Abstract
Many accessions (ecotypes) of Arabidopsis have been collected. Although few differences exist among their nucleotide sequences, these subtle differences induce large genetic variation in phenotypic traits such as stress tolerance and flowering time. To understand the natural variability in salt tolerance, large-scale soil pot experiments were performed to evaluate salt tolerance among 350 Arabidopsis thaliana accessions. The evaluation revealed a wide variation in the salt tolerance among accessions. Several accessions, including Bu-5, Bur-0, Ll-1, Wl-0, and Zu-0, exhibited marked stress tolerance compared with a salt-sensitive experimental accession, Col-0. The salt-tolerant accessions were also evaluated by agar plate assays. The data obtained by the large-scale assay correlated well with the results of a salt acclimation (SA) assay, in which plants were transferred to high-salinity medium following placement on moderate-salinity medium for 7 d. Genetic analyses indicated that the salt tolerance without SA is a quantitative trait under polygenic control, whereas salt tolerance with SA is regulated by a single gene located on chromosome 5 that is common among the markedly salt-tolerant accessions. These results provide important information for understanding the mechanisms underlying natural variation of salt tolerance in Arabidopsis.
Keywords: Acclimation, Arabidopsis thaliana accessions, mapping, natural variation, salt stress
Introduction
Arabidopsis is distributed widely across the world. Many accessions (ecotypes) have been collected from wild populations growing throughout the Northern Hemisphere in Europe, Asia, and Africa (Beck et al., 2008; Pico et al., 2008). The genus has also been found in North America, Australia, and Japan, where it was probably introduced from Europe (Alonso-Blanco and Koornneef, 2000). This broad geographic distribution encompasses substantial variation in growth environments, and phenotypic variation among accessions is expected to reflect the genetic variation that is important for adaptation to specific conditions. Considerable variation has been found for potentially adaptive traits in Arabidopsis, such as resistance to biotic stresses, including insects, fungi, bacteria, and viruses (Kunkel, 1996), and tolerance of abiotic stresses, such as high temperature, freezing, drought, high light, heavy metals, and ozone (Murphy and Taiz, 1995; Rao and Davis, 1999). Furthermore, variation has also been described for flowering time (Kowalski et al., 1994; Alonso-Blanco et al., 1998), circadian rhythms (Swarup et al., 1999), and seed size (Alonso-Blanco et al., 1999) in Arabidopsis accessions, suggesting that these accessions may be useful as a potential source of genetic resources (Alonso-Blanco et al., 2009).
Recent studies using this natural variation led to the identification of genes underlying ecologically relevant processes and complex traits. Several critical genes regulating traits such as freezing tolerance (Alonso-Blanco et al., 2005), growth, flowering (Balasubramanian et al., 2006), Na+ accumulation (Rus et al., 2006), powdery mildew resistance (Gollner et al., 2008), and copper tolerance (Kobayashi et al., 2008) have been successfully identified in studies using Arabidopsis accessions. These results provided new insights into aspects of genome evolution, differentiation among geographic population, and selective mechanisms that shape complex trait variation in nature (Mitchell-Olds and Schmitt, 2006).
Salt is a major abiotic stress causing both osmotic and ionic stress, which greatly affect plant growth and crop production. The reduction in shoot growth occurs in two phases: a rapid response to the increase in external osmotic pressure, and a slower response due to the accumulation of Na+ in leaves (Munns and Tester, 2008). Consequently, salinized plants are subjected to dehydration, metabolic toxicity, nutrient deficiencies, membrane dysfunction, and oxidative stress, which lead to tissue damage and early senescence (Essah et al., 2003). Plants have evolved a number of strategies to acclimatize to various stresses. Among others, salt acclimation (SA) responses include: (i) the maintenance of ion homeostasis; (ii) osmotic adjustment and compatible solute accumulation; (iii) water balance and control of transpiration; and (iv) structural and anatomical changes, for example the modification of apoplastic barriers (Hose et al., 2001; Essah et al., 2003; Sanchez et al., 2008). Much effort has been directed toward understanding the molecular mechanism for plant salt tolerance, with the ultimate goal of improving salt tolerance of crop plants. One important objective of these studies is to determine which genes are responsible for the stress tolerance.
Many genetic or reverse-genetic approaches with Arabidopsis as a model system have been adopted to identify stress tolerance genes. In response to high-salinity stress, various genes are up-regulated and their products are involved either directly or indirectly in plant protection. Some of the genes encoding enzymes for osmolyte synthesis, ion channels, receptors, and components of calcium signalling or some other regulatory signalling are able to confer salinity-tolerant phenotypes when transferred to salinity-sensitive plants (Mahajan et al., 2008). Genetic studies have revealed the essential genes for salt tolerance, including a plasma membrane Na+/H+ antiporter (SOS1), a vacuolar Na+/H+ antiporter (NHX1), and a plasma membrane Na+ transporter (HKT1). Each mutant shows a salt-hypersensitive phenotype (Shi et al., 2000; Apse et al., 2003; Sunarpi et al., 2005). Thus, evidence for salt tolerance mechanisms of the glycophytic plant Arabidopsis has been accumulating. However, how salt-tolerant plants acquire the salt tolerance in nature is poorly understood.
To examine natural variation in salt tolerance among Arabidopsis accessions, Quesada et al. (2002) performed a comparative study of salt tolerance at the germination stage using 102 accessions, and they reported a wide range of variation in salt tolerance among them. Salt-tolerant accessions were identified, and six quantitative trait loci (QTLs) contributing to salt tolerance at germination were detected. However, accessions that showed salt tolerance at the germination stage were very sensitive to salinity stress during vegetative growth. The map positions of the QTLs for salinity tolerance at germination were not coincident with those of the QTLs for salt tolerance during vegetative growth, suggesting that the mechanisms controlling salt tolerance during the growth stage are different from those at germination (Quesada et al., 2002).
In the present study, a large-scale evaluation of salt tolerance was performed during vegetative growth among 350 Arabidopsis thaliana accessions. The evaluation allowed the isolation of markedly salt-tolerant accessions after SA by comparison with a reference accession, Col-0. Genetic analyses revealed that the salt tolerance with SA is a monogenic trait common among salt-tolerant accessions, whereas salt tolerance without SA is a polygenic trait.
Materials and methods
Plant materials and growth conditions
The following A. thaliana accessions were used in the present study, where the JA number is the stock number of the RIKEN Bioresource Center: Aa-0 (JA001), Ag-0 (JA002), Ak-1 (JA003), Al-0 (JA004), Am-0 (JA005), An-1 (JA006), An-2 (JA265), Ang-0 (JA266), Ang-1 (JA007), Ba-1 (JA008), Bay-0 (JA009), Bch-1 (JA010), Bch-3 (JA267), Bch-4 (JA268), Bd-0 (JA011), Be-0 (JA012), Be-1 (JA269), Bl-1 (JA013), Bla-1 (JA014), Bla-2 (JA270), Bla-3 (JA271), Bla-4 (JA272), Bla-5 (JA273), Bla-10 (JA015), Bla-11 (JA274), Bla-12 (JA016), Bla-14 (JA017), Blh-1 (JA018), Blh-2 (JA275), Bn-0 (JA019), Bor-0 (JA020), Br-0 (JA021), Bs-1 (JA022), Bs-2 (JA276), Bs-5 (JA023), Bsch-0 (JA024), Bsch-2 (JA277), Bu-0 (JA025), Bu-1 (JA278), Bu-2 (JA026), Bu-3 (JA027), Bu-4 (JA028), Bu-5 (JA029), Bu-6 (JA279), Bu-7 (JA280), Bu-8 (JA281), Bu-9 (JA030), Bu-11 (JA031), Bu-13 (JA032), Bu-14 (JA033), Bu-14a (JA034), Bu-15 (JA035), Bu-17 (JA036), Bu-18 (JA282), Bu-19 (JA037), Bu-20 (JA038), Bu-21 (JA039), Bu-22 (JA040), Bu-23 (JA041), Bu-24 (JA042), Bu-25 (JA043), Bur-0 (JA044), Bus-0 (JA045), Bus-1 (JA046), Ca-0 (JA047), Cal-0 (JA048), Can-0 (JA049), Cen-0 (JA050), Cha-0 (JA051), Cha-1 (JA283), Chi-0 (JA053), Chi-1 (JA284), Chi-2 (JA285), Ci-0 (JA054), Cit-0 (JA055), Cl-0 (JA056), Co-0 (JA057), Co-2 (JA286), Co-3 (JA287), Co-4 (JA288), Col-0 (JA058), Ct-1 (JA059), Cvi-0 (JA060), Da-0 (JA061), Db-0 (JA062), Db-1 (JA289), Db-2 (JA290), Di-0 (JA063), Di-1 (JA064), Di-2 (JA065), Do-0 (JA066), Dr-0 (JA067), Dra-0 (JA068), Dra-1 (JA291), Dra-2 (JA292), Edi-0 (JA069), Ei-2 (JA070), Ei-4 (JA293), Ei-5 (JA294), Ei-6 (JA295), Eil-0 (JA071), El-0 (JA072), En-1 (JA296), En-2 (JA073), Ep-0 (JA074), Er-0 (JA075), Es-0 (JA076), Esc-0 (JA077), Est-0 (JA078), Est-1 (JA297), Et-0 (JA079), Fe-1 (JA080), Fi-0 (JA081), Fl-1 (JA082), Fl-3 (JA083), For-1 (JA084), For-2 (JA298), Fr-2 (JA085), Fr-3 (JA299), Fr-4 (JA300), Fr-5 (JA086), Fr-6 (JA301), Fr-7 (JA302), Ga-0 (JA303), Ga-2 (JA087), Gd-1 (JA088), Ge-0 (JA089), Ge-1 (JA090), Ge-2 (JA091), Gie-0 (JA092), Go-0 (JA093), Go-2 (JA094), Gr-1 (JA095), Gr-2 (JA304), Gr-3 (JA305), Gr-4 (JA306), Gr-5 (JA307), Gr-6 (JA308), Gre-0 (JA096), Gu-0 (JA097), Gu-1 (JA309), Gy-0 (JA098), Ha-0 (JA099), Hau-0 (JA100), Hel-1 (JA101), Hh-0 (JA102), Hi-0 (JA103), Hl-0 (JA104), Hl-2 (JA105), Hl-3 (JA106), Hm-0 (JA107), Hn-0 (JA108), Hs-0 (JA109), In-0 (JA110), Is-0 (JA111), Is-1 (JA310), Ita-0 (JA112), Je-0 (JA113), Jl-1 (JA114), Jl-2 (JA311), Jl-3 (JA312), Jl-4 (JA313), Jl-5 (JA314), Jm-0 (JA115), Jm-1 (JA116), Jm-2 (JA315), Ka-0 (JA117), Kas-1 (JA119), Kb-0 (JA120), Kil-0 (JA121), Kin-0 (JA122), Kl-0 (JA123), Kl-1 (JA316), Kl-2 (JA317), Kl-3 (JA318), Kl-4 (JA319), Kl-5 (JA124), Kn-0 (JA125), Ko-2 (JA320), Ko-3 (JA126), Ko-4 (JA127), Ko-5 (JA128), Kr-0 (JA129), Kro-0 (JA130), La-1 (JA131), Lan-0 (JA132), Lc-0 (JA133), Le-0 (JA134), Li-1 (JA321), Li-2 (JA135), Li-2–1 (JA136), Li-3 (JA322), Li-3–3 (JA137), Li-5 (JA138), Li-5–2 (JA323), Li-5–3 (JA324), Li-6 (JA139), Li-6–1 (JA325), Li-7 (JA326), Li-8 (JA327), Li-10 (JA328), Li-11 (JA329), Li-12 (JA330), Li-13 (JA331), Lip-0 (JA140), Ll-0 (JA141), Ll-1 (JA332), Ll-2 (JA333), Ll-3 (JA334), Lm-2 (JA142), Lo-1 (JA143), Lo-2 (JA335), Loh-0 (JA144), Lu-1 (JA145), Lz-0 (JA146), Ma-0 (JA147), Ma-2 (JA336), Map-0 (JA148), Mc-0 (JA149), Me-0 (JA150), Mh-0 (JA151), Mh-1 (JA152), Mir-0 (JA153), Mnz-0 (JA154), Mr-0 (JA155), Mrk-0 (JA156), Ms-0 (JA157), Mt-0 (JA158), Mv-0 (JA159), Mz-0 (JA160), Na-1 (JA161), Nc-1 (JA162), Nd-0 (JA163), Nie-0 (JA164), No-0 (JA165), Nok-0 (JA166), Nok-1 (JA167), Nok-2 (JA168), Nok-3 (JA169), Nok-4 (JA170), Np-0 (JA171), Nw-0 (JA172), Nw-1 (JA337), Nw-2 (JA338), Nw-3 (JA339), Nw-4 (JA340), Ob-0 (JA173), Ob-1 (JA174), Ob-2 (JA175), Ob-3 (JA341), Old-1 (JA176), Old-2 (JA177), Or-0 (JA178), Ost-0 (JA179), Ove-0 (JA180), Oy-0 (JA181), Pa-1 (JA182), Pa-2 (JA342), Pa-3 (JA343), Pdi-0 (JA183), Per-1 (JA184), Per-2 (JA185), Per-3 (JA186), Pf-0 (JA187), Pi-0 (JA188), Pi-2 (JA344), Pla-0 (JA189), Pla-1 (JA345), Pla-2 (JA346), Pla-3 (JA347), Pla-4 (JA348), Pn-0 (JA190), Po-0 (JA349), Po-1 (JA191), Pog-0 (JA192), Pr-0 (JA193), Pt-0 (JA194), Ra-0 (JA195), Rak-2 (JA196), Rd-0 (JA197), Ri-0 (JA198), Rou-0 (JA200), Rsch-0 (JA201), Rsch-4 (JA202), Ru-0 (JA203), Sah-0 (JA205), Sal-0 (JA204), Sap-0 (JA206), Sav-0 (JA207), Se-0 (JA208), Sei-0 (JA209), Set-0 (JA210), Sf-0 (JA211), Sf-1 (JA212), Sf-2 (JA213), Sg-1 (JA214), Sg-2 (JA215), Sh-0 (JA216), Si-0 (JA217), So-0 (JA218), Sol-0 (JA219), Sp-0 (JA220), Sr-0 (JA221), St-0 (JA222), Ste-0 (JA223), Stw-0 (JA224), Su-0 (JA225), Sue-0 (JA226), Sv-0 (JA227), Sy-0 (JA228), Ta-0 (JA229), Ts-1 (JA230), Ts-2 (JA231), Ts-3 (JA232), Ts-5 (JA233), Ts-6 (JA234), Ts-7 (JA235), Tsu-0 (JA236), Tu-0 (JA350), Tu-1 (JA237), Tul-0 (JA238), Ty-0 (JA239), Uk-1 (JA351), Uk-2 (JA240), Uk-3 (JA241), Uk-4 (JA242), Van-0 (JA243), Van-1 (JA352), Van-3 (JA353), Vi-0 (JA244), Wa-1 (JA245), Wc-1 (JA246), Wc-2 (JA247), Wil-1 (JA248), Wil-2 (JA249), Wil-3 (JA250), Wl-0 (JA251), Ws-0 (JA252), Wt-1 (JA253), Wt-2 (JA254), Wt-3 (JA255), Wt-4 (JA256), Wt-5 (JA257), Wu-0 (JA258), X-0 (JA259), Xx-0 (JA260), Xxx-0 (JA261), Yo-0 (JA262), Ze-0 (JA263), Zu-0 (JA264), Zu-1 (JA354).
Arabidopsis seeds were sown on Murahige and Skoog (MS) agar plates containing full-strength MS, 0.8% (w/v) agar, and 1% sucrose with vitamin mixture (10 mg l−1 myoinositol, 200 μg l−1 glycine, 50 μg l−1 nicotinic acid, 50 μg l−1 pyridoxine hydrochloride, 10 μg l−1 thiamin hydrochloride, pH 5.7), and then they were sealed with surgical tape. The seeds were stratified at 4 °C for 7 d and then transferred at 80 μmol m−2 s−1 irradiance and an 8 h/16 h day/night cycle at 22 °C for germination and growth. These growing conditions were used for all experiments described herein.
Large-scale salt tolerance evaluation using soil pots
Four 2-week-old seedlings grown on an MS agar plate were transferred into a plastic pot (8 cm, height 6.5 cm) filled with a 1:1 perlite:vermiculite mixture and watered with 1000-fold diluted Hyponex (Arditti et al., 1982). One week later, the pots were transferred into a container filled with 500 mM NaCl solution (2.0 l) from the bottom of the container to ∼1 cm. The height of the initial water level was noted, and every day just enough water was poured into the container to reach that initial water level. Salt tolerance of accessions was evaluated by the survival duration (days).
Stress treatment using agar plates
For the agar plate assay, 7-day-old seedlings grown on nylon mesh (990 μm) on an MS agar plate were transferred to a plate supplemented with 250 mM NaCl with the underlying mesh (hereafter ‘mesh transferred’) for 7 d (see Fig. 3).
Fig. 3.
Phenotypes of salt-tolerant accessions using the mesh-transferred assay. Seven-day-old Bu-5, Col-0, Ll-1, Bur-0, Wl-0, and Zu-0 seedlings grown on a nylon mesh on MS agar plates were mesh transferred to plates supplemented with 250 mM NaCl for 7 d.
For the agar plate assay with SA, 7-day-old seedlings grown on nylon mesh on an MS agar plate were mesh-transferred to a plate supplemented with 100 mM NaCl for 7 d. The 14-day-old seedlings were then mesh transferred to a plate supplemented with 300 mM NaCl, 5 mM CsCl, or 25 mM LiCl for 7 d, or 750 mM sorbitol for 14 d. On the other hand, for the assay without SA, 7-day-old seedlings grown on nylon mesh on an MS agar plate were mesh transferred to another MS agar plate without NaCl for 7 d. The 14-day-old seedlings were then mesh transferred to a plate supplemented with 300 mM NaCl, 5 mM CsCl, or 25 mM LiCl for 7 d, or 750 mM sorbitol for 14 d.
Chlorophyll determination
Aerial parts of Arabidopsis seedlings treated with 300 mM NaCl for 7 d with and without SA were harvested, and the seedlings of each accession were homogenized in cold acetone. Chlorophyll content was determined using the method described by Porra et al. (1989).
Measurement of Na+ and K+ contents
Seven-day-old Col-0 and Bu-5 seedlings grown on nylon mesh on MS agar plates were mesh transferred to plates supplemented with 100 mM NaCl for 7 d (SA) and subsequently mesh transferred to plates supplemented with 300 mM NaCl for 3 d. Aerial parts of Arabidopsis seedlings were harvested 0, 3, 7, and 10 d after transfer to the 100 mM medium. Plants were soaked in 5 ml of sterile distilled water for 5 s. The solution was then boiled for 15 min, passed through a 0.2 μm filter (Toyo Roshi Kaisha, Ltd), and diluted 20× with distilled water. The solution was analysed for Na+ and K+ contents using a Shim-pack IC-C3/C3 (S) column (Shimazu, Japan) on a PIA-1000 Personal Ion Analyzer (Shimazu).
Genetic mapping
Bu-5, Bur-0, Cal-0, Ll-1, and Zu-0 were crossed with a salt-sensitive accession, Col-0, and the resulting F1 progeny were selfed to generate F2 populations. Genomic DNA was prepared from individual F2 plants with the recessive phenotype for use as PCR templates. The simple sequence length polymorphism (SSLP) markers listed in Supplementary Tables S1 and S2 available at JXB online were used for mapping. PCR conditions were as follows: (94 °C for 2 min) ×1 cycle, (94 °C for 20 s, 52–55 °C for 20 s, 72 °C for 20 s ×40 cycles, and (72 °C for 2 min) ×1 cycle. The microsatellites were fractionated in 5–7% agarose gel, and the recombinant value was calculated from the band pattern.
Microarray analyses
Total RNA was isolated with RNAiso reagent (Takara, Japan) from 2-week-old Col-0 and Bu-5 seedlings with and without SA (100 mM NaCl for 7 d). mRNAs were prepared using the PolyATract mRNA Isolation System III (Promega). Arabidopsis ATH1 Genome Array (Affymetrix, USA) was used for microarray analyses. Preparation of fluorescent probes, microarray hybridization, and scanning were performed as described previously (Seki et al., 2002). Raw data were analysed by GeneSpring version 7 software (Silicon Genetics) and normalized using the Lowess normalization method (Cleveland, 1979). The genes showing a signal intensity >1000 were considered for RNA gel-blot analysis.
RNA gel-blot analysis
Total RNA was isolated with RNAiso reagent (Takara) from 2-week-old Col-0 and Bu-5 seedlings with SA (100 mM NaCl for 0, 3, and 7 d). Total RNA (10 μg) was fractionated in a 1% agarose gel containing formaldehyde and blotted onto a nylon membrane using 20× SSC. Probes were generated by PCR amplification from Col-0 genomic DNA using primers designed from specific sequences of target genes. The DNA fragments were labelled with [32P]dCTP using DNA Labeling Kit Version 2 (Takara). The membranes were then hybridized with 32P-labelled fragments at 65 °C overnight. The membranes were washed three times with 1× SSC and 1% SDS for 3 min at room temperature, once with 1× SSC and 1% SDS for 15 min at room temperature, and then twice with 0.1× SSC and 0.1% SDS for 15 min at 65 °C.
Measurement of amino acid contents
Seven-day-old Col-0 and Bu-5 plants grown on nylon mesh on MS agar plates were mesh transferred to plates supplemented with 100 mM NaCl for 7 d as SA. Aerial parts of Arabidopsis seedlings were harvested on 0, 3, and 7 d after transfer to the saline medium. Plants were homogenized with 2.5 ml of sterile distilled water and treated in a boiling bath for 5 min. The heat-treated homogenate was cooled in an ice bath and centrifuged at 15 000 rpm for 20 min. The supernatant fraction was analysed by an automatic amino acid analyser (Model L-7100 Series, Hitachi).
Results
Salt tolerance of Arabidopsis thaliana accessions grown in soil
RIKEN Bioresource Center preserves and distributes 350 A. thaliana accessions. To isolate salt-tolerant Arabidopsis accessions, the salt tolerance of 344 accessions was evaluated using the following assay. Three-week-old plants grown in soil pots were transferred to a container filled with 500 mM NaCl solution. Just enough water was added to the container every day to maintain the initial water level, and the number of survival days was measured from the day the stress began until complete chlorosis was observed in all plants in a pot. The results indicated a wide variation in the salt tolerance among the 344 accessions (Fig. 1, Supplementary Fig. S1 at JXB online). Six accessions did not germinate. In total, 288 accessions showed greater salt tolerance than the reference accession (Col-0 plants). Figure 2 shows the markedly salt-tolerant accessions, Bu-5, Bur-0, Ll-1, Wl-0, and Zu-0, and the salt-sensitive accessions, Bch-4, Cvi-0, Sg-2, Sh-0, and Col-0 before and after the stress treatment (Fig. 2). Only 56 accessions showed similar or greater sensitivity to salt stress compared with Col-0 plants, meaning that Col-0 is a salt-sensitive accession of Arabidopsis. On the other hand, there is no significant correlation between the salt tolerance and the flowering time under normal growth conditions (r=0.025, Supplementary Fig. S2 at JXB online).
Fig. 1.
Frequency distribution of days of survival of 344 Arabidopsis thaliana accessions under salt stress using soil pots. Three-week-old plants grown in soil pots were exposed to 500 mM NaCl in water. Survival days represent the number of days from the initiation of stress until complete chlorosis was observed in all plants in a pot. Arrows indicate the average (n=20) positions corresponding to a reference accession Col-0, and markedly salt-tolerant accessions including Bu-5, Bur-0, Ll-1, Wl-0, and Zu-0, respectively.
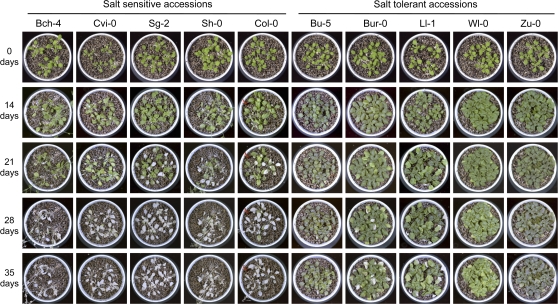
Fig. 2.
Weekly photographs of salt-tolerant or salt-sensitive accessions under salinity stress using the soil pot assay. Three-week-old Bch-4, Cvi-0, Sg-2, Sh-0, Col-0, Bu-5, Bur-0, Ll-1, and Zu-0 plants grown in soil pots were exposed to 500 mM NaCl in water for 35 d. It shows the weekly photographs from day 0 to the 35th day after salt stress treatment.
Several salt-tolerant accessions show superior salt-acclimated tolerance
To confirm the reliability of the salt tolerance assay, the salt-tolerant accessions were also evaluated by an assay using agar plates supplemented with NaCl. Some of the salt-tolerant accessions showed salt tolerance on the agar plates as well (Fig. 3, Supplementary Table S1 at JXB online). Compared with the salt tolerance assay using soil pots (Fig. 2), however, there was not a marked difference in salt tolerance between salt-tolerant accessions and Col-0 on agar plates.
Disagreement in salt tolerance between the two assays probably depends on the differences in how plants experience salinity stress and in the cation-exchange capacities or the level of transpiration occurring in the plants grown on the two media (Flowers, 2004). The salinity treatment on agar plates renders salinity shock to plants, whereas the treatment using soil pots exposes plants to a gradual increase of NaCl stress because the water contained in soil is gradually substituted with saline water. To confirm this idea, those accessions that failed to show marked salt tolerance on agar plates were reassessed using the SA assay, in which 7-day-old seedlings were transferred onto agar plates with 100 mM NaCl for 7 d (acclimation period), which does not affect plant growth significantly, and subsequently transferred to plates with 300 mM NaCl (Fig. 4A). Although there was no significant difference in salt tolerance between Col-0 and Bu-5 without SA, the salt-acclimated Bu-5 plants showed distinct salt tolerance compared with Col-0 plants, as also noted in the soil pot assay (Fig. 4B). Thus, the treatment, 100 mM NaCl for 7 d, was defined as SA treatment. The salt tolerance of Bu-5 was also confirmed by measuring chlorophyll content with and without SA (Fig. 4C). These results suggest that the salt tolerance assay using soil pots evaluates not only stress tolerance to salinity shock but also salt tolerance acquired during SA, and Bu-5 plants have an SA ability superior to that of Col-0 plants.
Fig. 4.
Salt tolerance after salt acclimation (SA) of Col-0 and Bu-5 plants. (A) Flowchart of the SA assay. The arrow shows a mesh transfer. (B) Phenotypes of Col-0 and Bu-5 seedlings with and without SA under 300 mM NaCl. (C) Chlorophyll content in shoots under 30 mM NaCl for 7 d with and without SA. The contents were assessed based on fresh weight (FW). Values are the mean ±SE; n=5.
Na+ shoot accumulation is comparable in Bu-5 and Col-0
For plants to achieve salt tolerance, it is important for them to limit the Na+ accumulation in their shoots. Using ion chromatography, Na+ content was examined in both Bu-5 and Col-0 plants during SA and a sequential high-salinity stress treatment. There was no discernible difference in Na+ content between the shoots of Bu-5 and Col-0 plants during SA and the following high-salinity period (Fig. 5). Although both Bu-5 and Col-0 plants translocate Na+ to their shoots, Bu-5 can survive the salt stress, suggesting that the Bu-5 plants have an enhanced defence mechanism against accumulated Na+ or to the osmotic stress involved in the salt stress during SA.
Fig. 5.
Na+ and K+ contents in Col-0 and Bu-5 during the SA assay. (A) Na+ contents in rosette leaves of Col-0 and Bu-5. (B) K+ contents in rosette leaves of Col-0 and Bu-5. Seven-day-old Col-0 and Bu-5 seedlings grown on nylon mesh on MS agar plates were mesh transferred to plates with 100 mM NaCl for 7 d as SA and subsequently mesh transferred to plates with 300 mM NaCl for 3 d. The dashed line shows the transfer point from 100 mM to 300 mM NaCl. Na+ and K+ contents were based on fresh weight (FW). Values are mean ±SE; n=5. Differences between Bu-5 and Col-0 were analysed by Student's t-test. *P <0.05; ***P <0.001.
Salt-tolerant plants, including halophytes, can maintain K+ uptake under salt stress, whereas excessive Na+ prevents K+ uptake. The Bu-5 plants also maintained higher K+ levels in their shoots during SA and the following high-salinity period in comparison with those of Col-0 plants, despite the fact that Na+ shoot accumulation was comparable between Bu-5 and Col-0 plants (Fig. 5). The maintenance of cellular K+ levels under salt stress may be one mechanism underlying the marked salt tolerance of Bu-5.
Bu-5 plants acquired osmotic stress tolerance during SA
To determine whether Bu-5 is tolerant of osmotic stress in general or of specific ions, salt-acclimated Bu-5 and Col-0 plants were treated with sorbitol, CsCl, or LiCl. There was no significant difference in their growth under Cs+ or Li+, more toxic analogues of Na+ (Mendoza et al., 1994). All of the salt-acclimated Bu-5 plants exhibited marked tolerance of 750 mM sorbitol stress, whereas Col-0 plants showed complete chlorosis (Fig. 6A). These results indicate that Bu-5 plants do not acquire tolerance of specific ions, but instead acquire general osmotic tolerance during SA.
Fig. 6.
Evaluation of stress tolerance of sorbitol, CsCl, and LiCl after SA of salt-tolerant accessions. (A) Salt-acclimated 2-week-old Col-0 and Bu-5 seedlings were mesh transferred to MS agar plates containing 750 mM sorbitol, 5 mM CsCl, or 25 mM LiCl. (B) Salt-acclimated Bu-5, Col-0, Ll-1, Bur-0, Wl-0, and Zu-0 plants were exposed to 750 mM sorbitol for 14 d.
The osmotic stress tolerance after SA of Bu-5, Bur-0, Ll-1, Wl-0, and Zu-0, which showed salt tolerance in soil pots, was also reassessed (Fig. 2). All of these salt-acclimated accessions except Wl-0 showed marked osmotic stress tolerance compared with Col-0 plants (Fig. 6B). This finding indicates that the data from the large-scale evaluation correlates well with the results of the osmotic tolerance assay after SA. The salt-acclimated Wl-0 plants showed higher salt tolerance than Col-0 plants in the soil pot assay or mesh-transferred assay, but not in the SA assay, suggesting that Wl-0 plants have a superior tolerance to salinity shock compared with Col-0 plants.
Microarray analysis of Bu-5 and Col-0 plants during SA
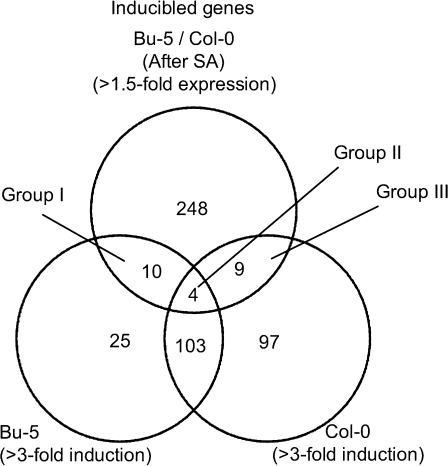
To analyse the expression profile of Bu-5 and Col-0 plants during SA, microarray analyses were performed using the Arabidopsis ATH1 Genome Array. RNA was extracted from non-acclimated plants and salt-acclimated plants treated with 100 mM NaCl for 7 d. The SA-inducible genes in Bu-5 and Col-0 were identified by comparing the expression profile of salt-acclimated plants with that of non-acclimated plants. A total of 261 SA-inducible genes (fold change, signal value of salt-acclimated plants divided by that of non-acclimated plants >3, signal value >1000) were identified in Bu-5, and 376 SA-inducible genes were identified in Col-0. Figure 7 shows the classification among these SA-inducible genes in Bu-5 and Col-0, as well as the genes expressed strongly in Bu-5 in comparison with Col-0 (ratio, signal value of salt-acclimated Bu-5 divided by that of salt-acclimated Col-0 >1.5). Fewer genes were up-regulated in Bu-5 than in Col-0 after 7 d of SA, suggesting that the salt tolerance mechanism of Bu-5 is not dependent on genome-wide transcriptional changes during the SA. Genes that overlapped in the Venn diagram (Table 1) were categorized into three groups: Group I, SA-inducible genes of Bu-5 that were expressed strongly in Bu-5 compared with Col-0; Group II, SA-inducible genes of both Bu-5 and Col-0 that were expressed strongly in Bu-5 compared with Col-0; and Group III, SA-inducible genes of Col-0 but with a higher signal intensity in Bu-5 than that in Col-0. SA-suppressed genes in Bu-5 and Col-0 were also identified (Supplementary Fig. S3 and Table S2 at JXB online).
Fig. 7.
Classification of SA-inducible genes in Col-0 and Bu-5. In total, 213 and 142 SA-inducible genes of Col-0 and Bu-5, respectively, were identified by microarray analyses. In addition, 271 genes expressed strongly in Bu-5 as compared with Col-0 on day 7 under SA were identified. These genes were categorized into seven groups: Group I, genes induced in Bu-5 and expressed strongly in salt-acclimated Bu-5 compared with salt-acclimated Col-0; Group II, genes induced in both Col-0 and Bu-5 and expressed strongly in salt-acclimated Bu-5 compared with salt-acclimated Col-0; Group III, genes induced in Col-0 and expressed strongly in salt-acclimated Bu-5 compared with salt-acclimated Col-0; Group IV, genes expressed strongly in salt-acclimated Bu-5 compared with salt-acclimated Col-0; Group V, genes induced in Bu-5; Group VI, genes induced in Col-0; Group VII, genes induced in both Col-0 and Bu-5. The number of SA-inducible genes whose expression ratios are >3-fold is indicated. In addition, the number of genes expressed 1.5 times more highly in Bu-5 than in Col-0 on day 7 under SA is indicated. Fold change was calculated from the normalized score based on the signal intensity.
Table 1.
List of genes that are induced by salt acclimation
| Areaa | Fold changeb |
MIPs | Gene name | Descriptionc | ||
| Col-0 with SA | Bu-5 with SA | Bu-5 with SA | ||||
| Col-0 without SA | Bu-5 without SA | Col-0 with SA | ||||
| Group I | 2.2 | 4.7 | 2.4 | At1g60590 | Polygalacturonase | |
| 0.8 | 4 | 1.9 | At4g19690 | 1RT1 | Fe(II) transport protein | |
| 2.6 | 6.1 | 1.7 | At3g02380 | COL2 | Zinc-finger proteins | |
| 2.8 | 5.3 | 1.7 | At2g47800 | ATMRP4 | ATPase transporter involved in multidrug transport | |
| 2.6 | 5.1 | 1.6 | At3g16530 | Lectin-like protein | ||
| 1.9 | 3.6 | 1.6 | At4g38960 | Zinc finger (B-box type) family protein | ||
| 2.6 | 3 | 1.6 | At1g31970 | STRS1 | ATP-dependent RNA helicase 5 | |
| 2.6 | 5.5 | 1.5 | At3g12320 | Unknown protein | ||
| 2.9 | 3.5 | 1.5 | At2g48020 | Sugar transporter ERD6-like 7 | ||
| 2.1 | 3.2 | 1.5 | At4g24700 | Unknown protein | ||
| Group II | 196.5 | 93.4 | 1.7 | At3g60140 | DIN2 member of glycoside hydrolase family 1 | |
| 16.3 | 12.5 | 1.6 | At3g28270 | Similar to AT14A | ||
| 21.2 | 11.9 | 1.6 | At2g04040 | A detoxifying efflux carrier | ||
| 3.5 | 3.9 | 1.5 | At2g39800 | P5CS1 | delta1-pyrroline-5-carboxylate synthase | |
| Group III | 3.3 | 2.9 | 2.3 | At1g15550 | GA4 | Gibberellic acid biosynthetic pathway |
| 3 | 0.6 | 2.2 | At1g52000 | Jacalin lectin family protein | ||
| 3.4 | 2.2 | 1.9 | At5g44670 | Unknown protein | ||
| 4.9 | 2.2 | 1.8 | At5g24160 | SQP1,2 | Squalene monooxygenase 1,2 | |
| 4 | 2.3 | 1.7 | At5g54250 | CNGC4 | Cyclic nucleotide gated channel family | |
| 3.7 | 2.1 | 1.7 | At4g28290 | Unknown protein | ||
| 3.4 | 2.8 | 1.5 | At5g08620 | STRS2 | DEAD-box RNA helicases | |
| 3.2 | 1.6 | 1.5 | At4g08870 | Arginase | ||
| 3 | 2.6 | 1.5 | At3g62310 | RNA helicase | ||
Classification shown in Fig. 8. Group I, genes induced in Bu-5 and expressed highly in salt-acclimated Bu-5 compared with salt-acclimated Col-0. Group II, genes induced in both Col-0 and Bu-5 and expressed highly in salt-acclimated Bu-5 compared with salt-acclimated Col-0. Group III, genes induced in Col-0 and expressed highly in salt-acclimated Bu-5 compared with salt-acclimated Col-0.
Fold change calculated from normalized score on the basis of intensity. SA, salt acclimation.
According to The Arabidopsis Information Resource.
RNA gel-blot analysis was performed for genes encoding arginase (At4g08870), polygalacturonase (At1g60590), ERD6-like sugar transporter (At2g48020), DIN2 (At3g60140), P5CS1 (At2g39800), and ProDH (At3g30775) to confirm the validity of the microarray analyses and to investigate the detailed expression pattern during SA (Fig. 8). The expression levels of all these genes on day 7 of SA were higher than those before SA. Thus, the expression data obtained by microarray analysis correlated well with those obtained by RNA gel-blot analysis. In addition to the strong expression of P5CS1 in Bu-5 plants, the expression level of ProDH, encoding a key enzyme for proline degradation, in Bu-5 was lower than that in Col-0. These results suggest that Bu-5 plants accumulate more proline during SA in comparison with Col-0 plants.
Fig. 8.
Expression of SA-inducible genes in Col-0 and Bu-5. Total RNA (10 μg per lane) was isolated from 7-day-old Col-0 and Bu-5 seedlings subjected to 100 mM NaCl as SA. The northern blots were sequentially probed with the indicated probes belonging to Groups I–III of the microarray analyses.
To analyse whether the higher expression of P5CS1 and the lower expression of ProDH in Bu-5 during SA led to higher proline accumulation, the endogenous levels of amino acids in Bu-5 and Col-0 plants under 100 mM NaCl (SA condition) were measured. Although no difference in proline content was found between Bu-5 and Col-0 plants under normal growth conditions, Bu-5 plants accumulated twice as much proline as Col-0 plants on day 7 of SA (Supplementary Fig. S4 at JXB online). The P5CS1 expression data obtained by microarray and RNA gel-blot analyses correlated well with the proline content. However, Col-0 plants accumulated slightly more proline than Bu-5 plants on day 3 of SA, suggesting that proline accumulation may not be a critical factor for the acquisition of superior SA ability in Bu-5, because the 3 d of SA enables the salt or osmotic stress tolerance to be increased in Bu-5 plants, but is not sufficient (data not shown). There was no significant difference in the other amino acid contents between Bu-5 and Col-0 plants on day 7 of SA.
Genetic analyses of salt or osmotic tolerance with and without SA
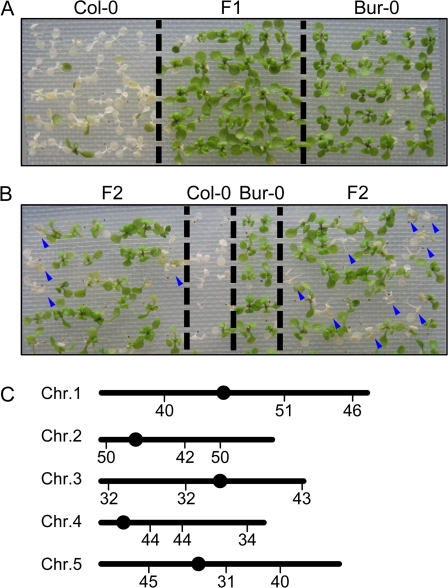
Bur-0, which showed clear salt tolerance on agar plates without SA (Fig. 3), was analysed genetically by crossing it with the salt-sensitive accession, Col-0. All the F1 progeny exhibited salt tolerance (Bur-0 phenotype; Fig. 9A). F2 plants were obtained by selfing and used to map the loci controlling the salt tolerance. DNA was isolated from F2 plants showing salt sensitivity (Col-0 phenotype; Fig. 9B and Table 2). Figure 9C shows the preliminary results using SSLP markers. The loci controlling salt tolerance were found on chromosomes 3, 4, and 5 whose recombination frequencies were 32, 34, and 31%, respectively. To define the positions of these loci further, new SSLP markers that can recognize polymorphisms between Bur-0 and Col-0 around these loci were established. However, no marker showed a recombination frequency <20%, suggesting that without SA the salt tolerance trait of Bur-0 is a polygenic trait.
Fig. 9.
Genetic architecture of NaCl tolerance without SA. (A) Salt tolerance of F1 progeny derived from crosses between Bur-0 and Col-0 plants. Two-week-old seedlings of Bur-0, Col-0, and their F1 progeny grown on a nylon mesh on MS agar plates were mesh transferred to plates with 225 mM NaCl for 7 d. (B) F2 segregation analysis. Two-week-old Bur-0, Col-0, and their F1 progeny grown on a nylon mesh on MS agar plates were mesh transferred to plates with 225 mM NaCl for 7 d. Arrowheads indicate F2 seedlings with the recessive phenotype. (C) Genetic mapping of loci controlling the differential response to salt stress without SA. Recombination frequency derives from the salt-sensitive F2 population. The scores indicate recombination frequencies (%). n=44. SSLP markers are described in Supplementary Table S2 available at JXB online. Dots represent centromeres.
Table 2.
Segregation of sensitivity and tolerance to high salinity and osmotic stress after salt acclimation in F2 progeny from a Col-0×Bur-0 and Col-0×Bu-5 cross
Individual seedling were evaluated for the sensitivity to salinity or osmotic stress and were scored as sensitive (S) or tolerant (T).
χ2 values show that the observed tolerant to sensitive ratio does not deviate from the expected ratio 3:1 at P=0.05 and 1 degree of freedom (1 df).
Phenotype of F1 and F2 populations transferred to 225 mM NaCl for 7 d without salt acclimation.
Phenotype of F1 and F2 populations transferred to 750 mM sorbitol for 14 d after salt acclimation.
To analyse genetically the osmotic tolerance after SA, F1 progeny were generated by crossing an osmotolerant accession, Bu-5, with a sensitive one, Col-0. The salt-acclimated F1 plants all exhibited osmotolerance (Fig. 10A). The osmotolerance of F1 plants was nearly identical to that of Bu-5 plants, whereas the osmotolerance of F1 was slightly lower than that of Bu-5, suggesting that the osmotolerance of Bu-5 plants after SA is semi-dominant: heterozygotes do not have an intermediate phenotype between those of the Col-0 and Bu-5 plants but are nearly identical to the tolerant Bu-5 phenotype. Osmosensitivity to 750 mM sorbitol of the salt-acclimated F2 seedlings segregated with a 3.12:1 ratio of tolerant to sensitive phenotypes (Fig. 10B, Table 2). χ2 analysis showed that the observed tolerant-to-sensitive ratio did not deviate from the expected ratio of 3:1 (Table 2). These results indicate a monogenic and semi-dominant trait controlling sensitivity to osmotic stress after SA.
Fig. 10.
Genetic architecture of osmotic stress tolerance after SA. (A) Salt tolerance of F1 progeny derived from crosses between Bu-5 and Col-0 plants. Two-week-old seedlings of Bu-5, Col-0, and their F1 progeny grown on a nylon mesh on MS agar plates were mesh transferred to plates with 100 mM NaCl (SA) and subsequently transferred to plates with 750 mM sorbitol for 14 d. (B) F2 segregation analysis. Salt-acclimated Bu-5, Col-0, and their F1 progeny were mesh transferred to MS agar plates with 750 mM sorbitol for 14 d. Arrowheads indicate F2 seedlings with the recessive phenotype. (C) Genetic mapping of loci controlling the differential response to osmotic stress after SA. The scores indicate recombination frequencies (%), which are derived from osmosensitive F2 populations [Col-0×Bu-5 (n=42–44), Col-0×Bur-0 (n=20), Col-0×Cal-0 (n=20), Col-0×Ll-1 (n=18), and Col-0×Zu-0 (n=20)]. nd indicates no polymorphism between Col-0 and the accession when the SSLP marker was used. The SSLP markers are described in Supplementary Table S3 at JXB online. Dots represent centromeres.
To map the locus responsible for the osmotolerance of Bu-5 after SA, DNA was isolated from salt-acclimated F2 plants exhibiting osmosensitivity under 750 mM sorbitol (Col-0 phenotype; Fig. 10B). The locus exhibited a strong linkage to At5g_013 on chromosome 5 (Fig. 10C), indicating that the osmotolerant locus of Bu-5 is located between At5g-102 and nga129. Because the osmotolerance of Bu-5 plants after SA is thought to be a semi-dominant phenotype, the osmotolerance locus was also mapped using F2 plants showing osmotolerance under 1250 mM sorbitol after SA (Bu-5 phenotype). In agreement with the mapping using osmosensitive F2 plants, the locus was mapped to the same position (data not shown). This result supports the idea that the Bu-5 allele for osmotolerance after SA is semi-dominant.
Experiments were also carried out to examine whether the osmotolerance locus on chromosome 5 is consistent with the salt tolerance locus of Bu-5. All the salt-acclimated F1 plants exhibited the salt-sensitive Col-0 phenotype under 325 mM NaCl, which is a lethal concentration for salt-acclimated Col-0 plants. Thus, DNA was isolated from salt-tolerant F2 plants after SA. The salt tolerance locus of Bu-5 was mapped to the same position on chromosome 5, coincident with the location of the osmotolerance locus of Bu-5. These results indicate that this locus determines the differential phenotype for salt or osmotic stress tolerance between the tolerant accession Bu-5 and the sensitive accession Col-0. Furthermore, to determine whether the locus responsible for salt-acclimated osmotolerance of Bu-5 is common in the other salt-tolerant accessions, the osmotolerance locus of Bur-0, Cal-0, Ll-1, and Zu-0 was mapped. The osmotolerance locus of these accessions also showed strong linkage to the same position (Fig. 10C), indicating that the locus on chromosome 5 plays an important role in osmotic stress tolerance after SA.
The genes responsible for the salt-acclimated osmotolerance located in the mapped region between At5g-102 and nga129, which is ∼18.5 Mbp and includes 517 genes, were examined. No gene could be identified as an SA-inducible gene by macroarray data. This suggests that the salt-acclimated osmotolerance mechanism of the tolerant accessions is not dependent on the expression level of the responsible gene between the tolerant accessions and the sensitive accession Col-0.
Discussion
Much effort has been directed toward understanding the molecular and cellular mechanisms underlying salt tolerance in plants. Molecular genetic approaches have proven to be an especially powerful tool for identifying the essential genes for plant salt tolerance (Wu et al., 1996; Liu and Zhu, 1998). However, most of these studies were performed on a typical glycophyte, such as A. thaliana Col-0, as significant technological advances have been made for this model plant, whereas genetic tools including molecular markers for positional cloning have not been constructed for halophytic plants. In this study, several A. thaliana accessions showing marked salt tolerance compared with the reference accession, Col-0, were isolated from 350 accessions stocked in the RIKEN Bioresource Center.
The salt tolerance among 350 A. thaliana accessions was evaluated by counting the days of survival from the initiation of salt stress until complete chlorosis was observed in all plants in soil pots exposed to saline water. Because the salt stress treatment using soil pots more gradually exposes plants to saline conditions than the assay using agar plates, the soil pot assay made it possible to detect subtle differences in salt tolerance among the accessions. As a result, 288 accessions with greater salt tolerance than that of Col-0 plants were identified, with some showing marked salt tolerance. To confirm the validity of the evaluation using soil pots, the salt-tolerant accessions were reassessed using agar medium supplied with NaCl. However, the salt tolerance of several accessions was not confirmed by the plate assay. The disagreement in the two salt tolerance assays led to the examination of the ability for SA in plants.
It was assumed that the assay using agar medium could detect differences in the natural salt tolerance among accessions because of the drastic change in NaCl concentration from 0 mM to 225 mM, an osmotic shock, whereas the soil pot assay could detect the salt tolerance induced by salt stress because of the gradual replacement of distilled water with saline water. Therefore, the SA assay was established using agar medium. Arabidopsis accessions, including the salt-sensitive accession Col-0, showed higher salt tolerance after SA than those without SA. Thus, all the accessions showed an ability for SA, but there were substantial differences in the degree of SA ability among them. Some accessions clearly exhibited salt tolerance after SA compared with Col-0, although these accessions failed to show higher salt tolerance than Col-0 without SA (Fig. 4B).
With regard to plant-acquired stress tolerance systems such as SA, acclimation to cold and heat stresses is well known. Most temperate plants, including Arabidopsis, have evolved adaptive mechanisms to increase their freezing tolerance in response to a period of low, but non-freezing, temperatures through a process known as cold acclimation (Warren et al., 1996; Xin and Browse, 1998; Fowler and Thomashow, 2002; Vogel et al., 2005; Xin et al., 2007). The expression of heat shock proteins induced by non-lethal heat treatment confers acquired thermotolerance to lethal temperatures. Recently, it was reported that Arabidopsis Hsa32 and HsfA2 play important roles in plant heat acclimation, and the acquired thermotolerance in both hsa32 and hsfA2 knockout mutants was compromised (Charng et al., 2006, 2007). These previous results for cold and heat acclimation suggest the existence of a regulatory component for acquired osmotic stress tolerance, with subtle differences in the component among Arabidopsis accessions causing large variation in the osmotic and salinity stress tolerance.
Salinity stress has two aspects, ion toxicity and osmotic stress. To reveal which stress tolerance was induced during SA in Bu-5, the ionic or osmotic stress tolerance after SA and Na+ content in the shoots of Bu-5 and Col-0 plants were analysed. Bu-5 plants did not tolerate ionic stress but showed osmotolerance after SA (Fig. 6A). These results suggest that the SA makes it possible for Bu-5 plants to survive osmotic stress, one aspect of salinity stress. Na+ transport plays important roles in plant salt tolerance. To prevent Na+ accumulation in the cytoplasm, plants use the following strategies: reduction of Na+ influx, vascular compartmentalization of Na+, and exclusion of Na+ (Ward et al., 2003). HKT1, NHX1, and SOS1 play essential roles in these mechanisms. Transgenic plants overexpressing HKT1, NHX1, or SOS1 were shown to confer salt tolerance in Arabidopsis and tomato with normal ion homeostasis under salinity stress (Apse et al., 1999; Zhang and Blumwald, 2001; Moller et al., 2009). However, the Na+ content in Bu-5 plants was at the same level as that in Col-0 plants throughout SA and the subsequent high-salinity condition. Moreover, there were no significant differences in the expression levels of HKT1, NHX1, and SOS1 between Bu-5 and Col-0, suggesting that the salt tolerance acquired by Bu-5 plants during SA is independent of these molecules.
An attempt was made to map the loci responsible for salt tolerance with or without SA in salt-tolerant accessions. The mapping using SSLP markers allowed identification of at least three loci on chromosomes 3, 4, and 5 that are responsible for salt tolerance in Bur-0 without SA. Similarly, Quesada et al. (2002) reported that four loci contribute to salt tolerance during the vegetative growth stage in Col-4, whereas the accession is not salt tolerant during the vegetative growth stage. One of the four loci was mapped on chromosome 5 at a position that is close to the location of SOS2, whose product is a Ser/Thr protein kinase required for K+ nutrition and NaCl tolerance during vegetative growth in Arabidopsis. In the present analysis, one of the mapped loci responsible for salt tolerance without SA in Bur-0 was also mapped on chromosome 5 near the SOS2 locus. As for the other two loci on chromosomes 3 and 4, these map positions were different from those of the salt tolerance loci found in Col-4, suggesting that natural variation in salt tolerance without SA is a polygenetic trait and that the genes responsible for salt tolerance differ among Arabidopsis accessions. On the other hand, only one locus responsible for salt tolerance after SA was linked to chromosome 5 in Bu-5. Interestingly, the locus for salt tolerance after SA of the other salt-tolerant accessions, Bur-0, Cal-0, Ll-1, and Zu-0, was mapped to the same locus on chromosome 5, suggesting that this salt tolerance mechanism is common among salt-tolerant accessions. NaCl stress consists of osmotic and ionic stress. It is known that osmotic stress has an immediate effect on growth rather than ionic stress. Ionic stress impacts on growth much later, and has less effect than the osmotic stress, especially at low to moderate salinity levels. Only at high salinity levels does the ionic effect dominate the osmotic effect (Munns and Tester, 2008). Plants subjected to high-salinity stress without SA, such as the mesh-transferred assay in this study, are likely to experience an osmotic shock as well as ionic stress. Thus, it is reasonable that the response is under polygenic control. In contrast, the SA assay and the soil pot assay expose plants to a gradual increase of NaCl stress or to a low salinity level (100 mM NaCl), suggesting that the osmotic effect dominates the ionic effect on plant growth.
The fine mapping of the locus allowed a marker to be found between At5g-102 and nga129 showing very low or zero recombination frequency. Some genes related to abiotic stress, including CYP707A3, SAD1, and three K+ transporters (KAT1, TPK2, and KCO3), are located in this genomic region. The major abscisic acid (ABA) catabolic pathway is triggered by ABA 8′-hydroxylation catalysed by the cytochrome P450 CYP707A family (Okamoto et al., 2006). Among four members of Arabidopsis CYP707As, the expression of CYP707A3 was most highly induced in response to both dehydration and subsequent rehydration. A T-DNA insertional cyp707a3-1 mutant contained higher ABA levels and showed a reduced transpiration rate and hypersensitivity to exogenous ABA during early seedling growth (Umezawa et al., 2006). SAD1 encodes a polypeptide similar to multifunctional Sm-like small nuclear ribonucleoproteins (snRNPs) that are required for mRNA splicing, export, and degradation. The Arabidopsis sad1 mutation causes hypersensitivity to ABA and drought and reduces the expression of some stress-responsive genes (Xiong et al., 2001). The sad1 plants are also defective in the positive feedback regulation of ABA biosynthesis genes by ABA and are impaired in drought stress induction of ABA biosynthesis. Although these genes may contribute to the osmotic stress tolerance after SA through ABA accumulation, significant induction of ABA-inducible genes was not observed in Bu-5 during SA compared with Col-0 (data not shown).
The chemico-physical similarity between Na+ and K+ generates pronounced effects on plant K+ nutrition during NaCl stress. The inhibitory effect of Na+ on K+ uptake mechanisms can create K+ deficiency, and accumulation of cytoplasmic Na+ can interfere with the catalytic role of K+ related to many metabolic processes. At the same time, alteration in K+ can disturb the osmotic balance function of stomata (Mahajan et al., 2008). Plants possess numerous active and passive transporters for the uptake, compartmentalization, and long-distance transport of K+ (Maathuis, 2006). Genes encoding the Shaker family K+ channel KAT1 and voltage-independent K+ channels TPK2 and KCO3 are located in the mapped region between At5g-102 and nga129 (Schachtman et al., 1992; Voelker et al., 2006). Obata et al. (2007) reported that Arabidopsis KAT1 and rice OsKAT1 suppressed the salt-sensitive phenotype of yeast (Saccharomyces cerevisiae) strain G19 (Δena1-4), which lacks a major component of Na+ efflux. Bu-5 plants were able to maintain K+ homeostasis during SA, whereas Col-0 plants were not, although Na+ uptake was similar in Bu-5 and Col-0 plants (Fig. 5). These K+ channels may lead to superior Na+ and K+ homeostasis in Bu-5 plants compared with that in Col-0 plants.
In this work, natural variations in salt tolerance among 350 A. thaliana accessions were analysed. The genetic analyses indicate that salt tolerance without SA is a quantitative trait under polygenic control, whereas salt tolerance with SA is regulated by a single gene common among salt-tolerant accessions. Mapping of the locus responsible for salt tolerance after SA indicated that the gene is located within the region between SSLP markers At5g-102 and nga129, which is ∼18.5 Mbp and includes 517 genes. Fine mapping with more markers and a larger number of F2 progeny is in progress to locate the locus more precisely. The findings will help to elucidate how salt-tolerant plants acquire that tolerance in nature.
Supplementary data
The following data are available at JXB online.
Supplementary Table S1. Evaluation of salt tolerance of Arabidopsis accessions using soil pots or an agar plate with or without salt acclimation (SA).
Supplementary Table S2. List of genes that are suppressed by salt acclimation.
Supplementary Table S3. Information on SSLP markers used for mapping loci controlling the differential response to salt stress without salt acclimation.
Supplementary Table S4. Information on SSLP markers used for mapping loci controlling the differential response to osmotic stress after salt acclimation.
Supplementary Fig. S1. Large-scale evaluation of salt tolerance among 350 Arabidopsis thaliana accessions.
Supplementary Fig. S2. Relationship between salt tolerance and flowering time under normal growth conditions in Arabidopsis accessions.
Supplementary Fig. S3. Classification of SA-suppressed genes in Col-0 and Bu-5.
Supplementary Fig. S4. Amino acid contents in Col-0 and Bu-5 during SA assay.
Supplementary Material
Acknowledgments
We thank Takayuki Nozu, Saki Hoshiyasu, and Megumi Mizukoshi of the Department of Bioscience, Tokyo University of Agriculture, for their technical assistance. We also thank Ms. Yuko Kobayashi of RIKEN Plant Science Center for her excellent technical assistance. The Arabidopsis accessions used in this study are maintained and provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan. This work was supported by a Grant-in-Aid for Young Scientists from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (TT), and by Advanced Research Project of Tokyo University of Agriculture.
References
- Alonso-Blanco C, Aarts MG, Bentsink L, Keurentjes JJ, Reymond M, Vreugdenhil D, Koornneef M. What has natural variation taught us about plant development, physiology, and adaptation? The Plant Cell. 2009;21:1877–1896. doi: 10.1105/tpc.109.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA. 1999;96:4710–4717. doi: 10.1073/pnas.96.8.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, El-Assal SE, Coupland G, Koornneef M. Analysis of natural allelic variation at flowering time loci in the Landsberg erecta and Cape Verde Islands ecotypes of Arabidopsis thaliana. Genetics. 1998;149:749–764. doi: 10.1093/genetics/149.2.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Gomez-Mena C, Llorente F, Koornneef M, Salinas J, Martinez-Zapater JM. Genetic and molecular analyses of natural variation indicate CBF2 as a candidate gene for underlying a freezing tolerance quantitative trait locus in Arabidopsis. Plant Physiology. 2005;139:1304–1312. doi: 10.1104/pp.105.068510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Koornneef M. Naturally occurring variation in Arabidopsis: an underexploited resource for plant genetics. Trends in Plant Science. 2000;5:22–29. doi: 10.1016/s1360-1385(99)01510-1. [DOI] [PubMed] [Google Scholar]
- Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- Apse MP, Sottosanto JB, Blumwald E. Vacuolar cation/H+ exchange, ion homeostasis, and leaf development are altered in a T-DNA insertional mutant of AtNHX1, the Arabidopsis vacuolar Na+/H+ antiporter. The Plant Journal. 2003;36:229–239. doi: 10.1046/j.1365-313x.2003.01871.x. [DOI] [PubMed] [Google Scholar]
- Arditti J, Clements MA, Fast G, Hadley G, Nishimura G, Ernst R. Orchid seed germination and seedling culture. Orchid Biology: Reviews and Perspectives. 1982;II:331–346. [Google Scholar]
- Balasubramanian S, Sureshkumar S, Agrawal M, Michael TP, Wessinger C, Maloof JN, Clark R, Warthmann N, Chory J, Weigel D. The PHYTOCHROME C photoreceptor gene mediates natural variation in flowering and growth responses of Arabidopsis thaliana. Nature Genetics. 2006;38:711–715. doi: 10.1038/ng1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck JB, Schmuths H, Schaal BA. Native range genetic variation in Arabidopsis thaliana is strongly geographically structured and reflects Pleistocene glacial dynamics. Molecular Ecology. 2008;17:902–915. doi: 10.1111/j.1365-294X.2007.03615.x. [DOI] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Chi WT, Wang CN, Chang SH, Wang TT. A heat-inducible transcription factor, HsfA2, is required for extension of acquired thermotolerance in Arabidopsis. Plant Physiology. 2007;143:251–262. doi: 10.1104/pp.106.091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charng YY, Liu HC, Liu NY, Hsu FC, Ko SS. Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. Plant Physiology. 2006;140:1297–1305. doi: 10.1104/pp.105.074898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. Journal of the American Statistical Association. 1979;74:829–836. [Google Scholar]
- Essah PA, Davenport R, Tester M. Sodium influx and accumulation in Arabidopsis. Plant Physiology. 2003;133:307–318. doi: 10.1104/pp.103.022178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ. Improving crop salt tolerance. Journal of Experimental Botany. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- Fowler S, Thomashow MF. Arabidopsis transcriptome profiling indicates that multiple regulatory pathways are activated during cold acclimation in addition to the CBF cold response pathway. The Plant Cell. 2002;14:1675–1690. doi: 10.1105/tpc.003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollner K, Schweizer P, Bai Y, Panstruga R. Natural genetic resources of Arabidopsis thaliana reveal a high prevalence and unexpected phenotypic plasticity of RPW8-mediated powdery mildew resistance. New Phytologist. 2008;177:725–742. doi: 10.1111/j.1469-8137.2007.02339.x. [DOI] [PubMed] [Google Scholar]
- Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W. The exodermis: a variable apoplastic barrier. Journal of Experimental Botany. 2001;52:2245–2264. doi: 10.1093/jexbot/52.365.2245. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kuroda K, Kimura K, Southron-Francis JL, Furuzawa A, Kimura K, Iuchi S, Kobayashi M, Taylor GJ, Koyama H. Amino acid polymorphisms in strictly conserved domains of a P-type ATPase HMA5 are involved in the mechanism of copper tolerance variation in Arabidopsis. Plant Physiology. 2008;148:969–980. doi: 10.1104/pp.108.119933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski SP, Lan TH, Feldmann KA, Paterson AH. QTL mapping of naturally-occurring variation in flowering time of Arabidopsis thaliana. Molecular and General Genetics. 1994;245:548–555. doi: 10.1007/BF00282217. [DOI] [PubMed] [Google Scholar]
- Kunkel BN. A useful weed put to work: genetic analysis of disease resistance in Arabidopsis thaliana. Trends in Genetics. 1996;12:63–69. doi: 10.1016/0168-9525(96)81402-8. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhu JK. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- Maathuis FJ. The role of monovalent cation transporters in plant responses to salinity. Journal of Experimental Botany. 2006;57:1137–1147. doi: 10.1093/jxb/erj001. [DOI] [PubMed] [Google Scholar]
- Mahajan S, Pandey GK, Tuteja N. Calcium- and salt-stress signaling in plants: shedding light on SOS pathway. Archives of Biochemistry and Biophysics. 2008;471:146–158. doi: 10.1016/j.abb.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Mendoza I, Rubio F, Rodriguez-Navarro A, Pardo JM. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. Journal of Biological Chemistry. 1994;269:8792–8796. [PubMed] [Google Scholar]
- Mitchell-Olds T, Schmitt J. Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis. Nature. 2006;441:947–952. doi: 10.1038/nature04878. [DOI] [PubMed] [Google Scholar]
- Moller IS, Gilliham M, Jha D, Mayo GM, Roy SJ, Coates JC, Haseloff J, Tester M. Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. The Plant Cell. 2009;21:2163–2178. doi: 10.1105/tpc.108.064568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L. A new vertical mesh transfer technique for metal-tolerance studies in Arabidopsis (ecotypic variation and copper-sensitive mutants) Plant Physiology. 1995;108:29–38. doi: 10.1104/pp.108.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata T, Kitamoto HK, Nakamura A, Fukuda A, Tanaka Y. Rice shaker potassium channel OsKAT1 confers tolerance to salinity stress on yeast and rice cells. Plant Physiology. 2007;144:1978–1985. doi: 10.1104/pp.107.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Kuwahara A, Seo M, Kushiro T, Asami T, Hirai N, Kamiya Y, Koshiba T, Nambara E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiology. 2006;141:97–107. doi: 10.1104/pp.106.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pico FX, Mendez-Vigo B, Martinez-Zapater JM, Alonso-Blanco C. Natural genetic variation of Arabidopsis thaliana is geographically structured in the Iberian peninsula. Genetics. 2008;180:1009–1021. doi: 10.1534/genetics.108.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochimica et Biophysica Acta. 1989;975:384–394. [Google Scholar]
- Quesada V, Garcia-Martinez S, Piqueras P, Ponce MR, Micol JL. Genetic architecture of NaCl tolerance in Arabidopsis. Plant Physiology. 2002;130:951–963. doi: 10.1104/pp.006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Davis KR. Ozone-induced cell death occurs via two distinct mechanisms in Arabidopsis: the role of salicylic acid. The Plant Journal. 1999;17:603–614. doi: 10.1046/j.1365-313x.1999.00400.x. [DOI] [PubMed] [Google Scholar]
- Rus A, Baxter I, Muthukumar B, Gustin J, Lahner B, Yakubova E, Salt DE. Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis. PLoS Genetics. 2006;2:e210. doi: 10.1371/journal.pgen.0020210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Neves AR, Cavalheiro J, dos Santos MM, Garcia-Quintans N, Lopez P, Santos H. Contribution of citrate metabolism to the growth of Lactococcus lactis CRL264 at low pH. Applied and Environmental Microbiology. 2008;74:1136–1144. doi: 10.1128/AEM.01061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI, Lucas WJ, Anderson JA, Gaber RF. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Ishida J, et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. The Plant Journal. 2002;31:279–292. doi: 10.1046/j.1365-313x.2002.01359.x. [DOI] [PubMed] [Google Scholar]
- Shi H, Ishitani M, Kim C, Zhu JK. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proceedings of the National Academy of Sciences, USA. 2000;97:6896–6901. doi: 10.1073/pnas.120170197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunarpi Horie T, Motoda J, et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. The Plant Journal. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- Swarup K, Alonso-Blanco C, Lynn JR, Michaels SD, Amasino RM, Koornneef M, Millar AJ. Natural allelic variation identifies new genes in the Arabidopsis circadian system. The Plant Journal. 1999;20:67–77. doi: 10.1046/j.1365-313x.1999.00577.x. [DOI] [PubMed] [Google Scholar]
- Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K. CYP707A3, a major ABA 8′-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. The Plant Journal. 2006;46:171–182. doi: 10.1111/j.1365-313X.2006.02683.x. [DOI] [PubMed] [Google Scholar]
- Voelker C, Schmidt D, Mueller-Roeber B, Czempinski K. Members of the Arabidopsis AtTPK/KCO family form homomeric vacuolar channels in planta. The Plant Journal. 2006;48:296–306. doi: 10.1111/j.1365-313X.2006.02868.x. [DOI] [PubMed] [Google Scholar]
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. The Plant Journal. 2005;41:195–211. doi: 10.1111/j.1365-313X.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- Ward JM, Hirschi KD, Sze H. Plants pass the salt. Trends in Plant Science. 2003;8:200–201. doi: 10.1016/S1360-1385(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Warren G, McKown R, Marin AL, Teutonico R. Isolation of mutations affecting the development of freezing tolerance in Arabidopsis thaliana (L.) Heynh. Plant Physiology. 1996;111:1011–1019. doi: 10.1104/pp.111.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SJ, Ding L, Zhu JK. SOS1, a genetic locus essential for salt tolerance and potassium acquisition. The Plant Cell. 1996;8:617–627. doi: 10.1105/tpc.8.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Browse J. Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proceedings of the National Academy of Sciences, USA. 1998;95:7799–7804. doi: 10.1073/pnas.95.13.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Z, Mandaokar A, Chen J, Last RL, Browse J. Arabidopsis ESK1 encodes a novel regulator of freezing tolerance. The Plant Journal. 2007;49:786–799. doi: 10.1111/j.1365-313X.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- Xiong L, Gong Z, Rock CD, Subramanian S, Guo Y, Xu W, Galbraith D, Zhu JK. Modulation of abscisic acid signal transduction and biosynthesis by an Sm-like protein in Arabidopsis. Developmental Sell. 2001;1:771–781. doi: 10.1016/s1534-5807(01)00087-9. [DOI] [PubMed] [Google Scholar]
- Zhang HX, Blumwald E. Transgenic salt-tolerant tomato plants accumulate salt in foliage but not in fruit. Nature Biotechnology. 2001;19:765–768. doi: 10.1038/90824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.