Abstract
Glutathione (GSH) and ascorbate (ASC) are important antioxidants that are involved in stress defence and cell proliferation of meristematic root cells. In principle, synthesis of ASC and GSH in the roots as well as ASC and GSH transport from the shoot to the roots by phloem mass flow is possible. However, it is not yet known whether the ASC and/or the GSH level in roots depends on the supply from the shoot. This was analysed by feeding mature leaves with [14C]ASC or [35S]GSH and subsequent detection of the radiolabel in different root fractions. Quantitative dependency of root ASC and GSH on shoot-derived ASC and GSH was investigated with poplar (Populus tremula×P. alba) trees interrupted in phloem transport. [35S]GSH is transported from mature leaves to the root tips, but is withdrawn from the phloem along the entire transport path. When phloem transport was interrupted, the GSH content in root tips halved within 3 d. [14C]ASC is also transported from mature leaves to the root tips but, in contrast to GSH, ASC is not removed from the phloem along the transport path. Accordingly, ASC accumulates in root tips. Interruption of phloem transport disturbed the level and the ASC redox state within the entire root system. Diminished total ASC levels were attributed mainly to a decline of dehydroascorbate (DHA). As the redox state of ASC is of particular significance for root growth and development, it is concluded that phloem transport of ASC may constitute a shoot to root signal to coordinate growth and development at the whole plant level.
Keywords: Ascorbate, glutathione, phloem transport, poplar, redox state, root growth
Introduction
Ascorbate (ASC) and glutathione (GSH) are important antioxidants that exhibit numerous functions in stress defence, regulation of plant metabolism, as well as growth and development (May et al., 1998; Meyer and Hell, 2005; Mullineaux and Rausch, 2005; Halliwell, 2006; Noctor, 2006; Meyer, 2008; Foyer and Noctor, 2009). Both ASC and GSH are involved in root development due to their function in redox regulation. However, this function is executed in an independent way because one cannot compensate for the absence of the other (Sánchez-Fernández et al., 1997; Potters et al., 2002, 2004; Jiang and Feldman, 2005). Maintenance of the root quiescent centre (QC) is accompanied by low total ASC content and high ascorbate oxidase activity (Kerk and Feldman, 1995; Liso et al., 2004); as a result the total ASC pool is dominated by dehydroascorbate (DHA) (Jiang et al., 2003). ASC is necessary for the transition from G1 to S in the cell cycle (Liso et al., 1988).
As a consequence, any changes in ASC content affect cell cycle activity. Hence, the G1 state is extended when the ASC content of the cells in the QC is low, as reviewed in Potters et al. (2002). Application of ASC induced cell division in Allium cepa roots (Liso et al., 1988). In the tobacco cultivar Bright Yellow 2 (BY-2), ASC stimulated cell division while DHA decreased the mitotic index (de Pinto et al., 1999) and slowed down cell cycle progression (Potters et al., 2004). The latter, however, was only observed when DHA was added in the G1 phase (Potters et al., 2004). ASC treatment of Arabidopsis roots resulted in a complete loss of a QC marker. Lee et al. (2007) concluded that ASC treatment might change the maintenance of cell type identities in roots, and affected cell type-specific gene expression. When auxin transport was inhibited by a specific inhibitor, DHA in the QC declined to approximately one-third compared to the controls, whereas ASC increased; these reactions were accompanied by an activation of the distal region in the QC (Jiang et al., 2003). These examples clearly demonstrate the importance of the ASC to DHA ratio and its adjustment for root growth and development.
GSH, though less intensively studied, is also important for root growth and development (Potters et al., 2002). GSH enhanced the number of meristematic cells undergoing mitosis, while depletion of GSH had the opposite effect (Sánchez-Fernández et al., 1997). Inhibition of GSH synthesis by the specific inhibitor buthionine sulphoximine (BSO) resulted in reduced root formation (Cobbett et al., 1998). In accordance with this, a mutant from Arabidopsis (rml1) deficient in γ-glutamylcysteine synthetase (γ-ECS) is unable to establish roots (Vernoux et al., 2000). γ-ECS catalyses the first step of glutathione synthesis. Thus this mutant revealed a markedly diminished GSH content.
Root hair development depends on formation of reactive oxygen species (ROS) by NADPH oxidase (Foreman et al., 2003). As ASC and GSH are involved in ROS detoxification (Noctor and Foyer 1998), the amounts of ASC and/or GSH as well as the maintenance of its redox state seem to be important for root hair growth. Indeed, in Arabidopsis roots, the GSH level is linked to root hair tip growth, and redox-dependent modulation is thought to be a crucial element in adjusting growth and development to the environment conditions (Sánchez-Fernández et al., 1997). In addition to the influences of ASC on cell division within the QC, it may also be involved in lateral root development, since a very low ASC content Arabidopsis mutant (vtc2) exhibited altered root growth with the number and length of lateral roots being increased (Olmos et al., 2006). As ASC removes ROS (Foyer and Halliwell, 1976; Noctor and Foyer, 1998), the low ASC in roots of the vtc2 mutant can improve lateral root development (Foreman et al., 2003; Olmos et al., 2006) probably by retaining ROS. Therefore, low ASC contents mediated by ROS scavenging under stress conditions may improve growth, as discussed by Olmos et al. (2006). From these observations it can be hypothesized that ASC and/or GSH transported from the shoot to the roots may affect the levels of these antioxidants in the roots and thereby function as a shoot to root signal for growth and development. A prerequisite for such a function is that the ASC and GSH level in the root tip depends on its long-distance transport from the shoot.
The highest level of ASC synthesis takes place in the leaves, but ASC synthesis seems to be apparent in all plant cells (see Hancock et al., 2003). Feeding of L-galactono-1,4-lactone, the precursor of ascorbate (Smirnoff et al., 2001), results in increased ASC contents mainly in mature leaves of Medicago sativa (Franceschi and Tarlyn, 2002) but also in Cucurbita maxima roots (Liso et al., 2004). ASC is a widespread constituent of phloem sap, and isolated phloem strands are competent for ASC biosynthesis (Hancock et al., 2003). Transport of ASC from leaves to sink tissues such as root tips and floral tissues has been demonstrated for three herbaceous plant species (Franceschi and Tarlyn, 2002). Since sulphate assimilation is a light-dependent process (Brunold, 1990) and because cysteine formation limits GSH synthesis (Strohm et al., 1995) it is assumed that GSH is mainly synthesized in the leaves. This is supported by the finding that GSH synthesis can be stimulated with increasing light intensity (Ogawa et al., 2004). However, glutathione production has also been found in other plant organs including the roots (Vauclare et al., 2002). Like ASC, GSH is a regular constituent of phloem sap (Rennenberg et al., 1979; Bonas et al., 1982; Lappartient and Touraine, 1996; Bourgis et al., 1999; Hartmann et al., 2000; Kuzuhara et al., 2000; Schulte et al., 2002) and is transported from mature leaves to the roots (Rennenberg et al., 1979; Bonas et al., 1982; Hartmann et al., 2000). However, it has not been established whether ASC and/or GSH levels in the roots and, hence, root growth and development depend on in situ synthesis of these antioxidants or its long-distance transport from the shoot in the phloem. The aim of the present study was to address these questions by two different approaches. Radiolabelled ASC or GSH was fed to a mature poplar leaf and the distribution of radioactivity in different root fractions was determined. In girdling experiments, where phloem transport to the root was interrupted at the transition between stem and root, the dependency of the ASC and GSH levels in different root fractions on shoot-derived ASC and GSH was determined.
Materials and methods
Plant material and growth conditions
Seedlings of the poplar hybrid Populus tremula×P. alba clone 717 1B4 (Institute National de la Recherche Agronomique, INRA) were micropropagated as described by Strohm et al. (1995) and Noctor et al. (1996). After 4 weeks, cuttings were transferred onto quartz sand (0.7–2 mm, Götz and Moritz, Freiburg, Germany) and were grown in a greenhouse (26±5 °C) under long day (16 h light) conditions and a light intensity that varied from 60 μmol m2 s−1 to 600 μmol m2 s−1 depending on the weather conditions. At full sunlight the greenhouse was shaded automatically. Seedlings were watered with 1/4 modified Hoagland solution combined with Long-Ashton medium (Strohm et al., 1995) consisting of 1.25 mM KNO3, 2.5 mM Ca(NO3)2, 0.5 mM MgSO4, 4.5 mM MgCl2, 0.25 mM KH2PO4, 2.3 μM MnCl2, 10 μM H3BO3, 0.08 μM CuCl2, 0.2 μM ZnCl2, 0.2 μM Na2MoO4, 0.04 μM CoCl2, 22.5 μM FeCl2, and 22.5 μM Na2EDTA. Plants were harvested after 8 weeks of growth. Since the bark of deciduous trees includes the phloem, interruption of phloem transport can be achieved by peeling off the bark (Mason and Maskell, 1928). In girdling experiments, 2 cm of the bark was peeled off at the stem–root transition around the entire plant.
Feeding of [35S]GSH and L-[14C]ASC to the leaves
[35S]GSH was fed to leaves using the flap feeding technique of Biddulph (1956). A flap was cut into a mature leaf so that the connection to the main vein was maintained in the direction of the petiole. For this purpose, the first two cuts of ∼ 1 mm were made lateral to the main leaf vein. The third cut was made to release the leaf vein from the leaf lamina. The flap that contained part of the main vein remained connected in the direction of the petiole. In this way GSH and ASC were fed directly into the phloem, and phloem transport out off the fed leaf was facilitated. During cutting of the flap, the leaf was submerged in potassium phosphate buffer (50 μM K2HPO4/KH2PO4 buffer, pH 6.2). After cutting, the flap was dipped immediately into a test tube containing the feeding solution, i.e. 15.6 μl of [35S]GSH {30 μCi [35S]GSH (Hartmann Analytic GmbH, Braunschweig, Germany) prepared from an aqueous solution containing 1075 Ci mmol−1 GSH and 10 mM dithiothreitol (DTT)} or 15 μl of [14C]ascorbic acid {30 μCi of L-[1-14C]ASC (American Radiolabeled Chemicals, Inc., St. Louis, MO, USA) prepared from solid ASC with 8.5 mCi mmol−1 in 50 μM K2HPO4/KH2PO4 buffer pH 6.2}. The feeding solutions were taken up completely within 30±5 min.
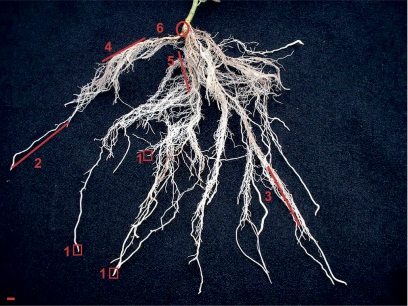
After a total incubation time of 2, 3, or 5 h in the case of [35S]GSH feeding, or after 5 h in the case of [14C]ASC feeding at room temperature (25±3 °C) and 600±30 μE m−2 s−1 PAR (Osram, HPS L 65W/150 ultra white and Osram, L Fluora 35W/77R, Osram, Munich, Germany) at plant height, incubation was terminated by cutting off the fed leaf. Subsequently, poplar trees were dissected into the apex, and the first, second, third, seventh, and 11th leaves counted from the apex. The trunk section basal to the fed leaf was divided into sections of 2 cm in length that were separated into bark and wood. The root system was separated into six root fractions of different developmental stages (Fig. 1). Fraction 6 (R6) was the main root that appears green. Smaller roots that showed secondary growth and appeared red were combined in fraction 5 (R5). Roots with secondary growth that appeared white constituted fraction 4 (R4). Fraction 3 (R3) contained roots with a diameter of ∼ 0.5–1 mm and small side roots. Long white roots without side roots that were ∼ 1 mm in diameter were combined in fraction 2 (R2). Root tips were sampled whenever possible and were combined in fraction 1 (R1). All samples were immediately frozen in liquid nitrogen and stored at –24 °C until analysis.
Fig. 1.
The root system of an 8-week-old poplar plant grown in sand culture. The root system was dissected into six fractions of different developmental stages. Fraction 6 (R6) was the main root that appears green. Smaller roots that revealed secondary growth and appeared red were indicated as fraction 5 (R5). Roots with secondary growth which appeared white constituted fraction 4 (R4). Fraction 3 (R3) contained the roots with a diameter of ∼ 0.5–1 mm and developed small side roots. The long white roots without side roots that were ∼ 1 mm in diameter comprised fraction 2 (R2). The root tips were sampled whenever possible and were combined in fraction 1 (R1). The bar indicates 5 mm.
35S and 14C analyses
35S and 14C radioactivity was determined in 20–100 mg of powdered (under liquid nitrogen) plant tissue as described by Herschbach and Rennenberg (1996). After solubilization with a tissue solubilizer (1 ml of Soluene 350, Packard Instruments, Frankfurt, Germany), samples were bleached with 200 μl of H2O2 (30%) overnight. After adding 5 ml of scintillation fluid (HiSafe 3, Packard Instruments, Frankfurt, Germany), radioactivity was determined using a liquid scintillation counter (Wallac System 1409, Wallac, Turku, Finland). Data were corrected for quenching.
Analyses of thiols and 35S-labelled metabolites
Thiols, i.e. cysteine, γ-EC, and GSH, were extracted, derivatized, and quantified as described by Strohm et al. (1995) and Herschbach et al. (2000). A 30 mg aliquot of leaf material powdered under liquid nitrogen or 100 mg of root and bark powder was homogenized in 750 μl of 0.1 M HCl containing 50 mg of insoluble polyvinylpolypyrrolidone (PVPP). Samples were centrifuged (14 000 g, 15 min) and 120 μl of the clear supernatant was added to 180 μl of 200 mM CHES buffer (pH 9.3). Reduction of reduced disulphides was performed with 30 μl of 15 mM DTT for 1 h at room temperature. Thiols were derivatized with 20 μl of 30 mM monobromobimane and stabilized by adding 240 μl of 10% (v/v) acetic acid after 15 min of derivatization. Aliquots of 150 μl were taken to separate bimane conjugates by HPLC analysis (SUPERCOSILTM LC-18, 25 cm×4.6 mm, 5 μm, Sigma-Aldrich) as described by Schupp and Rennenberg (1988) using 10% (v/v) methanol, 0.25% (v/v) acetic acid (pH 3.9) as solvent A and 90% (v/v) methanol, 0.25% (v/v) acetic acid (pH 3.9) as solvent B. Bimane derivatives were detected by fluorescence detection at 480 nm after excitation at 380 nm (Schupp and Rennenberg, 1988) and quantified by the use of external standards. During this analysis [35S]sulphate eluted prior to cysteine. To determine the amount of 35S in thiols, 1 ml fractions of the HPLC eluate were collected. After addition of 4 ml of scintillation fluid (HiSafe 3, Packard Instruments, Frankfurt, Germany), radioactivity was determined by liquid scintillation counting and classified by comparison with the fluorescent detector output.
Analyses of 14C-labelled metabolites
For 14C-labelled metabolite analysis in plants fed [14C]ASC a combination of HPLC analysis and liquid scintillation counting was applied. For this purpose, ASC was determined as described by Polle et al. (1990). ASC and DHA were extracted from 100 mg of root or wood tissue or from 50 mg of leaf tissue in 500 μl of meta-phosphoric acid (5%, v/v) plus 50 mg of PVPP at 4 °C. Total ASC was determined after enzymatic oxidation of ASC to DHA by ascorbate oxidase. Aliquots of 50 μl of tissue extracts were diluted with 100 μl of sodium acetate (200 mM, pH 6.2). After addition of 15 μl of ascorbate oxidase (1 mg ml−1 in 200 mM sodium acetate pH 6.2) the mixture was incubated for 15 min at 37 °C. Thereafter, 100 μl of 3.7 mM sodium acetate were added and the mixture was kept further for 30 min at room temperature. Thereafter, DHA was derivatized after addition of 50 μl of o-PDA (o-phenyldiamine, 1 mg ml−1 ethanol) during 30 min at room temperature in the dark. The final volume was adjusted to 655 μl. A 150 μl aliquot was taken to separate the DHA derivative by isocratic HPLC analysis with a solvent consisting of 80 mM K2HPO4 and 20% (v/v) methanol (pH 7.8 adjusted with ortho-phosphoric acid) on a reversed phase column (ODS 15×4.6 mm, 5 μm Ultrasphere™, Beckman Lincoln, Krefeld, Germany). Fluorescence of the DHA derivate was measured at 450 nm after excitation at 350 nm. DHA was quantified using external standards. Radioactivity within the eluate was determined with a liquid scintillation counter in 1 min fractions after adding 4 ml of scintillation fluid (HiSafe 3, Packard Instruments, Frankfurt, Germany).
ASC assay
ASC in non-radioactive samples was determined photometrically using the method of Okamura (1980) as described by Haberer et al. (2007). ASC and DHA were extracted from 20–25 mg of plant tissue, which was frozen and powdered under liquid nitrogen, with 500 μl of meta-phosphoric acid (5%; w/v) at 4 °C on ice. The mixture was stirred and centrifuged (14 000 g, 30 min, 4 °C). The supernatant (100 μl) was neutralized with triethanolamine (20 μl, 1.5 mM) and mixed with sodium phosphate buffer (100 μl, 150 mM, pH 7.4). The ASC in the assay was measured directly, while total ASC was measured after complete reduction by DTT (50 μl, 10 mM, 30 min). Excess DTT was removed with N-ethylmaleimide (50 μl of NEM, 0.5%). Samples for ASC analysis were treated in the same way as described by Haberer et al. (2007). ASC reduces ferric ions to ferrous ions which coupled with 2,2′-dipyridyl to form a complex with a characteristic absorption at 525 nm, allowing quantification (Okamura, 1980). DHA was then calculated by subtraction of readings for ASC from readings for total ASC.
Data analysis
Significant differences in ASC, DHA, and GSH contents between treatments (n=3) and between root sections (n=3) of girdled trees were analysed with the statistics program SPSS 16.0 for windows (Chicago, IL, USA). Prior to the test of significance with the Turkey test, the normality and homogeneity of the data were tested. Normality of the data was tested with the Kolmogorov–Smirnov test that includes correction of significance after Lilliefors and Shapiro-Wilk. Homogeneity of variance was tested with the Levene test. If homogeneity was not given, values were transferred using the natural logarithm. If homogeneity was still not given, the Games–Howell test was applied. Significant differences at P <0.05 are indicated.
Results
Translocation of 35S after [35S]GSH feeding to a leaf
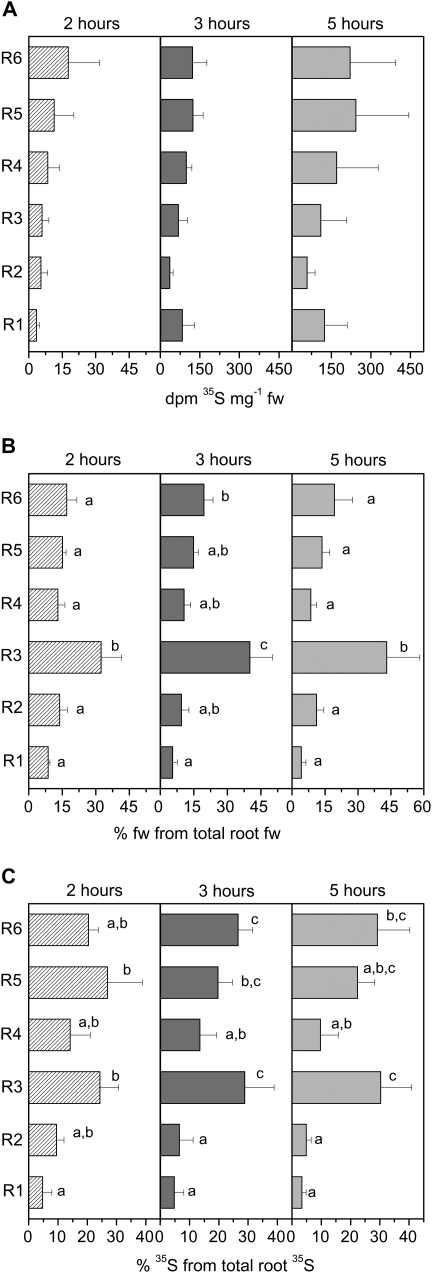
One leaf of poplar plants (one of the ninth to 14th leaf counted from the apex) was fed with [35S]GSH by flap feeding (Biddulph, 1956) and the subsequent 35S transport out of the fed leaf was terminated after 2, 3, and 5 h. Along the transport path, the highest labelling in bark and wood was detected just below the fed leaf, and labelling decreased with increasing distance from the fed leaf (data not shown). After 2 h of incubation, only low levels of 35S were detected in the root tips or any other root fraction (Fig. 2A). 35S reached the root tips 3 h after starting [35S]GSH feeding. A slight enrichment of 35S in the root tips compared to the roots of fraction 2 was observed after 5 h of incubation. As the root tips only amounted to 4.2–8.7% of total root mass (Fig. 2B), the distribution of 35S within the root system was calculated (Fig. 2C). From this calculation, it appears that within the root system, 35S is preferentially found in the roots of fraction R3 and in the main root (fraction R6). This distribution correlates well with the biomass of different root fractions that was highest in roots of fraction R3 followed by the main root (R6) (Fig. 2B).
Fig. 2.
35S in root fractions (A), relative root fresh weight of each fraction (B), and relative 35S distribution between root fractions (C). (A) 35S radioactivity in the six root fractions (see Fig. 1) 2, 3, and 5 h after feeding [35S]GSH to a mature leaf. (B) Contribution of the six different root fractions to total root fresh weight. Total root fresh weight of the individual poplar plants was set to 100% and the percentage of each fraction was calculated. (C) The contribution of 35S detected in each of the six root fractions to total root 35S radioactivity per root system of poplar plants fed with [35S]GSH to a mature leaf. Total 35S determined in the whole root system was set to 100%. The contribution of each root fraction was calculated. Data given are mean values from three poplar plants investigated per time point. Different indices indicate significant differences between root fractions at P <0.05.
Along the transport path from the fed leaf to the roots, the [35S]GSH to [35S]sulphate ratio decreased. This indicates that with increasing distance from the fed leaf, the amount of labelled GSH declined in comparison with the amount of labelled sulphate. Whereas the [35S]GSH to [35S]sulphate ratio in the fed leaf ranged from 1.0 to 1.5, it amounted to 0.2 in the bark, 0.4 in the wood, and ranged from 0.17 to 0.33 in the roots.
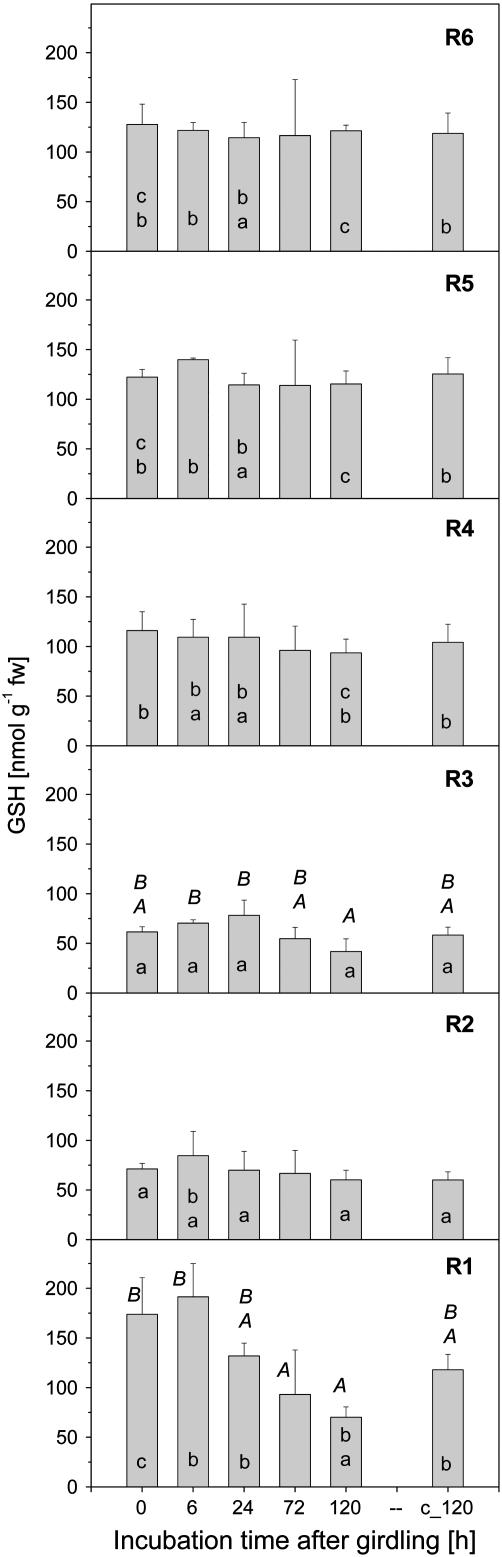
GSH contents in roots after stem girdling
Poplar plants were girdled at the transition of the stem to the roots to disrupt glutathione transport in the phloem. Glutathione contents in roots of different fractions were analysed 6 h, 24 h, 3 d, and 5 d after girdling (Fig. 3). Roots from control poplar plants without a bark girdle were harvested twice; first at time zero and secondly after 120 h, i.e. 5 d after starting the experiment. The GSH content without girdling was similar in roots of fraction R4, R5, and R6, but only half as high in roots of fraction R3 and R2 (Fig. 3). GSH contents in the root tip (R1) were ∼ 30% higher compared to the main root (R6). Disruption of phloem transport did not affect the glutathione content of the roots of fraction R2, R3, R4, R5, or R6, but GSH in the root tips (fraction R1) declined with increasing time of disrupted phloem transport. After 3 d of disruption of phloem transport, the GSH content of the root tips was diminished to about half the level determined prior to girdling. Compared with control roots which were harvested on day 0 and day 5, the GSH content in root tips after 5 d of phloem transport interruption was diminished to 40% and 60%, respectively. GSSG contents in roots were not affected by girdling in any root fraction either 6 h or 5 d of girdling (data not shown).
Fig. 3.
GSH contents in the six different root fractions of 8-week-old poplar plants girdled for different periods of time at the transition between stem and root. c_120 indicates an additional control harvested at day 5 after starting the experiment. Data given are mean values ±SD from three plants at the time indicated. (a) Indices indicate significant differences between the six root fractions during one harvest time at P <0.05; (A) indicates significant differences between sampling dates along one root fraction at P <0.05. The absence of indices indicated that significant differences were not found.
Translocation of 14C after [14C]ASC feeding to a leaf
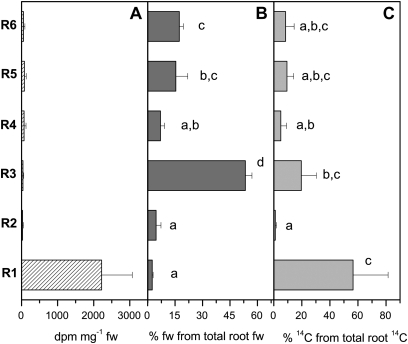
After 5 h incubation, the 14C from [14C]ASC fed to a mature leaf (16th or 18th counted from the apex) was detected mainly in the root tips (Fig. 4A). Preferential translocation of 14C label to the root tip was also obvious when the absolute distribution of 14C within the root system was calculated. Although the contribution of root tips to total root fresh weight was only 2.5±0.5% (Fig. 4B), the proportion of 14C within the root tip fraction amounted to 56.5±25.0% (Fig. 4C). This means that root tips are the preferential sink for the 14C transported from the fed leaf to the root system. 14C was also detected in the apex (46±48 dpm mg−1 fw) and in young developing leaves (76±81 dpm mg−1 fw); however, at amounts comparable with those found in the main root fraction (R6) (53±39 dpm mg−1 fw). In both the apex and the main root, the ASC determined by HPLC analysis correlated with the 14C in the corresponding fraction of the HPLC eluate (data not shown). Apparently, [14C]ASC is transported in both the acropetal and basipetal direction. These results indicate that [14C]ASC is transported from the fed leaf to the roots by phloem transport, but, in contrast to [35S]GSH, ASC was not taken up from the phloem along the transport path and was preferentially transported to the root tips.
Fig. 4.
Distribution of 14C within the root system 5 h after [14C]ASC was fed to a mature leaf. Data given in A are mean values of dpm mg−1 fresh weight from the three trees analysed. Data given in B are mean values ±SD of the percentage of each root fraction from total root fresh weight. Data given in C represent the amount of 14C from each root fraction relative to total 14C determined in the whole root system that was set to 100%. Different indices indicate significant differences between root fractions at P <0.05.
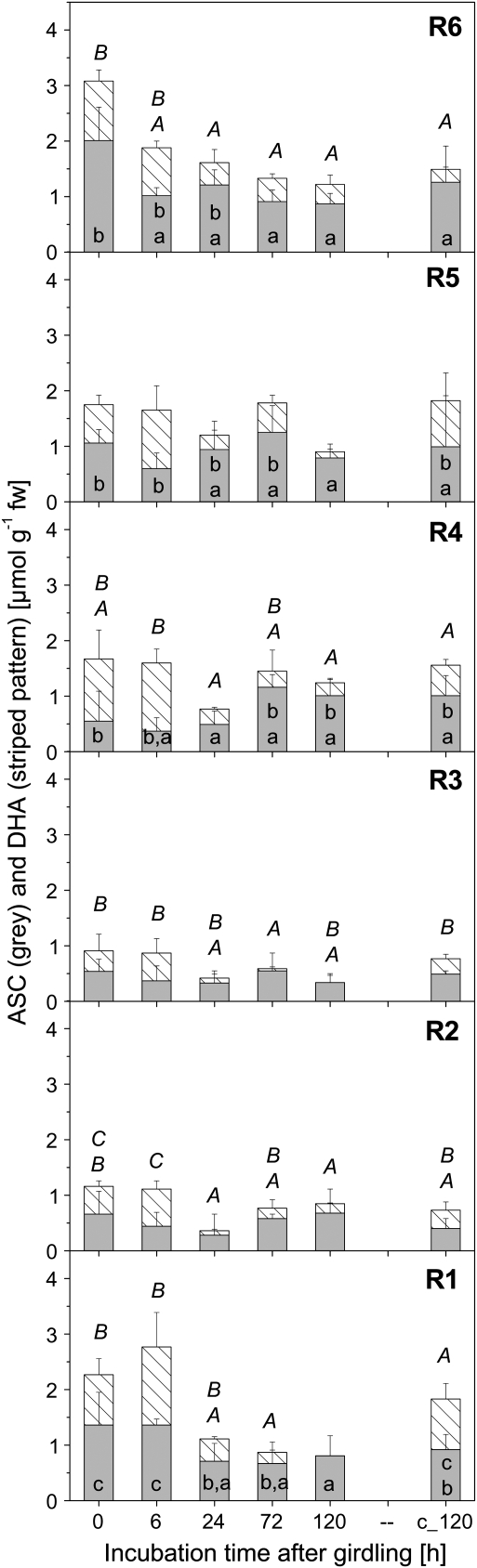
ASC contents in roots after stem girdling
ASC and DHA were analysed in the root system after disruption of phloem transport by girdling (Fig. 5). Total ASC levels in roots reached only 10–40% of the levels in leaves (∼ 6–8 μmol ASC g−1 leaf fw). The lowest amounts of total ASC were found in roots of fractions R2 and R3, while total ASC levels were higher in roots of fractions R6, R5, R4, and R1 (Fig. 5). Girdling resulted in a significant decline in total ASC in root tips (R1) and in roots from fraction R3 and R6. In these fractions, total ASC was diminished to ∼ 40% of that in controls after 5 d of girdling. The contribution of oxidized to total ASC was 30–60% prior to girdling and 6 h after phloem disruption by girdling. This was also true for the root tips, where 40% and 50% of the ASC was found to be oxidized, respectively (Fig. 5). After 24 h of girdling the contribution of DHA to total ASC declined significantly. After 120 h of phloem disruption by girdling, the pool of DHA was significantly diminished in all root fractions. This was clearly an effect of girdling, as non-girdled control poplar plants did not show such an effect.
Fig. 5.
Total ascorbate (complete bar), ASC (grey parts), and DHA (striped pattern) in different root fractions of 8-week-old poplar plants girdled for different periods of time at the transition between stem and root. Total ascorbate and ASC were determined photometrically as described by Haberer et al. (2007). DHA contents were calculated as the difference between these two parameters. c_120 indicates an additional control harvested at day 5 after starting the experiment. Data given are means ±SD from three individual plants. (a) Indices indicate significant differences of total ASC within one root fraction between treatments at P <0.05 and (A) indicates significant differences of DHA of a given root fraction between treatments at P <0.05. Absence of indices indicates that significant differences were not found.
Discussion
In poplar plants ASC is transported from mature leaves to the roots. As previously observed for herbaceous plants (Franceschi and Tarlyn, 2002) leaf fed [14C]ASC accumulated in poplar root tips. In contrast to the two herbaceous species Medicago sativa and Arabidopsis where young leaves, flower buds, and siliques are strong sinks for the applied [14C]ASC, this was not observed with poplar. Although [14C]ASC labelling was found in the poplar apex (46±48 dpm mg−1 fw) and in young developing leaves (76±81 dpm mg−1 fw) 5 h after feeding, this labelling was negligible compared to the labelling of root tips (R1) (2219±849 dpm mg−1 fw). Whether flowers or seeds of poplar are sinks for ASC from mature leaves could not be answered in the present study, because poplar trees switch from juvenile growth to reproductive growth after only 7–10 years (Hsu et al., 2006). Thus, it cannot be excluded that during flowering and seed development phloem transport into apical sink tissues also takes place in poplar, as found for herbaceous plants.
During ASC transport to the roots ASC was not withdrawn from the phloem and was thus not stored in bark or wood tissues, or in any other root fraction. This was indicated by a lack of [14C]ASC accumulation in these tissues (data not shown). Thus it can be concluded that root tips of poplar trees are a preferential sink of shoot-derived ASC. Contrasting results were found for GSH. Along the transport path, 35S from the 35S fed as GSH to a mature leaf decreased continuously up to the main root and accumulated in the bark and wood of the trunk (data not shown). Simultaneously, the proportion of radiolabelled sulphate compared to GSH increased. Previous experiments also showed that radiolabelled GSH in the phloem declined during its transport from source to sink, whereas sulphate remained more or less constant (Hartmann et al., 2000). Nevertheless, GSH is a regular sulphur constituent of phloem exudates from poplar (Herschbach et al., 1998) and thus it is transported from the shoot to the roots (present study). Thus, although GSH was transported up to fine roots, specific accumulation of [35S]GSH in root tips was not found 5 h after [35S]GSH was fed to a mature leaf. In conclusion, root tips of poplar trees are a preferential sink of shoot-derived ASC, but not for shoot-derived GSH or sulphate.
Beside transport to the roots it must be considered that ASC could be synthesized in roots. When Cucurbita maxima roots were fed with the ASC precursor L-galactono-1,4-lactone, the ASC level increased (Liso et al., 2004); and the enzyme catalysing the final step of ascorbate synthesis (L-galactono-1,4-lactone dehydrogenase) was detected both in leaves and in roots (Groten et al., 2005; Matamoros et al., 2006). ASC levels in roots are usually low compared to leaves (Franceschi and Tarlyn, 2002; Matamoros et al., 2006) as also found in the present study (7.0±1.1 μmol ASC g−1 fw in leaves versus 2.3±0.3 μmol ASC g−1 fw in fine roots). The girdling experiments with poplar indicate that — despite ASC synthesis in roots (Liso et al., 2004; Groten et al., 2005; Matamoros et al., 2006) — even these low root ASC levels are largely maintained by phloem transport of ASC. Approximately half of the ASC was lost in root fractions R1, R3, R5, and R6 24 h after disruption of phloem transport. This decline is consistent with the ASC turnover rate of 2.5% h−1 previously reported in Arabidopsis leaves (Conklin et al., 1997). In germinating pea seedlings an even faster turnover of ASC of 13% h−1 has been reported (Pallanca and Smirnoff, 2000). Phloem transport interruption simultaneously induced a decline in DHA content of poplar roots. Apparently, phloem transport of ASC plays a decisive role in ASC homeostasis in poplar roots.
Kerk and Feldmann (1995) established a direct correlation between the ASC redox state and cell proliferation rates. High DHA contents blocked the transition from G1 to S in the QC of Zea mays roots that correlated with low ASC contents in the QC and prevented cell proliferation, while higher ASC levels induced cell division in surrounding meristematic initials. In tobacco cell cultures (BY-2), peak values of ascorbate, but not GSH, coincided with a peak in the mitotic index (de Pinto et al., 1999). Similar to decreasing DHA during the G1 phase that may shorten the cell cycle in tobacco cell cultures (Kato and Esada, 1999), the reduction of DHA in roots as found in the present study after girdling could trigger cell division and thus may change root growth and development. This was supported by an ASC-deficient mutant (vtc2) that revealed changes in the root system (Olmos et al., 2006). The most noticeable root phenotype is the aberrant gravitropic response of the primary root and lateral roots of the vtc2 mutant. Thus, phloem-transported ASC may be considered as a signal controlling root growth and development. Still, a phloem-transported signal that controls root ASC synthesis cannot be excluded from the present experiments. However, ASC re-synthesis in the roots from ASC degradation products synthesized in the fed leaf is unlikely because specific labelling of 14C coincides with the HPLC ASC peak (data not shown).
The low shoot-derived GSH supply to root tips may be the result of significant sulphate assimilation so that the roots are largely independed of reduced sulphur from the shoot. Tips of maize roots showed the highest levels of adenosine 5′-phosphosulphate reductase activity (Kopriva et al., 2001), the enzyme that catalyses the regulatory step for sulphate reduction (Kopriva and Koprivova, 2004; Martin et al., 2005). GSH synthesis from sulphate has been demonstrated in excised root of Arabidopsis (Vauclare et al., 2002) and poplar roots (Scheerer et al., 2009). Therefore, it seems possible that roots are self-sufficient in sulphate reduction and thus in GSH synthesis. Another explanation for the lack of preferential translocation of shoot-derived GSH to the root tip may be GSH degradation in the fed leaf and GSH re-synthesis in the roots. As cysteine is not the main transport form of reduced sulphur in the phloem of poplar (Herschbach et al., 1998, 2000) and radiolabelling of the cysteine pool was not observed in phloem exudates from poplar (Hartmann et al., 2000), it seems improbable that cysteine is the transport form of reduced sulphur from mature leaves to roots. Hence GSH re-synthesis from shoot-derived cysteine within the roots seems unlikely, but GSH synthesis from shoot-derived glutamate and/or glycine cannot be excluded. Nevertheless the GSH content in root tips declined after 3 d of phloem transport interruption, indicating that part of the GSH in root tips originates from the shoot. The observed decline suggests a contribution of long-distance transport to the compensation of GSH turnover in the root tips of ∼ 0.5% h−1. Apparently, this low rate of delivery by phloem transport is required to maintain the GSH level in the root tips that do not seem from these experiments to be completely self-sufficient in GSH synthesis. As an alternative explanation, a shoot signal delivered by phloem transport may be necessary for full capacity of GSH biosynthesis in root tips.
It seems from the present experiments that poplar root tips are dependent on GSH and ascorbate from the shoot. However, the rates are different (around 10% losses of GSH, but even 50% loss of total ASC after 24 h phloem interruption). As the ASC content in roots was one order of magnitude higher than that of GSH it may be speculated that the effect of ASC on redox-dependent root growth is more relevant. Maintenance of the cellular redox state does not only depend on the concentration of an antioxidant, but — among other factors — also on the rate constant of its conversion in disturbing reactions. As the rate constant for superoxide radical scavenging is higher for GSH than for ASC (Rennenberg and Polle, 1994), ASC may be a less potent chemical antioxidant than GSH at similar concentrations. This may be overcome by higher ASC concentrations, as frequently observed in plant tissues including roots (Rennenberg and Polle, 1994; Noctor, 2006; Rennenberg et al., 2007), thereby supporting the relevance of ASC for redox-dependent root growth under these conditions. This is supported by an Arabidopsis mutant (vtc) low in ASC. This mutant showed changes in hormone levels and primary root development, and also changed stress sensitivity. It can therefore be assumed that the ASC level is one possible internal signal allowing plants to respond to environmental stimuli by adjusting growth and development (Kotchoni et al., 2009).
Since both GSH and ASC are subject to compartmentation, the subcellular distribution of GSH and ASC seems to be essential for its functions in plant growth and development. This point is even more important when it is considered that both antioxidants affect root growth and development in different ways. Whereas ASC/DHA seems more effective in cell proliferation, GSH affects cell size determination in tobacco cell cultures (de Pinto et al., 1999) and root hair formation that could not be mimicked by ASC in Arabidopsis roots (Sánchez-Fernández et al., 1997). The latter corresponds to high GSH contents in the root epidermis and in rapidly dividing cells (Fricker et al., 2000). Nevertheless, GSH depletion blocks cell proliferation in BY-2 tobacco cell cultures (Vernoux et al., 2000). Potters et al. (2002) concluded that a sufficient GSH concentration is necessary for regular cell division. This view is supported by results from studies with Arabidopsis mutants. Whereas root development was not found in the rml1 mutant that exhibited only 3% of the GSH detected in control roots (Vernoux et al., 2000), cell division in roots, i.e. normal root development, was observed in the cad2-1 mutant which exhibits 30% of the GSH compared to the control (Howden et al., 1995).
From these studies it can be concluded that not only the redox state but also the absolute amounts of GSH and ASC are important for the development of plants in a changing environment. The present study shows that shoot to root transport in the phloem is required to maintain the GSH and ASC levels in the roots. Thus, phloem transport of these antioxidants may constitute an important signal for the adjustment of root growth and development to changing environmental conditions. Further experiments under distinct environmental growth conditions that include transgenic plants modified in phloem transport of ASC or GSH and in root ASC or GSH biosynthesis are required to test these assumptions.
Acknowledgments
Financial support by the University of Freiburg within the programme ‘Support of evaluated research projects’ is gratefully acknowledged.
References
- Biddulph SF. Visual indications of 35S and 32P translocation in the phloem. American Journal of Botany. 1956;43:143–148. [Google Scholar]
- Bonas U, Schmitz K, Rennenberg H, Bergmann L. Phloem transport of sulphur in Ricinus. Planta. 1982;155:82–88. doi: 10.1007/BF00402936. [DOI] [PubMed] [Google Scholar]
- Bourgis F, Roje S, Nuccio ML, et al. S-Methylmethionine plays a major role in phloem sulphur transport and is synthesized by a novel type of methyltransferases. The Plant Cell. 1999;11:1485–1497. doi: 10.1105/tpc.11.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunold C. Reduction of sulfate to sulphide. In: Rennenberg H, Brunold C, De Kok LJ, Stulen I, editors. Sulphur nutrition and sulphur assimilation in higher plants. The Hague: SPB Academic Publishing; 1990. pp. 13–31. [Google Scholar]
- Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium-sensitive mutant, cad2-1, of Arabidopsis thaliana is deficient in γ-glutamylcysteine synthetase. The Plant Journal. 1998;16:73–78. doi: 10.1046/j.1365-313x.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- Conklin PL, Pallanca JE, Last RL, Smirnoff N. L-Ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiology. 1997;115:1277–1285. doi: 10.1104/pp.115.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pinto MC, Francis D, De Gara L. The redox state of the ascorbate–dehydroascorbate pair as a specific sensor of cell division in tobacco BY-2 cells. Protoplasma. 1999;209:90–97. doi: 10.1007/BF01415704. [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, et al. Reactive oxygen species produced by NADPH oxidase regulated plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signalling, acclimation, and practical implications. Antioxidants and Redox Signalling. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Tarlyn NM. L-Ascorbic acid is accumulated in source leaf phloem and transported to sink tissues in plants. Plant Physiology. 2002;130:649–656. doi: 10.1104/pp.007062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker MD, May M, Meyer AJ, Sheard N, Whithe NS. Measurements of glutathione levels in intact roots of Arabidopsis. Journal of Microscopy. 2000;198:162–173. doi: 10.1046/j.1365-2818.2000.00696.x. [DOI] [PubMed] [Google Scholar]
- Groten K, Vanacker H, Dutilleul C, Bastian F, Bernard S, Carzaniga R, Foyer CH. The roles of redox processes in pea nodule development and senescence. Plant, Cell and Environment. 2005;28:1293–1304. [Google Scholar]
- Haberer K, Herbinger K, Alexou M, Tausz M, Rennenberg H. Antioxidative defense of old growth beech (Fagus sylvatica) under double ambient O3 concentrations in a free-air exposure system. Plant Biology. 2007;9:215–226. doi: 10.1055/s-2007-964824. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RD, NcRae D, Haupt S, Viola R. Synthesis of L-ascorbic acid in the phloem. BMC Plant Biology. 2003;3:7. doi: 10.1186/1471-2229-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T, Mult S, Suter M, Rennenberg H, Herschbach C. Leaf age-dependent differences in sulphur assimilation and allocation in poplar (Populus tremula×P. alba) leaves. Journal of Experimental Botany. 2000;51:1077–1088. doi: 10.1093/jexbot/51.347.1077. [DOI] [PubMed] [Google Scholar]
- Herschbach C, Jouanin L, Rennenberg H. Overexpression of γ-glutamylcysteine synthetase, but not glutathione synthetase, elevates glutathione allocation in the phloem of transgenic poplar trees. Plant and Cell Physiology. 1998;39:447–451. [Google Scholar]
- Herschbach C, Rennenberg H. Storage and remobilization of sulphur in beech trees (Fagus sylvatica) Physiologia Plantarum. 1996;98:125–132. [Google Scholar]
- Herschbach C, van der Zalm E, Schneider A, Jouanin L, De Kok LJ, Rennenberg H. Regulation of sulfur nutrition in wild-type and transgenic poplar over-expressing γ-glutamylcysteine synthetase in the cytosol as affected by atmospheric H2S. Plant Physiology. 2000;124:461–473. doi: 10.1104/pp.124.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden R, Andersen CR, Goldsbrough PB, Cobbett CS. Cadmium sensitive, glutathione deficient mutant of Arabidopsis thaliana. Plant Physiology. 1995;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C-Y, Liu Y, Luthe DS, Yuceer C. Poplar FT2 shortens the juvenile phase and promotes seasonal flowering. The Plant Cell. 2006;18:1846–1861. doi: 10.1105/tpc.106.041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang K, Feldman LJ. Regulation of apical meristem development. Annual Review of Cell and Developmental Biology. 2005;21:485–509. doi: 10.1146/annurev.cellbio.21.122303.114753. [DOI] [PubMed] [Google Scholar]
- Jiang K, Meng YL, Feldman LJ. Quiescent centre formation in maize roots is associated with an auxin-regulated oxidizing environment. Development. 2003;130:1429–1438. doi: 10.1242/dev.00359. [DOI] [PubMed] [Google Scholar]
- Kato N, Esaka M. Changes in ascorbate oxidase gene expression and ascorbate levels in cell division and cell elongation in tobacco cells. Physiologia Plantarum. 1999;105:321–329. [Google Scholar]
- Kerk NM, Feldman LJ. A biochemical model for the initiation and maintenance of the quiescent centre: implications for organization of root meristem. Development. 1995;121:2825–2833. [Google Scholar]
- Kopriva S, Jones S, Koprivova A, Suter M, von Ballmoos P, Brander K, Flückiger J, Brunold C. Influence of chilling stress on the intercellular distribution of assimilatory sulphate reduction and thiols in Zea mays. Plant Biology. 2001;3:24–31. [Google Scholar]
- Kopriva S, Koprivova A. Plant adenosine 5′-phosphosulphate reductase: the past, the present, and the future. Journal of Experimental Botany. 2004;55:1775–1783. doi: 10.1093/jxb/erh185. [DOI] [PubMed] [Google Scholar]
- Kotchoni SO, Larrimore KE, Mukherjee M, Kempinski CF, Barth C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiology. 2009;149:803–815. doi: 10.1104/pp.108.132324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzuhara Y, Isobe A, Awazuhara M, Fujiwara T, Hayashi H. Glutathione levels in phloem sap of rice plants under sulphur deficient conditions. Soil Science and Plant Nutrition. 2000;46:265–270. [Google Scholar]
- Lappartient AG, Touraine B. Demand-driven control of root ATP sulphurylase activity and SO42– uptake in intact canola. Plant Physiology. 1996;111:147–157. doi: 10.1104/pp.111.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim MW, Kim SH. Cell type identity in Arabidopsis roots is altered by both ascorbic acid-induced changes in the redox environment and the resultant endogenous auxin response. Journal of Plant Biology. 2007;50:484–489. [Google Scholar]
- Liso R, Innocenti AM, Bitonti MB, Arrigoni O. Ascorbic acid-induced progression of quiescent center cells from G1 to S phase. New Phytologist. 1988;110:469–471. [Google Scholar]
- Liso R, De Tullio MC, Ciraci S, Balestrini R, La Rocca N, Bruno L, Chiappetta A, Bitonti B, Bonfante P, Arrigoni O. Localisation of ascorbic acid, ascorbic acid oxidase, and glutathione in roots of Cucurbita maxima L. Journal of Experimental Botany. 2004;55:2589–2597. doi: 10.1093/jxb/erh262. [DOI] [PubMed] [Google Scholar]
- Martin MN, Tarczynski MC, Shen B, Leustek T. The role of 5′adenylylsulfate reductase in controlling sulfate reduction in plants. Photosynthesis Research. 2005;86:309–323. doi: 10.1007/s11120-005-9006-z. [DOI] [PubMed] [Google Scholar]
- Mason TG, Maskell EJ. Studies on the transport of carbohydrates in the cotton plant. I. A study of diurnal variation in the carbohydrates of leaf, bark, and wood, and of the effects of ringing. Annals of Botany. 1928;42:189–253. [Google Scholar]
- Matamoros MA, Loscos J, Coronado MJ, Ramos J, Sato S, Testillano PS, Tabata S, Becana M. Biosynthesis of ascorbic acid in legume root nodules. Plant Physiology. 2006;141:1068–1077. doi: 10.1104/pp.106.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May MJ, Vernoux T, Leaver C, Van Montagu M, Inzé D. Glutathione homeostasis in plants: implications for environmental sensing and plant development. Journal of Experimental Botany. 1998;49:649–667. [Google Scholar]
- Meyer AJ. The integration of glutathione homeostasis and redox signaling. Journal of Plant Physiology. 2008;165:1390–1403. doi: 10.1016/j.jplph.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Meyer AJ, Hell R. Glutathione homeostasis and redox-regulation by sulfhydryl groups. Photosynthesis Research. 2005;86:435–457. doi: 10.1007/s11120-005-8425-1. [DOI] [PubMed] [Google Scholar]
- Mullineaux PM, Rausch T. Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynthesis Research. 2005;86:459–474. doi: 10.1007/s11120-005-8811-8. [DOI] [PubMed] [Google Scholar]
- Noctor G. Metabolic signalling in defence and stress: the central roles of soluble redox couples. Plant, Cell and Environment. 2006;29:409–425. doi: 10.1111/j.1365-3040.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Noctor G, Strohm M, Jouanin L, Kunert K-J, Foyer CH, Rennenberg H. Synthesis of glutathione in leaves of transgenic poplar overexpressing γ-glutamylcysteine synthetase. Plant Physiology. 1996;112:1071–1078. doi: 10.1104/pp.112.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Hatano-Iwasaki A, Yanagida M, Iwabuchi M. Level of glutathione is regulated by ATP-dependent ligation of glutamate and cysteine through photosynthesis in Arabidopsis thaliana: mechanism of strong interaction of light intensity with flowering. Plant and Cell Physiology. 2004;45:1–8. doi: 10.1093/pcp/pch008. [DOI] [PubMed] [Google Scholar]
- Okamura M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clinica et Chimica Acta. 1980;103:259–268. doi: 10.1016/0009-8981(80)90144-8. [DOI] [PubMed] [Google Scholar]
- Olmos E, Kiddle G, Pellny TK, Kumar S, Foyer CH. Modulation of plant morphology, root architecture, and cell structure by low vitamin C in Arabidopsis thaliana. Journal of Experimental Botany. 2006;57:1645–1655. doi: 10.1093/jxb/erl010. [DOI] [PubMed] [Google Scholar]
- Pallanca JE, Smirnoff N. The control of ascorbic acid synthesis and turnover in pea seedlings. Journal of Experimental Botany. 2000;51:669–674. [PubMed] [Google Scholar]
- Polle A, Chakrabarti K, Schürmann W, Rennenberg H. Composition and properties of hydrogen peroxide decomposition systems in extracellular and total extracts from needles of Norway spruce (Picea abies L., Karst) Plant Physiology. 1990;94:312–319. doi: 10.1104/pp.94.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiology and Biochemistry. 2002;40:537–548. [Google Scholar]
- Potters G, Horemans N, Bellone S, Caubergs RJ, Trost P, Guisez Y, Asard H. Dehydroascorbate influences the plant cell cycle through a glutathione-independent reduction mechanism. Plant Physiology. 2004;134:1479–1487. doi: 10.1104/pp.103.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennenberg H, Herschbach C, Haberer K, Kopriva S. Sulphur metabolism in plants: are trees different? Plant Biology. 2007;9:620–637. doi: 10.1055/s-2007-965248. [DOI] [PubMed] [Google Scholar]
- Rennenberg P, Polle A. Metabolic consequences of atmospheric sulfur influx into plants. In: Alscher R, Wellburn AR, editors. Plants responses to the gaseous environment. Molecular, metabolic and physiological aspects. London: Chapman and Hall; 1994. pp. 165–180. [Google Scholar]
- Rennenberg H, Schmitz K, Bergmann L. Long-distance transport of sulphur in Nicotiana tabacum. Planta. 1979;147:57–62. doi: 10.1007/BF00384591. [DOI] [PubMed] [Google Scholar]
- Sánchez-Fernández R, Fricker M, Corben LB, White NS, Sheard N, Leaver CJ, Van Montagu M, Inzé D, May MJ. Cell proliferation and hair tip growth in the Arabidopsis root are under mechanistically different forms of redox control. Proceedings of National Academy of Sciences, USA. 1997;94:2745–2750. doi: 10.1073/pnas.94.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer U, Haensch R, Mendel R, Kopriva S, Rennenberg H, Herschbach C. Sulphur flux through the sulphate assimilation pathway is differently controlled by adenosine 5′-phosphosulphate reductase under stress and in transgenic poplar plants overexpressing γ-ECS, SO or APR. Journal of Experimental Botany. 2009 doi: 10.1093/jxb/erp327. doi: 10.1093/jxb/erp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte M, von Ballmoos P, Rennenberg H, Herschbach C. Life-long growth of Quercus ilex L. at natural CO2 springs acclimates sulphur, nitrogen and carbohydrate metabolism of the progeny to elevated pCO2. Plant, Cell and Environment. 2002;25:1715–1727. [Google Scholar]
- Schupp R, Rennenberg H. Diurnal changes in the glutathione concentration of spruce needles (Picea abies L.) Plant Science. 1988;57:113–117. [Google Scholar]
- Smirnoff N, Conklin PL, Loewus FA. Biosynthesis of ascorbic acid in plants: a renaissance. Annual Reviews of Plant Physiology and Plant Molecular Biology. 2001;52:437–467. doi: 10.1146/annurev.arplant.52.1.437. [DOI] [PubMed] [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of GSH synthesis in leaves of transgenic poplar (Populus tremula×P. alba) overexpressing GSH synthetase. The Plant Journal. 1995;7:141–145. [Google Scholar]
- Vauclare P, Kopriva S, Fell D, Suter M, Sticher L, von Ballmoos P, Krähenbühl U, Op den Camp R, Brunold C. Flux control of sulphate assimilation in Arabidopsis thaliana: adenosine 5′-phosphosulfate reductase is more susceptible than ATP sulfurylase to negative control by thiols. The Plant Journal. 2002;31:729–740. doi: 10.1046/j.1365-313x.2002.01391.x. [DOI] [PubMed] [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, et al. The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. The Plant Cell. 2000;12:97–109. doi: 10.1105/tpc.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]