Abstract
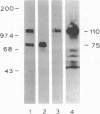
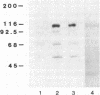
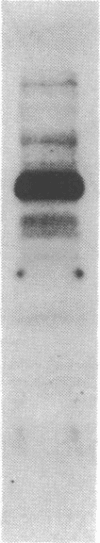
Treatment of intact human platelets with the adenylate cyclase agonist forskolin (100 microM) resulted in an increase in cAMP phosphodiesterase activity in freeze-thaw lysates. When the low-Km (high affinity), cGMP-inhibited cAMP phosphodiesterase was isolated from such lysates by blue dextran-Sepharose chromatography, the specific activity of the enzyme was increased an average of 11-fold over similarly processed control platelets. The increase in the low-Km, cGMP-inhibited cAMP phosphodiesterase activity was inhibited when platelets were incubated with the protein kinase inhibitor H-8 prior to treatment with forskolin, suggesting that the stimulation of cAMP phosphodiesterase activity involved a cAMP-dependent phosphorylation. When intact platelets that had been prelabeled with 32Pi were treated with forskolin and the low-Km, cGMP-inhibited phosphodiesterase was isolated by blue dextran-Sepharose chromatography, a protein of 110,000 kDa was phosphorylated. By using a monospecific antiserum to the purified phosphodiesterase, this protein was shown to be the low-Km, cGMP-inhibited cAMP phosphodiesterase by electrophoretic transfer blot (Western blot) analysis and by immunoprecipitation. The stable prostacyclin analog iloprost also stimulated the low-Km cAMP phosphodiesterase activity about 2-fold and caused phosphorylation of the enzyme. These results suggest that phosphorylation of the low-Km, cGMP-inhibited phosphodiesterase may be an important regulatory mechanism for this enzyme in platelets.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez R., Taylor A., Fazzari J. J., Jacobs J. R. Regulation of cyclic AMP metabolism in human platelets. Sequential activation of adenylate cyclase and cyclic AMP phosphodiesterase by prostaglandins. Mol Pharmacol. 1981 Sep;20(2):302–309. [PubMed] [Google Scholar]
- Ashby B. Cyclic AMP turnover in response to prostaglandins in intact platelets: evidence for separate stimulatory and inhibitory prostaglandin receptors. Second Messengers Phosphoproteins. 1988;12(1):45–57. [PubMed] [Google Scholar]
- Beavo J. A., Hansen R. S., Harrison S. A., Hurwitz R. L., Martins T. J., Mumby M. C. Identification and properties of cyclic nucleotide phosphodiesterases. Mol Cell Endocrinol. 1982 Nov-Dec;28(3):387–410. doi: 10.1016/0303-7207(82)90135-6. [DOI] [PubMed] [Google Scholar]
- Brothers V. M., Walker N., Bourne H. R. Increased cyclic nucleotide phosphodiesterase activity in a mutant S49 lymphoma cell. Characterization and comparison with wild type enzyme activity. J Biol Chem. 1982 Aug 25;257(16):9349–9355. [PubMed] [Google Scholar]
- Degerman E., Belfrage P., Newman A. H., Rice K. C., Manganiello V. C. Purification of the putative hormone-sensitive cyclic AMP phosphodiesterase from rat adipose tissue using a derivative of cilostamide as a novel affinity ligand. J Biol Chem. 1987 Apr 25;262(12):5797–5807. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Galvagno M. A., Moreno S., Cantore M. L., Passeron S. Cyclic adenosine 3',5'-monophosphate phosphodiesterase from Mucor rouxii: regulation of enzyme activity by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 1979 Aug 13;89(3):779–785. doi: 10.1016/0006-291x(79)91846-1. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Grant P. G., Colman R. W. Purification and characterization of a human platelet cyclic nucleotide phosphodiesterase. Biochemistry. 1984 Apr 10;23(8):1801–1807. doi: 10.1021/bi00303a034. [DOI] [PubMed] [Google Scholar]
- Hamet P., Franks D. J., Tremblay J., Coquil J. F. Rapid activation of cAMP phosphodiesterase in rat platelets. Can J Biochem Cell Biol. 1983 Nov;61(11):1158–1165. doi: 10.1139/o83-149. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Haslam R. J. Interactions of the pharmacological receptors of blood platelets with adenylate cyclase. Ser Haematol. 1973;6(3):333–350. [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Asano T. Human blood platelet 3': 5'-cyclic nucleotide phosphodiesterase. Isolation of low-Km and high-Km phosphodiesterase. Biochim Biophys Acta. 1976 Apr 8;429(2):485–497. doi: 10.1016/0005-2744(76)90296-5. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Wallace A. V., Marchmont R. J., Martin B. R., Heyworth C. M. Insulin controls intracellular cyclic AMP concentrations in hepatocytes by activating specific cyclic AMP phosphodiesterases: phosphorylation of the peripheral plasma membrane enzyme. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:159–176. [PubMed] [Google Scholar]
- Kerner N., Moreno S., Passeron S. Regulation of cyclic AMP phosphodiesterase from Mucor rouxii by phosphorylation and proteolysis. Interrelationship of the activatable and insensitive forms of the enzyme. Biochem J. 1984 Apr 1;219(1):293–299. doi: 10.1042/bj2190293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Macphee C. H., Harrison S. A., Beavo J. A. Immunological identification of the major platelet low-Km cAMP phosphodiesterase: probable target for anti-thrombotic agents. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6660–6663. doi: 10.1073/pnas.83.17.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee C. H., Reifsnyder D. H., Moore T. A., Beavo J. A. Intact cell and cell-free phosphorylation and concomitant activation of a low Km, cAMP phosphodiesterase found in human platelets. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(7):487–496. [PubMed] [Google Scholar]
- Manganiello V. C., Yamamoto T., Elks M., Lin M. C., Vaughan M. Regulation of specific forms of cyclic nucleotide phosphodiesterases in cultured cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:291–301. [PubMed] [Google Scholar]
- Marchmont R. J., Houslay M. D. Characterization of the phosphorylated form of the insulin-stimulated cyclic AMP phosphodiesterase from rat liver plasma membranes. Biochem J. 1981 Jun 1;195(3):653–660. doi: 10.1042/bj1950653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Vane J. R. Arachidonic acid metabolites and the interactions between platelets and blood-vessel walls. N Engl J Med. 1979 May 17;300(20):1142–1147. doi: 10.1056/NEJM197905173002006. [DOI] [PubMed] [Google Scholar]
- Moreno S., Galvagno M. A., Passeron S. Control of Mucor rouxii adenosine 3':5'-monophosphate phosphodiesterase by phosphorylation--dephosphorylation and proteolysis. Arch Biochem Biophys. 1982 Apr 1;214(2):573–580. doi: 10.1016/0003-9861(82)90062-5. [DOI] [PubMed] [Google Scholar]
- Salzman E. W., Weisenberger H. Role of cyclic AMP in platelet function. Adv Cyclic Nucleotide Res. 1972;1:231–247. [PubMed] [Google Scholar]
- Schrör K., Darius H., Matzky R., Ohlendorf R. The antiplatelet and cardiovascular actions of a new carbacyclin derivative (ZK 36 374)--equipotent to PGI2 in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1981 Jun;316(3):252–255. doi: 10.1007/BF00505658. [DOI] [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Calmodulin and Ca2+-dependent phosphorylation and dephosphorylation of 63-kDa subunit-containing bovine brain calmodulin-stimulated cyclic nucleotide phosphodiesterase isozyme. J Biol Chem. 1986 Jan 25;261(3):1322–1328. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Differential regulation of bovine brain calmodulin-dependent cyclic nucleotide phosphodiesterase isoenzymes by cyclic AMP-dependent protein kinase and calmodulin-dependent phosphatase. Proc Natl Acad Sci U S A. 1985 May;82(9):2603–2607. doi: 10.1073/pnas.82.9.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegl A. M., Daly J. W., Smith J. B. Inhibition of aggregation and stimulation of cyclic AMP generation in intact human platelets by the diterpene forskolin. Mol Pharmacol. 1982 May;21(3):680–687. [PubMed] [Google Scholar]
- Sinha A. K., Shattil S. J., Colman R. W. Cyclic AMP metabolism in cholesterol-rich platelets. J Biol Chem. 1977 May 25;252(10):3310–3314. [PubMed] [Google Scholar]
- Thompson W. J., Terasaki W. L., Epstein P. M., Strada S. J. Assay of cyclic nucleotide phosphodiesterase and resolution of multiple molecular forms of the enzyme. Adv Cyclic Nucleotide Res. 1979;10:69–92. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay J., Lachance B., Hamet P. Activation of cyclic GMP-binding and cyclic AMP-specific phosphodiesterases of rat platelets by a mechanism involving cyclic AMP-dependent phosphorylation. J Cyclic Nucleotide Protein Phosphor Res. 1985;10(4):397–411. [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Lieberman F., Osborne J. C., Jr, Manganiello V. C., Vaughan M., Hidaka H. Selective inhibition of two soluble adenosine cyclic 3',5'-phosphate phosphodiesterases partially purified from calf liver. Biochemistry. 1984 Feb 14;23(4):670–675. doi: 10.1021/bi00299a013. [DOI] [PubMed] [Google Scholar]