to analyse trichome morphology, size, and density. All measurements were performed on WT and hl plants grown side by side in the same growth chamber. Scanning electron microscopy (SEM) was performed with a JEOL 6400V microscope (Tokyo, Japan). Tissues were fixed for 24 h in a solution of 2.5% paraformaldehyde, 2.5% glutaraldehyde buffered with 0.1 M sodium cacodylate, pH 7.4 (Electron Microscopy Sciences, Hatfield, PA, USA). Samples were dehydrated in a graduated ethanol series, critical point dried with CO2 (Bal-Tec CPD, Balzers, Lichtenstein), mounted, and sputter coated with 30 nm gold particles (EMSCOPE SC500 sputter coater, Ashford, UK). Samples were examined with a 15 kV accelerating voltage and the resulting images were captured digitally.
CryoSEM analysis was conducted as previously described (Ahlstrand, 1996; Esch et al., 2003). Briefly, leaf tissue was frozen in liquid nitrogen and then sputter coated with gold (∼20 nm) using the Emitech K1150 cryo-preparation system (Emitech, http://www.quorumtech.com/) containing an airlock interface with a Hitachi S3500N SEM (Hitachi, http://www.hitachi-hta.com/). Images were captured using 5 kV to minimize surface charging of the trichomes. The Quartz PCI digital imaging system (Quartz, http://www.qrtz.com/index.html) was used for initial image processing followed by further modifications using Adobe Photoshop (Adobe, http://www.adobe.com/) to prepare images for publication.
Terpene analysis
Analysis of volatile terpenes was performed as described by Schilmiller et al. (2009), with minor modifications. Four-week-old plants were used to obtain trichome exudates from either whole leaves (leaf dip method) or isolated type VI trichomes. For the former method, single leaflets were incubated at room temperature in 1 ml of methyl tertiary-butyl ether (MTBE) containing 10 ng μl−1 of tetradecane internal standard. Following a 5 min incubation period with gentle shaking, the leaf was removed and its dry weight was determined. The resulting MTBE solution (2 μl) was used directly for capillary gas chromatography–mass spectrometry (GC-MS) analysis as described below. For direct analysis of type VI glands, a stretched Pasteur pipette was used to collect type VI glandular heads from the adaxial leaf surface. Collected glands, which readily stick to the glass surface, were dissolved in 100 μl of MTBE containing 10 ng μl−1 tetradecane as an internal standard. A small portion (2 μl) of this extract was analysed by GC-MS on a DB-5 fused-silica column (10 m length, 0.1 mm i.d., 0.34 μm thick stationary phase; Agilent, Santa Clara, CA, USA). The GC program used an injector temperature of 280 °C. The initial column temperature was held at 40 °C for 1 min and then ramped at 40 °C min−1 to 90 °C, 15 °C min−1 to 110 °C, 25 °C min−1 to 250 °C, and finally at 40 °C min−1 to 320 °C, which was maintained for 2 min. The helium carrier gas flow was set to 0.4 ml min−1. All compounds were analysed with an Agilent 6890N GC system interfaced to a 5975B quadrupole mass spectrometer (Santa Clara, CA, USA) operated using 70 eV electron ionization and mixed selected ion monitoring (m/z 85 and 93) per scan (m/z 33–350) mode. The terpene content in leaf dip samples was normalized to the dried weight of the tissue used for each extraction. The terpene content in type VI gland exudates was normalized to a specific number of isolated glands. Under the GC conditions employed, β-phellandrene co-eluted with minor amounts of limonene (data not shown). 2-Carene and α-humulene were used as standards to determine response factors for monoterpenes and sesquiterpenes, respectively.
Analysis of non-terpenoid metabolites
Leaves from 4-week-old plants were used to prepare leaf dip or type VI trichome exudates as described above. Single leaflets were incubated in 1 ml of isopropanol:acetonitrile:water (3:3:2) containing 10 μM of internal standard propyl-4-hydroxybenzoate (synthesized in the laboratory of ADJ) for 5 min, with gentle shaking. Alternatively, type VI glandular heads collected with a Pasteur pipette were suspended in 100 μl of isopropanol:acetonitrile:water (3:3:2) containing 10 μM of internal standard propyl-4-hydroxybenzoate. The resulting extracts (10 μl) were analysed by liquid chromatography–mass spectrometry (LC-MS) using a Waters (Milford, MA, USA) LCT Premier mass spectrometer coupled to a Shimadzu (Columbia, MD, USA) LC-20AD HPLC ternary pump and SIL-5000 autosampler. Metabolites were detected using electrospray ionization in negative mode over the mass range of m/z 50–1500. Analyses were performed with rapid switching of the Aperture 1 voltage in the ion transit region of the mass spectrometer, which provides quasi-simultaneous generation of spectra under fragmenting and non-fragmenting conditions. This analytical approach is an extension of LC/MSE (Plumb et al., 2006), and provides exact mass measurements of molecular and fragment ions. The acquisition of spectra using multiple severities of fragmentation facilitates post-acquisition data mining and improved annotations of low level metabolites that co-elute with more abundant compounds. Mass spectra were acquired in centroid format with ‘dynamic range enhancement’ (DRE) enabled. Reverse-phase liquid chromatographic separation was performed using a fused core Ascentis Express C18 column (2.1×50 mm, 2.7 μm particles) maintained at 30 °C. A steep elution gradient was used to facilitate rapid metabolite analysis, with a solvent system consisting of 0.15% formic acid in MilliQ water (solvent A) and methanol (solvent B) and a run time of 5 min per sample. The gradient profile used an initial condition of 10% B, a 2 min linear gradient to 60% B, a 3 min ramp to achieve 100% B, a 4 min hold at 100% B, and return to 10% B over 5 min. The flow rate was 0.4 ml min−1.
Absolute quantitative analyses were performed using calibration curves derived from external standards, assuming all metabolites of each class shared a common response factor with the external standard for the class. External standards of rutin and tomatine (Sigma) were used to quantify flavonoids and alkaloids, respectively, and chlorogenic acid was used to quantify chlorogenic acid and quinic acid levels in extracts of leaves and trichomes. Glycoalkaloids were quantified based on integrated peak areas for the formate adduct ion ([M+formate]–); all other metabolites were quantified based on deprotonated molecules ([M–H]– ions). Since no commercially available standards were available for the acyl sugar metabolites, quantitative determinations are based on the assumption that all acyl sugars have the same response factor as the internal standard propyl-4-hydroxybenzoate. Secondary metabolites were normalized to the dried weight of the tissue used for each extraction. Peak areas of extracted ion chromatograms for characteristic masses of each metabolite, the internal standard, and external standards were integrated, and calibration curves were calculated using Waters QuanLynx software. Measured levels of secondary metabolites were normalized to the dried weight of tissue used for each extraction, or to the number of type VI trichomes used for each analysis.
Proteinase inhibitor assays
Proteinase inhibitor II (PI-II) levels in tomato leaves were determined by a radial immunodiffusion assay as previously described (Li and Howe, 2001; Li et al., 2003). A haemostat was used to make crushing-type wounds on all leaflets of the lower (oldest) leaf of 15-day-old tomato plants that contained two expanded leaves and a third emerging leaf. Wounded plants were incubated for 2 d under standard growth conditions, after which the wounded leaf was harvested for determination of PI-II protein levels.
Insect feeding trials
Tobacco hornworm (Manduca sexta) eggs and artificial diet were obtained from the Department of Entomology, North Carolina State University (Raleigh, NC, USA). Eggs were hatched at 26 °C as recommended by the supplier. Hatched larvae were reared on artificial diet for 4 d before transfer to 4-week-old tomato plants. Challenged plants were maintained in a growth chamber for the duration of the feeding trial.
Statistical analysis
All data were expressed as the mean ±SE. Data were analysed using the unpaired t-test by SigmaStat (version 3.1; Systat Software Inc., Point Richmond, CA, USA).
Results
Effect of hl on trichome development
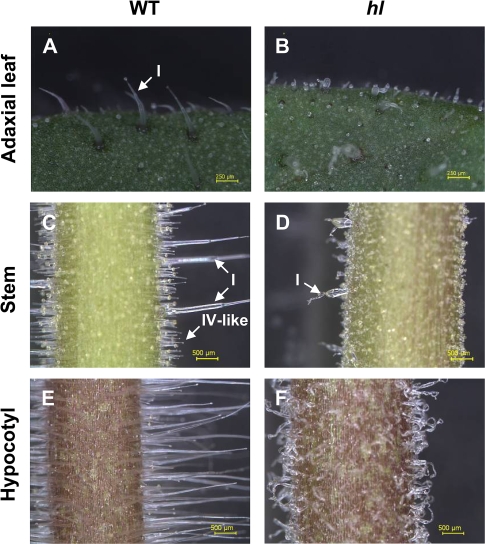
Light microscopy was used to compare the morphology of trichomes on stems, leaves, and hypocotyls of the hl mutant with its wild-type parent (cv Alisa Craig) (Fig. 1). The most conspicuous hl phenotypes were the absence of normal type I trichomes and the presence of highly twisted and swollen trichomes. The identity of the distorted structures as type I trichomes was confirmed by cryoelectron microscopy (CEM) and SEM. This analysis showed that type I trichomes on the hl mutant contain highly swollen cells that fail to orient perpendicular to the leaf surface, resulting in highly distorted and twisted structures (Fig. 2; Supplementary Fig. S1 available at JXB online). The effect of hl on trichome morphology was not specific to type I trichomes, but rather extended to other trichome types as well (Fig. 2; Supplementary Figs S1 and S2 at JXB online). The type VI trichome, which contains a short neck cell that connects the 4-celled glandular head to the stem, showed irregular patterns of cell division on the hl mutant (Fig. 2). The neck cell of many mutant type VI trichomes protruded from the side of the stem, resulting in a glandular head that appeared to lay down on the leaf surface (Fig. 2E; Supplementary Fig. S1 at JXB online). Expansion of these abnormal stem cells often gave the appearance of a branched trichome (Supplementary Fig. S1D at JXB online). Similar defects in trichome morphology were observed on hl stems, hypocotyls, sepals, and petals (Supplementary Figs S1, S2 at JXB online). SEM studies also showed that hl affects the morphology of type V and VII trichomes (Supplementary Figs S1, S2 at JXB online). The size and shape of epidermal pavement cells on hl leaves appeared normal. Although hl plants exhibited a modest reduction in shoot size, other obvious phenotypes related to development, leaf pigmentation, or fertility were not observed (Supplementary Fig. S3 at JXB online).
Fig. 1.
Light micrographs of trichomes on the leaf, stem, and hypocotyl of wild-type (WT) and hl plants. The edge of the adaxial leaf surface of WT (A) and hl (B) plants. Stem of WT (C) and hl (D) plants. Hypocotyl region of WT (E) and hl (F) plants. Scale bars represent 250 μm in (A) and (B), and 500 μm in (C–F). Three-week-old plants were used for all photographs. The arrows indicate various trichome types.
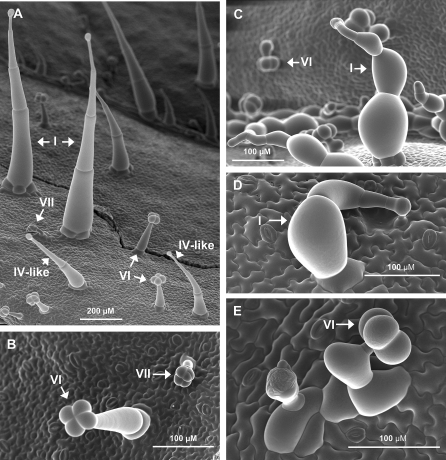
Fig. 2.
Cryoelectron micrographs of leaf trichomes on wild-type (WT) and hl plants. (A and B) Adaxial surface of a WT leaf. (C–E) Adaxial leaf surface of hl plants. Three-week-old plants were used for all images. Various trichome types, including type IV-like trichomes (A), are indicated by arrows.
An F2 population derived from a cross between hl and its wild-type parent was scored at the seedling stage (21-day-old plants) for the trichome distortion phenotype. Among 132 F2 plants examined by light microscopy, 34 plants exhibited trichome distortion, whereas the remaining F2 plants appeared normal. This ratio (2.9:1) is in good agreement with that predicted for a single recessive mutation (χ2=0.04; P=0.84). It is concluded that hl-related phenotypes are relatively specific for trichomes, and that this single recessive mutation affects the development of multiple trichome types on all aerial tissues.
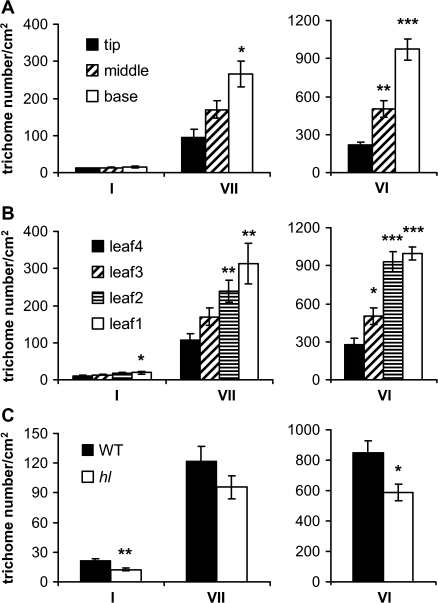
The effect of hl on trichome morphology prompted the determination of whether the mutation also alters trichome density. Four-week-old WT plants were used to develop a standardized procedure for trichome counting (Supplementary Fig. S4 at JXB online). Specified regions on the adaxial surface of lateral leaflets (excluding the midvein) were used to count glandular trichomes (types I, VI, and VII), which can be unambiguously identified with a dissecting light microscope. Type VI trichomes were the most abundant structures on the adaxial leaf surface (Fig. 3A). Trichome densities were higher in developmentally younger regions (base) of the leaflet compared with more developed regions (tip) that had undergone cell expansion. Higher trichome densities on younger leaves compared with older leaves from the same plant were also observed (Fig. 3B). All trichome types observed on WT plants were present on the hl mutant. Comparison of WT and hl mutant plants of a similar developmental age showed that the densities of type I and VI trichomes on the mutant were reduced to 57% and 70% of that on WT plants, respectively, whereas the densities of type VII trichomes on the two genotypes were similar (Fig. 3C). The densities of non-glandular trichomes (types III and V) on the adaxial leaf surfaces were also similar in WT and hl plants (data not shown). The pattern of trichome spacing on hl leaves appeared normal, although examples of abnormal clustering were observed infrequently (e.g. Fig. 2E). It is concluded that the major effect of hl on leaf pubescence results from alterations in trichome morphology rather than from changes in trichome density or spacing.
Fig. 3.
hl has minor effects on trichome density. (A) Density of glandular trichomes (types I, VI, and VII) in the base, middle, and tip region of leaflets from 4-week-old wild-type (WT) plants. Data show the mean (±SE) trichome number of six replicate leaves taken from position L3 (see Supplementary Fig. S4 at JXB online). Asterisks represent significant differences between trichome density in the tip region compared with either the middle or base region (unpaired t-test: *P <0.05; **P <0.01; ***P <0.001). (B) Effect of leaf developmental age on trichome density in WT plants. Trichome counts were performed on the middle region of lateral leaflets (Supplementary Fig. S4C at JXB online). Data show the mean (±SE) trichome number of six replicate leaves. Asterisks represent significant differences between the oldest leaf (leaf 4) and each of three developmentally younger leaves (unpaired t-test: *P <0.05; **P <0.01; ***P <0.001). Leaf 1 corresponds to the newly emerging, youngest leaf. (C) Leaf trichome density in WT and hl plants. Mean (±SE) trichome number of six replicate leaves on 4-week-old WT and hl plants. Asterisks represent significant differences between WT and hl plants (unpaired t-test: *P <0.05; **P <0.01).
Occurrence of a type IV-like trichome in S. lycopersicum
Cultivated tomato species have been reported to lack type IV trichomes (Luckwill, 1943; Antonious, 2001; Simmons and Gurr, 2005; Saeidi et al., 2007). CEM and SEM analysis of leaf tissue from cv Alisa Craig, however, revealed a type IV-like structure consisting of a small glandular tip, multicellular stem (varying in length from 0.2 mm to 0.4 mm), and a simple uni- or bicellular base (Fig. 2A; Supplementary Figs S1, S5 at JXB online). Although these leaf trichomes were not readily apparent by light microscopy, morphologically similar structures (∼0.5 mm in length) were apparent on stems and hypocotyls (Fig. 1C, E). The highly distorted trichomes on hl plants precluded the identification of type IV-like trichomes on the mutant. The possibility that the type IV-like trichomes observed represent a developmentally immature stage of the type I trichome cannot be excluded.
Effect of hl on accumulation of trichome-borne metabolites
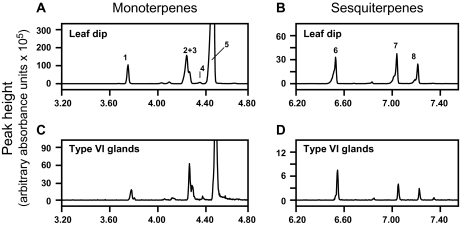
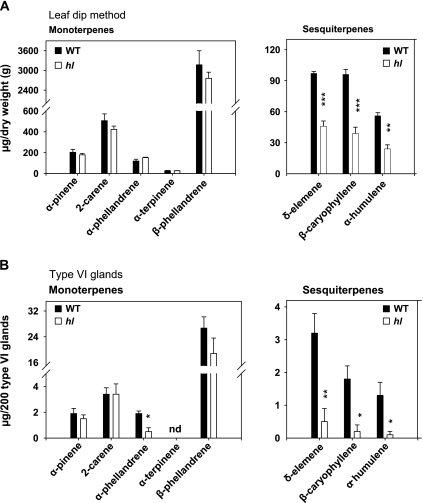
The question of whether hl affects the chemical composition of leaf glandular trichomes was next addressed. Initial experiments were focused on the analysis of terpenoids, which in S. lycopersicum are produced mainly in type VI glands on the surface of leaves, stems, and green fruits (Colby et al., 1998; van der Hoeven et al., 2000; Li et al., 2004; van Schie et al., 2007; Besser et al., 2009; Schilmiller et al., 2009). Detached leaflets were briefly immersed in MTBE and the resulting extracts were analysed by GC-MS. Under the GC conditions used, five peaks corresponding to the monoterpenes α-pinene, 2-carene, α-phellandrene, α-terpinene, and β-phellandrene were detected, with β-phellandrene being the most abundant (Fig. 4A). Lower amounts of three sesquiterpenes, δ-elemene, β-caryophyllene, and α-humulene, were identified in the same extracts (Fig. 4B). The terpene content of type VI glands was also profiled by using a stretched glass pipette to collect individual glands selectively into MTBE followed by GC-MS analysis (Fig. 4C, D). The mono- and sesquiterpene profile observed in collected type VI glands was nearly identical to that observed with the leaf dip procedure, indicating that type VI trichomes are the main source of tomato leaf terpenes. Comparison of the terpene profile in WT and hl leaf dips showed that the surface of hl leaves contains normal levels of monoterpenes but has significantly reduced levels of all three sesquiterpenes (Fig. 5A). Analysis of type VI glands yielded similar results; the sesquiterpene content of type VI glands isolated from the adaxial surface of hl leaves was <20% of that in glands collected from WT leaves (Fig. 5B). Measurements of the size of tetrabolate heads on WT and hl type VI trichomes indicated that the sesquiterpene deficiency in hl cannot be accounted for simply by reduced volume of type VI glands.
Fig. 4.
Terpene profiles in leaf dips and isolated type VI glands. (A and B) Detached leaflets were immersed in MTBE and the resulting extracts were analysed by GC-MS. (C and D) Terpenes were extracted from isolated type VI glands collected with a glass pipette. For the latter analysis, the amount of material injected for each GC-MS run was equivalent to 200 type VI glands. The indicated monoterpene peaks (A and C) correspond to the following compounds: 1, α-pinene; 2, 2-carene; 3, α-phellandrene; 4, α-terpinene; 5, β-phellandrene. Under the GC conditions used, minor amounts of limonene co-eluted with β-phellandrene. The sesquiterpene peaks (B and D) correspond to δ-elemene (6), β-caryophyllene (7), and α-humulene (8).
Fig. 5.
Comparison of terpene levels in wild-type (WT) and hl leaves. (A) Analysis of monoterpenes (left panel) and sesquiterpenes (right panel) extracted from WT (filled bar) and hl (open bar) plants (4 weeks old) using the leaf dip method. (B) Analysis of monoterpenes (left panel) and sesquiterpenes (right panel) obtained from collected type VI glands. Peak areas for each terpene compound were normalized to leaflet weight (A) or to 200 type VI glands (B). Under the GC conditions used, minor amounts of limonene co-eluted with β-phellandrene. Each mean represents data from four replicates. Asterisks denote significant differences between wild-type and hl (unpaired t-test: *P <0.05; **P <0.01; ***P <0.001). nd, not detected.
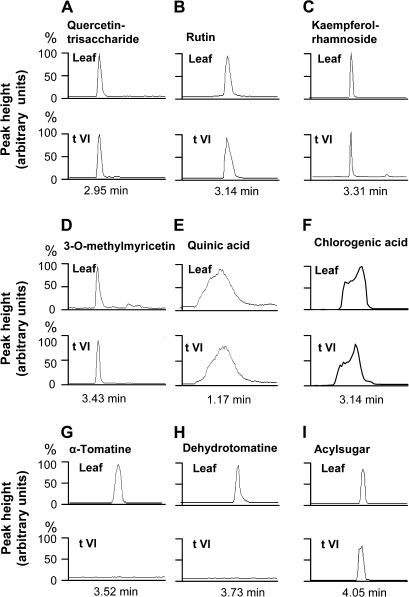
To profile the composition of non-volatile metabolites, excised leaflets were briefly immersed in a solution containing isopropanol/acetonitrile/water and the extracted compounds were analysed by LC-MS. Nine metabolites belonging to three general classes of compounds were identified (Fig. 6; Leaf). These included the phenolic-based compounds quercetin-trisaccharide (a hexose–deoxyhexose–pentose conjugate), rutin, kaempferol-rhamnoside, 3-O-methylmyricetin, quinic acid, and chlorogenic acid (Fig. 6A–F), two alkaloids (α-tomatine and dehydrotomatine; Fig. 6G, H), and an acyl sugar identified as a tetra-acylsucrose with three C5 fatty ester groups and one acetate ester (Fig. 6I). To determine whether these compounds are produced in type VI trichomes, the LC-MS analysis was performed on isolated type VI glands collected directly into the isopropanol/acetonitrile/water solvent. Analysis of injection volumes corresponding to 1000 type VI trichomes detected all six phenolic compounds (Fig. 6A–F) and the acyl sugar (Fig. 6I). In contrast, α-tomatine and dehydrotomatine were not detected in extracts obtained from isolated type VI glands (Fig. 6G, H). These results indicate that S. lycopersicum type VI leaf trichomes are major reservoirs for catecholic phenolics and acyl sugars but not the tomatine derivatives.
Fig. 6.
LC-MS analysis of non-volatile leaf metabolites. Extracted ion chromatograms were obtained for various metabolites extracted from wild-type leaves using either the leaflet dip procedure (Leaf) or collected type VI glands (t VI). For analysis of isolated type VI glands, the amount of material injected for each LC-MS run was equivalent to 1000 type VI glands. Chromatograms are for the respective [M–H]– ions with the exception of G–I (α-tomatine, dehydrotomatine, and acyl sugar), which correspond to the [M+HCOO]– ion. The identified acyl sugar contains a sucrose tetraester substituted with three C5 and one C2 fatty acyl chain, and is the major acyl sugar derivative identified in S. lycopersicum.
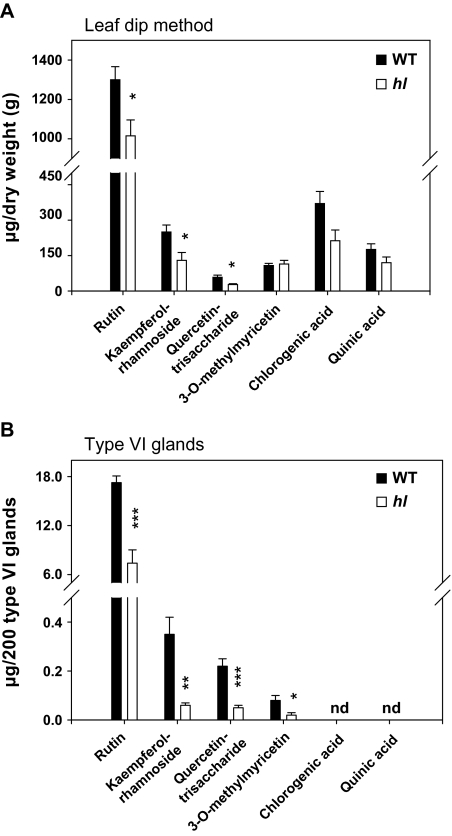
In comparison with the WT, leaf dip extracts from hl leaves contained reduced levels (46–72% of WT levels) of rutin, kaempferol-rhamnoside, and quercetin-trisaccharide (Fig. 7A). Analysis of extracts from isolated glands confirmed this finding, and also indicated that hl type VI trichomes contain ∼25% WT levels of 3-O-methylmyricetin (Fig. 7B). Analysis of leaf dip extracts showed that hl contains WT levels of α-tomatine, dehydrotomatine, and acyl sugar (data not shown). The glycoalkaloids were not detected in type VI glands from either mutant or WT plants, indicating that these compounds are produced elsewhere on the leaf surface. In summary, type VI trichomes from hl leaves are deficient in various sesquiterpene and flavonoid compounds, but contain normal levels of other specialized metabolites (e.g. monoterpenes and acyl sugars).
Fig. 7.
Relative levels of non-volatile metabolites in wild-type (WT) and hl leaves. (A) Results obtained from analysis of compounds in leaf dip extracts. (B) Results obtained from analysis of compounds in isolated type VI glands. Peak areas for each compound were normalized to leaflet weight (A) or to 200 type VI glands (B). Each data point represents the mean ±SE of four replicates. Asterisks represent significant differences between WT and hl plants (unpaired t-test: *P <0.05; **P <0.01; ***P <0.001). nd, not detected.
The hl mutant is compromised in resistance to an insect herbivore
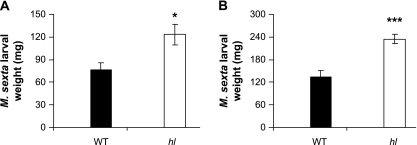
The effect of hl on the morphology and chemical composition of leaf trichomes suggested that the mutation might affect host plant interactions with insect herbivores. To address this question, two independent feeding trials were conducted to compare the performance of the solanaceous specialist M. sexta on WT and hl plants. At the end of each trial, M. sexta larvae growing on hl plants were significantly heavier than larvae reared on WT plants (Fig. 8A, B). The JA signalling pathway plays a central role in controlling induced resistance of cultivated tomato to lepidopteran insects, as well as various aspects of glandular trichome function (Howe et al., 1996; Li et al., 2004). To test whether the increased performance of M. sexta on hl plants results from a defect in JA signalling, the level of the wound-inducible serine proteinase inhibitor (PI-II), a well-characterized anti-insect protein whose induced expression depends on JA (L Li et al., 2002, 2004; Howe et al., 1996), was measured. The PI-II content in undamaged WT and hl leaves was near the detection limit of the assay (∼2 μg PI-II ml−1 leaf juice). In response to mechanical wounding, WT and hl leaves accumulated 45±5 and 53±3 μg PI-II ml−1 leaf juice, respectively (mean ±SE; n=5 per genotype). This finding indicates that JA signalling is activated upon herbivory, and the increased susceptibility of hl plants to herbivore attack cannot be attributed to a specific defect in PI-II production or to a general defect in the JA-mediated resistance pathway.
Fig. 8.
hl plants are compromised in resistance to feeding by Manduca sexta larvae. (A) Mean (±SE) mass of M. sexta larvae (n=14) reared for 8 d on either wild-type (WT) or hl plants. Each larva was grown on a single plant. (B) Mean (±SE) mass of M. sexta larvae (n=24) reared for 10 d on either WT or hl plants. Each plant was challenged with three larvae. Asterisks represent significant differences between WT and hl plants (unpaired t-test: *P <0.05; ***P <0.001).
Discussion
Previous light microscopic studies described the tomato hl mutant as having twisted and bent hairs on the stem (Rick and Butler, 1956; Reeves, 1977). The present results confirm and extend these observations by showing that hl severely affects the development of all trichome types on the aerial epidermis of leaves, stems, hypocotyls, and flowers. The most pronounced effect of hl was the production of swollen and irregularly shaped trichomes, with only minor effects on the density of some trichome types. Aberrant trichome cell expansion in the hl mutant appears to disrupt normal patterns of cell division, resulting in twisted and irregularly shaped multicellular trichomes. These observations, together with the lack of other obvious phenotypes, indicate that Hl function is relatively specific for trichome development.
The altered trichome morphology of hl plants is similar to phenotypes observed in the so-called distorted (DIS) group of trichome mutants in Arabidopsis. Molecular genetic studies have shown that many DIS genes have roles in actin-dependent cell morphogenesis. Proteins encoded by these genes participate in the formation of various multiprotein complexes that control the assembly of the actin cytoskeleton (Mathur et al., 2003; Schwab et al., 2003; Basu et al., 2005; Szymanski, 2005). Similar to what was observed in the hl mutant, loss of DIS function typically has negligible or only modest effects on other aspects of plant growth and development (Szymanski, 2005). It is also possible that Hl plays a role in the synthesis or deposition of new cell wall material in developing trichomes. In fact, previous studies noted increased fragility of the stems of tomato hl mutants and suggested that such brittleness could result from cell wall defects in phloem fibres of the vascular bundle (Dempsey and Sherif, 1987). Mutations that disrupt primary cell wall synthesis in Arabidposis cause severe swelling of root hair cells but do not appear to affect trichome development (Cavalier et al., 2008). Root hair development in the hl mutant appeared to be normal (data not shown). A more precise understanding of the role of Hl in trichome cell morphogenesis will be facilitated by molecular cloning of the gene.
Glandular trichomes produce a diverse array of compounds that provide direct or indirect protection against herbivores and pathogens. In Solanaceous plants, these compounds include terpenes, acyl sugars, alkaloids, and defence-related proteins (Duffey, 1986; Ranger et al., 2004; Shepherd et al., 2005; Antonious and Snyder, 2006; Shepherd and Wagner, 2007). Targeted metabolite profiling by GC-MS showed that type VI glands isolated from hl leaves contain normal levels of monoterpenes but are deficient in sesquiterpenes. Because the mono- and sesquiterpene compounds identified here are most probably synthesized in the plastid and cytosol, respectively (Colby et al., 1998; Besser et al., 2009; Sallaud et al., 2009), it was postulated that hl may disrupt a cellular function (e.g. formation of the actin cytoskeleton) required for optimal production of sesquiterpenes in the cytosol. This hypothesis may also explain the hl-mediated deficiency in various flavonoids, which are also synthesized by metabolic pathways in the cytosol (Winkel-Shirley, 2001). Little is known about the subcellular location of biosynthetic pathways for acyl sugars and the glycoalkaloids α-tomatine and dehydrotomatine. The levels of these compounds on the surface of hl and WT plants were similar. Interestingly, α-tomatine and dehydrotomatine were detected in leaf dip extracts but not in extracts from isolated type VI glands, including preparations of ∼10,000 pooled glands from WT leaves (data not shown). This finding indicates that type VI trichomes are not a major site of glycoalkaloid synthesis in the tomato leaf.
No-choice bioassays showed that M. sexta larvae, a natural herbivore of tomato, perform better on hl than on WT plants. These results indicate that hl impairs one or more cellular processes that are important for host defence against insect herbivores. The ability of hl plants to produce PI-II in response to wounding indicates that the mutant is probably not defective in JA-induced changes in leaf chemistry. Reduced resistance of hl plants to herbivory, however, was correlated with decreased levels of various trichome-borne metabolites that are known to exert repellant or toxic effects on insect herbivores. Previous studies with tomato have implicated sesquiterpenes, including elemene, humulene, and caryophyllene, in direct defence against lepidopteran and arachnid herbivores (Eigenbrode et al., 1994; Antonious and Snyder, 2006). The hl-mediated deficiency in rutin, 3-O-methylmyricetin, and conjugated forms of quercetin and kaempferol may also contribute to increased susceptibility of hl plants, as these compounds (or their derivatives) retard the growth of lepidopteran insects (Elliger et al., 1981; Isman and Duffey, 1982; Koul, 2005). The anti-insect activity of tomato leaf phenolics has been attributed to their accumulation in type VI glands (Duffey and Isman, 1981; Duffey, 1986). These compounds may be directly toxic to insects that rupture leaf glands during feeding. Alternatively, phenolics may be substrates for oxidation reactions catalysed by polyphenol oxidase (PPO), which is abundant in type VI glands of cultivated tomato (Li and Steffens, 2002). Highly reactive quinonoid products generated by PPO may also reduce the quality of dietary protein available to leaf-eating insects (Duffey and Isman, 1981; Eigenbrode et al., 1994; Summers and Felton, 1994; Thipyapong et al., 2007). It is also possible that increased susceptibility of hl plants to insect attack results from other factors, including changes in trichome morphology or altered chemical composition of the leaf lamina. Further analysis of tomato trichome mutants will help to define the role of trichomes in tomato–insect interactions.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Scanning electron micrographs of trichomes on leaf, stem, and hypocotyl of wild-type and hl plants.
Figure S2. Trichome morphology on flowers of wild-type and hl plants.
Figure S3. Phenotypic appearance of wild-type and hl plants.
Figure S4. Experimental set-up for trichome density measurements.
Figure S5. Type IV-like trichomes on the leaf surface of cultivated tomato.
Supplementary Material
Acknowledgments
We thank Erin Beach for assistance with plant growth and trichome density measurements, Guanghui Liu for performing genetic crosses, Tony Schilmiller for assistance with GC-MS analysis, and Ewa Danielewicz for expert assistance with SEM. We also thank members of the tomato trichome group for helpful discussions during the course of this work. We acknowledge the C.M. Rick Tomato Genetics Resource Center (University of California at Davis) for kindly providing tomato seed stocks. This research was supported by grants from the National Science Foundation (DBI-0604336) and the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy (grant DE-FG02-91ER20021).
References
- Ahlstrand G. Low-temperature low-voltage scanning microscopy (LTLVSEM) of uncoated frozen biological materials: a simple alternative. In: Bailey G, Corbett J, Dimlich R, Michael J, Zaluzec N, editors. Proceedings of Microscopy Microanalysis. San Francisco, CA: San Francisco Press; 1996. 918. [Google Scholar]
- Antonious GF. Production and quantification of methyl ketones in wild tomato accessions. Journal of Environmental Science and Health Part B. 2001;36:835–848. doi: 10.1081/PFC-100107416. [DOI] [PubMed] [Google Scholar]
- Antonious GF, Snyder JC. Natural products: repellency and toxicity of wild tomato leaf extracts to the two-spotted spider mite, Tetranychus urticae Koch. Journal of Environmental Science and Health Part B. 2006;41:43–55. doi: 10.1080/03601230500234893. [DOI] [PubMed] [Google Scholar]
- Basu D, Le J, El-Essal Sel D, Huang S, Zhang C, Mallery EL, Koliantz G, Staiger CJ, Szymanski DB. DISTORTED3/SCAR2 is a putative Arabidopsis WAVE complex subunit that activates the Arp2/3 complex and is required for epidermal morphogenesis. The Plant Cell. 2005;17:502–524. doi: 10.1105/tpc.104.027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser K, Harper A, Welsby N, Schauvinhold I, Slocombe S, Li Y, Dixon RA, Broun P. Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiology. 2009;149:499–514. doi: 10.1104/pp.108.126276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauth SL, Churchill GA, Mutschler MA. Identification of quantitative trait loci associated with acylsugar accumulation using intraspecific populations of the wild tomato, Lycopersicon pennellii. Theoretical and Applied Genetics. 1998;96:458–467. doi: 10.1007/s001220050762. [DOI] [PubMed] [Google Scholar]
- Bostock RM. Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annual Review of Phytopathology. 2005;43:545–580. doi: 10.1146/annurev.phyto.41.052002.095505. [DOI] [PubMed] [Google Scholar]
- Boughton AJ, Hoover K, Felton GW. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. Journal of Chemical Ecology. 2005;31:2211–2216. doi: 10.1007/s10886-005-6228-7. [DOI] [PubMed] [Google Scholar]
- Carter CD, Gianfagna TJ, Sacalis JN. Sesquiterpenes in glandular trichomes of a wild tomato species and toxicity to the Colorado potato beetle. Journal of Agricultural and Food Chemistry. 1989;37:1425–1428. [Google Scholar]
- Cavalier DM, Lerouxel O, Neumetzler L, et al. Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. The Plant Cell. 2008;20:1519–1537. doi: 10.1105/tpc.108.059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby SM, Crock J, Dowdle-Rizzo B, Lemaux PG, Croteau R. Germacrene C synthase from Lycopersicon esculentum cv. VFNT Cherry tomato: cDNA isolation, characterization, and bacterial expression of the multiple product sesquiterpene cyclase. Proceedings of the National Academy of Sciences, USA. 1998;95:2216–2221. doi: 10.1073/pnas.95.5.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey WH, Sherif HI. Brittleness in the stem of the seven ‘hairless’ mutants. Report of the Tomato Genetics Cooperative. 1987;37:43. [Google Scholar]
- Duffey SS. Plant glandular trichomes: their partial role in defence against insects. In: Juniper B, Southwood R, editors. Insects and the plant surface. London: Edward Arnold; 1986. pp. 151–172. [Google Scholar]
- Duffey SS, Isman MB. Inhibition of insect larval growth by phenolics in glandular trichomes of tomato leaves. Experientia. 1981;37:574–576. [Google Scholar]
- Duffey SS, Stout MJ. Antinutritive and toxic components of plant defense against insects. Archives of Insect Biochemistry and Physiology. 1996;32:3–37. [Google Scholar]
- Duke SO, Canel C, Rimando AM, Tellez MR, Duke MV, Paul RN. Current and potential exploitation of plant glandular trichome productivity. Advances in Botanical Research. 2000;31:121–151. [Google Scholar]
- Eigenbrode SD, Trumble JT, Millar JG, White KK. Topical toxicity of tomato sesquiterpenes to the beet armyworm and the role of these compounds in resistance derived from an accession of Lycopersicon hirsutum f. typicum. Journal of Agricultural and Food Chemistry. 1994;42:807–810. [Google Scholar]
- Elliger CA, Wong Y, Chan BG, Waiss AC. Growth inhibitors in tomato (Lycopersicon) to tomato fruitworm (Heliothis zea) Journal of Chemical Ecology. 1981;7:753–758. doi: 10.1007/BF00990307. [DOI] [PubMed] [Google Scholar]
- Enya J, Shinohara H, Yoshida S, Negishi TTH, Suyama K, Tsushima S. Culturable leaf-associated bacteria on tomato plants and their potential as biological control agents. Microbial Ecology. 2007;53:524–536. doi: 10.1007/s00248-006-9085-1. [DOI] [PubMed] [Google Scholar]
- Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, Idelkope B, Neizer J, Marks MD. A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development. 2003;130:5885–5894. doi: 10.1242/dev.00812. [DOI] [PubMed] [Google Scholar]
- Gentile AG, Stoner AK. Resistance in Lycopersicon and Solanum species to potato aphid. Journal of Economic Entomology. 1968;61:1152–1154. [Google Scholar]
- Goffreda JC, Szymkowiak EJ, Sussex IM, Mutschler MA. Chimeric tomato plants show that aphid resistance and triacylglucose production are epidermal autonomous characters. The Plant Cell. 1990;2:643–649. doi: 10.1105/tpc.2.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman JB, St Clair DA. Combining ability for beet armyworm, Spodoptera exigua, resistance and horticultural traits of selected Lycopersicon pennellii-derived inbred backcross lines of tomato. Plant Breeding. 1999;118:523–530. [Google Scholar]
- Howe GA. Jasmonates as signals in the wound response. Journal of Plant Growth Regulation. 2004;23:223–237. [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Annual Review of Plant Biology. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA. An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. The Plant Cell. 1996;8:2067–2077. doi: 10.1105/tpc.8.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isman MB, Duffey SS. Toxicity of tomato phenolic compounds to the fruitworm, Heliothis zea. Entomologia Experimentalis et Applicata. 1982;31:370–376. [Google Scholar]
- Juvik JA, Shapiro JA, Young TE, Mutschler MA. Acylglucoses from wild tomatoes alter behavior and reduce growth and survival of Helicoverpa zea and Spodoptera exigua (Lepidoptera, Noctuidae) Journal of Economic Entomology. 1994;87:482–492. [Google Scholar]
- Kennedy GG. Tomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annual Review of Entomology. 2003;48:51–72. doi: 10.1146/annurev.ento.48.091801.112733. [DOI] [PubMed] [Google Scholar]
- Koul O. Insect antifeedants. Boca Raton, FL: CRC Press; 2005. [Google Scholar]
- Kowalski SP, Domek JM, Sanford LL, Deahl KL. Effect of α-tomatine and tomatidine on the growth and development of the Colorado potato beetle (Coleoptera: Chrysomelidae): studies using synthetic diets. Journal of Entomological Science. 2000;35:290–300. [Google Scholar]
- Kozukue N, Han JS, Lee KR, Friedman M. Dehydrotomatine and α-tomatine content in tomato fruits and vegetative plant tissues. Journal of Agricultural and Food Chemistry. 2004;52:2079–2083. doi: 10.1021/jf0306845. [DOI] [PubMed] [Google Scholar]
- Lange WH, Bronson L. Insect pests of tomatoes. Annual Review of Entomology. 1981;26:345–371. [Google Scholar]
- Larkin JC, Brown ML, Schiefelbein J. How do cells know what they want to be when they grow up? Lessons from epidermal patterning in Arabidopsis. Annual Review of Plant Biology. 2003;54:403–430. doi: 10.1146/annurev.arplant.54.031902.134823. [DOI] [PubMed] [Google Scholar]
- Li L, Howe GA. Alternative splicing of prosystemin pre-mRNA in tomato generates two active forms of the prosystemin wound signal. Plant Molecular Biology. 2001;46:409–419. doi: 10.1023/a:1010645330275. [DOI] [PubMed] [Google Scholar]
- Li C, Williams MM, Loh YT, Lee GI, Howe GA. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiology. 2002;130:494–503. doi: 10.1104/pp.005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Liu G, Xu C, Lee GI, Bauer P, Ling HQ, Ganal MW, Howe GA. The tomato Suppressor of prosystemin-mediated responses2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. The Plant Cell. 2003;15:1646–1661. doi: 10.1105/tpc.012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li C, Lee GI, Howe GA. Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proceedings of the National Academy of Sciences, USA. 2002;99:6416–6421. doi: 10.1073/pnas.072072599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Steffens JC. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta. 2002;215:239–247. doi: 10.1007/s00425-002-0750-4. [DOI] [PubMed] [Google Scholar]
- Li L, Zhao Y, McCaig BC, Wingerd BA, Wang J, Whalon ME, Pichersky E, Howe GA. The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. The Plant Cell. 2004;16:126–143. doi: 10.1105/tpc.017954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckwill LC. The genus Lycopersicon: a historical, biological and taxonomic survey of the wild and cultivated tomatoes. UK: University of Aberdeen; 1943. [Google Scholar]
- Maluf WR, Campos GA, Cardoso MD. Relationships between trichome types and spider mite (Tetranychus evansi) repellence in tomatoes with respect to foliar zingiberene contents. Euphytica. 2001;121:73–80. [Google Scholar]
- Martin C, Glover BJ. Functional aspects of cell patterning in aerial epidermis. Current Opinion in Plant Biology. 2007;10:70–82. doi: 10.1016/j.pbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Mathur J, Mathur N, Kernebeck B, Hulskamp M. Mutations in actin-related proteins 2 and 3 affect cell shape development in Arabidopsis. The Plant Cell. 2003;15:1632–1645. doi: 10.1105/tpc.011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb RS, Johnson KA, Rainville P, Smith BW, Wilson ID, Castro-Perez JM, Nicholson JK. UPLC-MSE: a new approach for generating molecular fragment information for biomarker structure elucidation. Rapid Communications in Mass Spectrometry. 2006;20:1989–1994. doi: 10.1002/rcm.2550. [DOI] [PubMed] [Google Scholar]
- Ranger CM, Backus EA, Winter RE, Rottinghaus GE, Ellersieck MR, Johnson DW. Glandular trichome extracts from Medicago sativa deter settling by the potato leafhopper Empoasca fabae. Journal of Chemical Ecology. 2004;30:927–943. doi: 10.1023/b:joec.0000028459.45035.90. [DOI] [PubMed] [Google Scholar]
- Reeves AF. Tomato trichomes and mutations affecting their development. American Journal of Botany. 1977;64:186–189. [Google Scholar]
- Rick CM, Butler L. Cytogenetics of the tomato. Advances in Genetics. 1956;8:267–382. [Google Scholar]
- Rodriguez AE, Tingey WM, Mutschler MA. Acylsugars of Lycopersicon pennellii deter settling and feeding of the green peach aphid (Homoptera, Aphididae) Journal of Economic Entomology. 1993;86:34–39. [Google Scholar]
- Saeidi Z, Mallik B, Kulkarni RS. Inheritance of glandular trichomes and two-spotted spider mite resistance in cross Lycopersicon esculentum ‘Nandi’ and L. pennellii ‘LA2963’. Euphytica. 2007;154:231–238. [Google Scholar]
- Sallaud C, Rontein D, Onillon S, et al. A novel pathway for sesquiterpene biosynthesis from Z,Z-farnesyl pyrophosphate in the wild tomato Solanum habrochaites. The Plant Cell. 2009;21:301–317. doi: 10.1105/tpc.107.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Last RL, Pichersky E. Harnessing plant trichome biochemistry for the production of useful compounds. The Plant Journal. 2008;54:702–711. doi: 10.1111/j.1365-313X.2008.03432.x. [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Schauvinhold I, Larson M, Xu R, Charbonneau AL, Schmidt A, Wilkerson C, Last RL, Pichersky E. Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proceedings of the National Academy of Sciences, USA. 2009;106:10865–10870. doi: 10.1073/pnas.0904113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab B, Mathur J, Saedler R, Schwarz H, Frey B, Scheidegger C, Hulskamp M. Regulation of cell expansion by the DISTORTED genes in Arabidopsis thaliana: actin controls the spatial organization of microtubules. Molecular Genetics and Genomics. 2003;269:350–360. doi: 10.1007/s00438-003-0843-1. [DOI] [PubMed] [Google Scholar]
- Shepherd RW, Bass WT, Houtz RL, Wagner GJ. Phylloplanins of tobacco are defensive proteins deployed on aerial surfaces by short glandular trichomes. The Plant Cell. 2005;17:1851–1861. doi: 10.1105/tpc.105.031559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RW, Wagner GJ. Phylloplane proteins: emerging defenses at the aerial frontline? Trends in Plant Science. 2007;12:51–56. doi: 10.1016/j.tplants.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Simmons AT, Gurr GM. Trichomes of Lycopersicon species and their hybrids: effects on pests and natural enemies. Agricultural and Forest Entomology. 2005;7:265–276. [Google Scholar]
- Simmons AT, McGrath D, Gurr GM. Trichome characteristics of F1 Lycopersicon esculentum×L. cheesmanii f. minor and L. esculentum×L. pennellii hybrids and effects on Myzus persicae. Euphytica. 2005;144:313–320. [Google Scholar]
- Summers CB, Felton GW. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera, Noctuidae): potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochemistry and Molecular Biology. 1994;24:943–953. [Google Scholar]
- Szymanski DB. Breaking the WAVE complex: the point of Arabidopsis trichomes. Current Opinion in Plant Biology. 2005;8:103–112. doi: 10.1016/j.pbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Thipyapong P, Stout MJ, Attajarusit J. Functional analysis of polyphenol oxidases by antisense/sense technology. Molecules. 2007;12:1569–1595. doi: 10.3390/12081569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JS. Jasmonate-inducible plant defences cause increased parasitism of herbivores. Nature. 1999;399:686–688. [Google Scholar]
- van der Hoeven RS, Monforte AJ, Breeden D, Tanksley SD, Steffens JC. Genetic control and evolution of sesquiterpene biosynthesis in Lycopersicon esculentum and L. hirsutum. The Plant Cell. 2000;12:2283–2294. doi: 10.1105/tpc.12.11.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Schie CC, Haring MA, Schuurink RC. Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Molecular Biology. 2007;64:251–263. doi: 10.1007/s11103-007-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ. Secreting glandular trichomes: more than just hairs. Plant Physiology. 1991;96:675–679. doi: 10.1104/pp.96.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GJ, Wang E, Shepherd RW. New approaches for studying and exploiting an old protuberance, the plant trichome. Annals of Botany. 2004;93:3–11. doi: 10.1093/aob/mch011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams WG, Kennedy GG, Yamamoto RT, Thacker JD, Bordner J. 2-Tridecanone: a naturally occurring insecticide from the wild tomato Lycopersicon hirsutum f. glabratum. Science. 1980;207:888–889. doi: 10.1126/science.207.4433.888. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiology. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.