Abstract
Grain weight is one of the most important components of cereal yield and quality. A clearer understanding of the physiological and molecular determinants of this complex trait would provide an insight into the potential benefits for plant breeding. In the present study, the dynamics of dry matter accumulation, water uptake, and grain size in parallel with the expression of expansins during grain growth in wheat were analysed. The stabilized water content of grains showed a strong association with final grain weight (r2=0.88, P <0.01). Grain length was found to be the trait that best correlated with final grain weight (r2=0.98, P <0.01) and volume (r2=0.94, P <0.01). The main events that defined final grain weight occurred during the first third of grain-filling when maternal tissues (the pericarp of grains) undergo considerable expansion. Eight expansin coding sequences were isolated from pericarp RNA and the temporal profiles of accumulation of these transcripts were monitored. Sequences showing high homology with TaExpA6 were notably abundant during early grain expansion and declined as maturity was reached. RNA in situ hybridization studies revealed that the transcript for TaExpA6 was principally found in the pericarp during early growth in grain development and, subsequently, in both the endosperm and pericarp. The signal in these images is likely to be the sum of the transcript levels of all three sequences with high similarity to the TaExpA6 gene. The early part of the expression profile of this putative expansin gene correlates well with the critical periods of early grain expansion, suggesting it as a possible factor in the final determination of grain size.
Keywords: Expansin, gene family, grain growth, in situ hybridization, semi-quantitative RT-PCR, Triticum aestivum
Introduction
Molecular assisted breeding in crops needs to bridge the gap between knowledge in ecophysiology and advances in functional genomics. Uncovering the importance of particular genes in the determination of complex traits represents a key challenge for interdisciplinary efforts in improving efficiency in plant breeding of crops for food production. In wheat, grain weight (GW) is important not only for yield, but also in determining seed and grain quality (Marshall et al., 1986; Richards and Lukacs, 2002). Indeed, future increases of wheat yield potential could be achieved by improving individual grain weight as a breeding strategy aimed at avoiding the setting of smaller grains in more distal positions of the spike with a lower grain nutrient concentration (Calderini and Ortiz-Monasterio, 2003). However, the physiological mechanisms involved in the determination of grain weight and size are still poorly understood.
A number of approaches have been attempted in wheat and other crops to understand the determination of grain weight potential (GWP). The simplest way to address this complex trait has been through the monitoring of the rate of dry matter accumulation and the duration of the grain-filling period (Asana and Williams, 1965; Sofield et al., 1977; Wiegand and Cuellar, 1981). It has also been found that endosperm cell number plays an important role in GWP since the study by Brocklehurst (1977). The association between grain weight and grain volume at physiological maturity or harvest has also been reported by different authors (Dunstone and Evans, 1974; Millet and Pinthus, 1984; Saini and Westgate, 2000) indicating that grain density is a conservative trait of wheat (Borrás et al., 2004, and references therein). In addition, grain weight and maximum water content, which is reached early in the grain-filling period, have been found to be associated in wheat (Millet and Pinthus, 1984; Saini and Westgate, 2000), triticale (Saini and Westgate, 2000), maize (Borrás et al., 2004; Gambín et al., 2007a; Sala et al., 2007), sorghum (Gambin et al., 2007b; Yang et al., 2009), and sunflower (Lindstöm et al., 2006; Rondanini et al., 2007). More recently, gene transcript profiles during the grain-filling period in wheat (Laudencia-Chingcuanco et al., 2007), molecular co-ordination among grain tissues (Berger et al., 2006), and maps of quantitative trait loci associated with grain size in barley (see review by Coventry et al., 2003) have been published. Although these important advances provide promising clues towards their identification, the traits determining GWP remain elusive.
Water and dry matter dynamics are closely related in the growing grains of wheat (Schnyder and Baum, 1992; Calderini et al., 2000; Borrás et al., 2004; Pepler et al., 2005). The maximum water content is reached before grains accumulate 40% of their final dry weight (Borrás et al., 2004), indicating that the initial grain expansion occurs prior to storage reserve accumulation. Since the expansive growth of plant organs is often determined by the growth of the outer tissues (Kutschera and Niklas, 2007), it seems likely that a similar growth pattern occurs in wheat grains. During early development, the endosperm is largely acellular and the seed coat and pericarp form the outer layers of the developing grain (Rogers and Quatrano, 1983; Lopes and Larkins, 1993). The most sensitive period for grain weight determination before anthesis is after booting, when the carpels of the florets are expanding rapidly (Calderini et al., 1999a, 2001) and a positive association between final GW and the size of floret cavities in wheat was reported by Millet (1986). In addition, final GW and carpel weight at anthesis are associated in barley (Scott et al., 1983), rye grass (Warringa et al., 1998), sunflower (Cantagallo et al., 2004), and wheat (Calderini et al., 1999a; Calderini and Reynolds, 2000). From these observations, it seems possible that the maternal tissues, which form the pericarp of grains, affect the determination of grain weight, perhaps by constraining grain expansion and, consequently, grain volume (Calderini et al., 1999a, Ugarte et al., 2007).
The plant cell wall is a key determinant of cell expansion, shape, and volume. Cell walls have to be extremely strong to bear the stresses imposed by the internal turgor pressure of plant cells, but must also maintain the ability to extend during cell growth (Cosgrove, 1997). While the cellulose–hemicellulose network determines the extensibility of cell walls, proteins that act on this network control the process of cell growth. Among the proteins thought to regulate cell expansion, expansins are the only ones shown directly to induce cell wall extension (McQueen-Mason et al., 1992). The mechanism of expansin action involves the disruption of hydrogen bonds between cellulose microfibrils and cross-linking glycans in the cell wall, permitting turgor-driven cell wall extension (McQueen-Mason and Cosgrove, 1994, 1995). Two general classes of expansins are recognized in plants, with the α-expansins having a clear role in growth (Cosgrove, 2000; Li et al., 2003), whilst the roles of β-expansins are less clearly understood. Expansins are encoded by substantial multi-gene families, with more than 30 genes encoded in the genomes of both Arabidopsis and rice (Li et al., 2003). The number of expansin genes in a hexaploid cereal such as wheat is likely to be even higher. Recently, Liu et al. (2007) reported 168 wheat ESTs, representing putative α-expansin gene family members and, according to the differences in the deduced amino acid sequences of the selected ESTs, they estimated that, in the hexaploid wheat genome, there may be at least 30 α-expansins. Lin et al. (2005) examined the expression of nine α-expansins in different tissues of wheat and concluded that several different expansin genes are often expressed at similar levels in a single tissue, suggesting that some expansin genes may have overlapping biological functions. In a recent examination the expression of a small number of expansin transcripts in wheat (Liu et al., 2007) showed high expression of α-expansins in the rapidly elongating anther filament and in the stem.
In the present study, the dynamics of grain dry matter accumulation, water uptake, and grain size were analysed in parallel with the expression of expansins during those processes. Expansin genes co-expressed with key events in grain expansion were identified and their spatial expression pattern in grain was characterized.
Materials and methods
Plant material and field conditions
An experiment evaluating the spring wheat cultivar Bacanora, (released by CIMMYT) was sown in the field for two growing seasons at the Experimental Station of Universidad Austral de Chile in Valdivia (39°47′ S, 73°14′ W), Chile. Sowing dates were 31 August 2005 (season 1) and the 1 September 2006 (season 2). The experimental plots consisted of nine 2 m rows, spaced 15 cm apart. Seeding rates were 350 plants m−2 in both seasons. Plots were arranged in a randomized design with three replications. Fertilization rate was 300 kg N ha−1 and 150 kg P2O5 ha−1 incorporated during land preparation. The plots were surface-irrigated as required until maturity. Weeds were removed periodically by hand, while diseases (powdery mildew) and insects (aphids) were controlled by Priori (i.a. Azoxystrobin, Syngenta Agrobussines SA) and Karate (i.a. Lambdacitralotrina, Syngenta Agrobussines SA), respectively.
Grain measurements
The dates when the crop reached anthesis (stage 65) and physiological maturity (stage 95) were recorded using the scale proposed by Zadoks et al. (1974). At anthesis, 75 similar spikes were tagged. Fresh and dry weight and grain size (length, width, and height) of grain position 2 (the second grain from the rachis) of two central spikelets of the spike in season 1, and positions 1–4 in season 2 were measured from anthesis onwards, twice weekly until c. 50 d after anthesis (DAA). Grain volume was calculated from grain dimensions as shown below. Individual grains were weighed immediately following harvest and after oven drying for 48 h at 65 °C with an electronic balance (Mettler Toledo, XP205DR, Greifensee, Switzerland) to record fresh and dry weights, respectively. At harvest, 10 tagged spikes per plot were sampled to register final dry grain weight, dimensions, and volume of all grain positions (1–4) from the two central spikelets by the same procedure as during the grain-filling period.
Final grain weight, rate of grain-filling, and timing of physiological maturity were estimated using a linear model subjected to boundary conditions (i.e. grain weight is described by two equations with one break point) as in Calderini et al. (1999a). This break point represents the thermal time from anthesis to physiological maturity (when grain-filling ceased) and was calculated as the sum of daily average temperature [(Tmax+Tmin)/2] with a base of 0 °C (Hay and Kirby, 1991; Slafer et al., 1994; Calderini et al., 1996).
Length, width, and height of each grain were measured with an electronic caliper (6 inch/150 mm Digital Calipers, China). Grain volume (GV) was calculated from these measurements assuming the grain as an ellipsoid according to the Pikunov (1978) and Granville (1952) equations, respectively, as in a previous study (Miralles et al., 1998). The maximum value of the dimensions and volume, and the timing when these were reached, were calculated using the same model as that of grain weight dynamics.
Taking into account that grain water content follows a parabolic curve, a trilineal model, similar to that used by Pepler et al. (2005), was fitted to the water content data. The model was fitted using the iterative optimization technique of Table curve V 2.0 (Jandel, 1991).
Data of final grain weight and variables from grain growth dynamics (grain-filling rate, increase in water content rate, time of maximum grain weight, and time to stabilized water content) were assessed by two-way analysis of variance. Regression analyses were used to evaluate the degree of association between variables.
Cloning of pericarp-specific expansin cDNAs and sequence analysis
Total RNA was extracted with the RNeasy Plant kit (Quiagen, Basel, Switzerland) from 100 mg of isolated pericarps of wheat grains cv. Bacanora, harvested at 14 DAA from an experiment carried out under field conditions in 2004. The management of the experimental plots was as described above for seasons 1 (2005) and 2 (2006).
First strand cDNA was synthesized from total RNA (1 μg) from isolated wheat pericarps using Superscript reverse transcriptase following the manufacturer's specification (Invitrogen, Carlsbad, CA, USA). α-expansins sequences were amplified by PCR using degenerate primers (5′-GCG CTG AGC ACG GCS CTS TTC-3′ with 5′-CTG CCA GWT NDS GCC CCA GTT-3′ or 5′-GTG TCG TAS ACC YSG CCG GCG-3′), designed to conserve amino acid domain sequences as described by Jones and McQueen-Mason (2004). 1 μl cDNA was used as the template in a PCR of 35 cycles (94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min) and one cycle of 72 °C for 10 min and the reaction products were analysed on a 1% agarose gel. A single band was purified by a Promega gel purification kit (Wizard SV Gel and PCR Clean-Up System, Promega). PCR products were cloned into TOPO TA vectors following the manufacturer's instructions (Invitrogen); 40 putative expansin clones were digested with EcoR1, and subsequently sequenced in both directions by Lark Technologies (UK). The DNA sequences were used in BLASTn searches to confirm that they represented expansin transcripts, and aligned using the program ClustalX (version 2.0) to reveal the number of unique sequences. Sequences were searched against the non-redundant GenBank DNA and protein database using BLASTn and BLASTX (Altschul et al., 1990, 1997) and against the Uni Prot database (Release 1.5, TrEMBL, Swiss-Prot, and PIR, http://www.ebi.ac.uk/uniprot) resources using BLASTX. The best matches were used as the basis for obtaining annotations based on sequence identity.
Analysis of expansin gene expression by RT-PCR
Specific PCR primer pairs were designed for five novel expansin sequences that were cloned and consensus primers pairs were designed to discriminate three subgroups of sequences with high homology to one another using Primer3 software (Table 1). In season 1, all primers pairs were used to cDNA amplifications to obtain a screen of the pattern expression of the isolated expansin sequences. In season 2, five representative sequences were selected to the expression analysis. A fragment of the constitutively expressed 18S ribosomal RNA gene was amplified with the primers (Kong et al., 2007) and used as a control.
Table 1.
Primers used for the amplification of cDNA fragments encoding putative expansins of wheat pericarps cv. Bacanora
| Accession number of cloned sequence | Cloned sequence | Accession number | Expansin gene | Identity with expansin gene | Forward primer sequence (5′–3′) | Reverse primer sequence (5′–3′) |
| FN556064 | pTaExpA1 | AY589583 | TaExpA1 | 77% | GTG CGG TCA GTG CTA CAA GA | ATA GTT AGG CGG GCA AAG G |
| FN556065 | TaExpA2 | AY589584 | TaExpA2 | 98% | CCA CCA TGA TGT GTT GTT CC | AGT AGG AGT GGC CGT TGA TG |
| FN556066 | pTaExpA4 | AY543530 | TaExpA4 | 87% | AAC TTC TGC CCG TCG AAC TA | CCC TTC ATG GTG AAC CTC AT |
| FN556067 | pTaExpA5 | AY543531 | TaExpA5 | 74% | ACC ACA TCC ACA CAC GAG AG | CCA CCA GCT CGA AGT AGT CC |
| FN556069 | pTaExpA6 | AY543532 | TaExpA6 | 92% | CAA TCC TCC CCG CGA AC | GGT CCC CTT CAC CGA CAT |
| FN556070 | pTaExpA6 (a)a | AY543532 | TaExpA6 | 84% | GTG CAA CCC TCC TCG ACA C | GGT CCC CTT CAC CGA CAT |
| FN556071 | pTaExpA6 (b)a | AY543532 | TaExpA6 | 84% | GCA ACC CTC CCC GCG TC | GGT CCC CTT CAC CGA CAT |
| FN556068 | pTaExpA8 | AY543534 | TaExpA8 | 94% | ACT ACG CAC TCC CCA ACA AC | AGA GCT CAA GTC ACC GAT GC |
| 18S rRNA | GTG ACG GGT GAC GGA GAA TT | GAC ACT AAT GCG CCC GGT AT |
The table shows the maximum identity between sequences cloned and wheat expansins reported.
(a) and (b) represent groups of sequences with homology to TaExpA6 but different between them. (a) has 88% identity with (b).
The expansin sequences cloned in the present study had only been isolated from the pericarp of wheat grains in a preliminary study (Calderini et al., 2006). Expression profiles of these expansin transcripts were assessed in both isolated pericarps and whole grains and similar expression was found (data not shown). For these reasons, and in order to avoid excessive manipulation of grain tissues that may result in the loss of mRNA, expression mRNA analysis of whole grains was carried out in the present study. Total RNA was extracted from eight whole grains from two central spikelets of four spikes per plot, harvested at different development stages (2–20 DAA in season 1 and 6–34 DAA in season 2). Only the second grain from the spike rachis was sampled in season 1 and the second and third grains in season 2. RNA was isolated using TRIZOL (Invitrogen) and treated with DNAse I, RNase Free (Fermentas, Ontario, Canada). The quality and concentration of RNA was measured by UV-spectrometry with Nanodrop (nd-1000, Thermo Fisher Scientific, USA). RNA aliquots were analysed by electrophoresis in 2% agarose gels and visualized with UV light. First-strand cDNA was synthesized from 2 μg of total RNA in 20 μl of reaction volume, using M-MLV reverse transcriptase (Invitrogen). One-tenth of the first-strand cDNA was used as a template in a 20 μl PCR of 25 cycles (94 °C for 45 s, 60 °C for 45 s, and 72 °C for 45 s) using gene-specific or consensus primers. PCR products were analysed by electrophoresis in 2% agarose gels and visualized with UV light.
Sequence analysis of PCR products
0.1 ml PCR product was re-amplified by PCR with Taq DNA polimerase (Fermentas) and sequenced by 3730xl DNA analyser (Macrogen, Korea). The sequence data obtained was corrected by Chromas Lite 2.01 and comparated to other sequences in the NCBI Blast search program (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Probe synthesis
pTaEXPA6 probes were produced with specific primers (5′-CAA TCC TCC CCG CGA AC-3′ and 5′-GCC ATG TGG AGA AGG TCT GT-3′). RT-PCR products (350 bp) were cloned into the pGEM-T Easy Vector (Promega). Purified plasmid fragment was linearized with NcoI (Promega) or SalI (Biolabs) restriction enzymes. Probes were DIG labelled by in vitro transcription. Antisense and sense RNA probes were generated using SP6 and T7 RNA-polymerase (Invitrogen) depending on the orientation of the inserts.
In situ hybridization
To know the grain localization of expansin transcripts, in situ hybridization was performed. Samples of grains from position 2 were assessed at two development stages (5 DAA and 10 DAA) in season 2. In situ hybridization was performed following Jackson (1992), Coen et al. (1990), and Fobert et al. (1994). Grains were fixed in ice-cold 4% paraformaldehyde in PBS (Sigma) through vacuum infiltration, dehydrated with ethanol, then exchanged with Histoclear (National Diagnostics) and embedded into wax (Paramat Extra BDH), forming blocks containing 8–12 grains each. Transverse sections (10 μm thick) were mounted on Superfrost plus glass slides (BHD) prewarmed to 42 °C. Tissue on the slides was dewaxed, rehydrated, incubated for 10 min in proteinase K (1 μg ml−1) at 37 °C, and then hybridized with appropriate DIG-labelled probes (200 ng per slide) overnight at 55 °C using a coverslip (Hybri-slip, Sigma) over the slides. Antisense and sense probes were used in parallel hybridizations. Post-hybridization, washing at graded stringency (2× SSC at 55 °C and 1× NTE at 37 °C) was followed by detection. Antibody detection was performed by enzyme-linked immunoassay using a DIG Nucleic Acid Detection Kit (Roche), according to the manufacturer's instructions. The colour reaction was developed in the dark for between 3–5 h, stopped by immersing the slides in TE buffer, and the slides analysed under visible light with a Nikon optiphot microscope.
Results
Dynamics of grain dry matter accumulation
The final grain weight of all grain positions from two central spikelets measured at harvest in two crop seasons showed a wide range, between 32 mg and 56 mg (Table 2). This trait was affected (P <0.01) by both the growing season and the grain position within the spikelet (Table 2). Between seasons, differences in grain weight could be ascribed to mean temperatures during the anthesis–physiological maturity period as similar temperatures were found between booting and anthesis (14.5 °C). In season 1, mean temperature during the grain-filling period was 16.7 °C, while it was 15.5 °C in season 2. Because temperature is the main environmental driver of wheat development at this phase, grain fill duration was accordingly shorter in season 1 (36 d) than in season 2 (45 d). To avoid the effect of temperature on grain development, the time-course of the data are shown in thermal time units (°Cd) instead of days (Fig. 1). Taking into account grain positions, G2 reached the highest weight and G4 the lowest in both seasons as expected (Table 2). The final grain weight showed a close relationship (r2=0.94, P <0.01) with final grain volume.
Table 2.
Final grain weight of different grain positions from the rachis of the two central spikelets of wheat cv. Bacanora in growing seasons 1 and 2
| Grain position | Season 1a | Season 2a | |
| G1 | 44.9 c | 51.6 b | |
| G2 | 49.8 b | 56.3 a | |
| G3 | 43.9 c | 51.6 b | |
| G4 | 31.6 e | 37.4 d | |
| SEMb | 1.64 | ||
| Season | 6.35 E-07 | ||
| Grain position | 3.60 E−10 | ||
| S×GPc | 0.880 |
Different letters within the same column or between columns indicate statistically significant difference at P <0.05.
SEM stands for the standard error of the means.
S×Gp corresponds to Season×Grain position interaction effects. Values are means of three replicates. For each replicate 20 grains were evaluated.
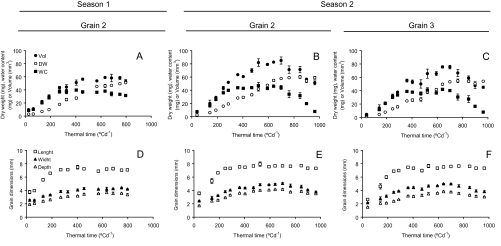
Fig. 1.
Volume (filled circles), dry weight (open circles), water content (filled squares), and grain dimensions [length (open squares), width (filled triangles), and height (open triangles)] of grain position 2 in growing seasons 1 (A, D) and 2 (B, E) and grain position 3 (C, F) in season 2.
Starting from anthesis, the dynamics of dry weight and water content (G2 in season 1 and G2 and G3 in season 2) are shown in Fig. 1 and Table 3. Averaged across grains, the rate of water uptake to stabilized grain water content (SGWC) was 35% higher than dry matter in season 1 and 46% higher in season 2 (Fig. 1). In addition, stabilized water content was reached at 275 °Cd (18 DAA) and 320 °Cd (22 DAA) after anthesis in seasons 1 and 2, respectively (Table 3). At that time grain weight was only 38% of its final value (Fig. 1A, B, C). Thus, it is clear that the water content of grains increased in advance of dry matter accumulation, indicating that grain expansion precedes filling (Fig. 1). From around 300 °Cd, water content remained at relatively constant values until shortly before physiological maturity. Similar to water content, grain volume increased up to 59% of its maximum value early (around 300 °Cd) during the grain-filling period in season 1 and 48% in season 2, however, maximum volume of grains was reached late in this phenological phase and close to physiological maturity, i.e. between 70% and 94% of the whole grain-filling period (Fig. 1).
Table 3.
Final grain length (FGL) stabilized grain water content (SGWC), maximum volume (MV), increased rates to FGL, SGWC, and MV, and thermal units when each stage was reached for grain positions 2 and 3 in the growing seasons 1 and 2
| Season | Grain position | FGL (mm) | SGWC (mg) | MV (mm3) | Increase in rate to |
Thermal units (°Cd) to reach: |
||||
| FGL (mm °Cd−1) | SGWC (mg °Cd−1) | MV (mm3 °Cd−1) | FGL | SGWC | MV | |||||
| 1 | 2 | 7.2 b | 37.3 b | 60.5 c | 0.018 a | 0.122 a | 0.134 a | 222 b | 275 b | 426 b |
| 2 | 2 | 7.6 a | 44.4 a | 91.3 a | 0.020 a | 0.139 a | 0.142 a | 246 ab | 319 ab | 613 a |
| 3 | 7.4 b | 38.7 b | 80.1 b | 0.021 a | 0.129 a | 0.117 a | 264 a | 327 a | 668 a | |
| SEM | 0.068 | 1.164 | 4.581 | 0.000 | 0.006 | 0.004 | 8.223 | 10.85 | 41.97 | |
| p | 0.009 | 0.003 | 0.000 | 0.514 | 0.367 | 0.321 | 0.089 | 0.082 | 0.014 | |
Different letters within the same column indicate statistically significant difference at P <0.05. SEM stands for the standard error of the means. Values are the means of three replicates.
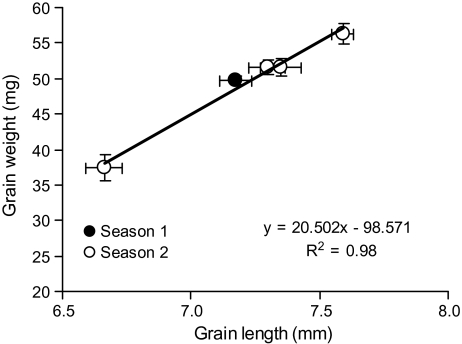
Grain dimensions showed contrasting dynamics. Grain length reached its final value in advance of grain width and height, but it is noteworthy to highlight that grain length consistently reached its final value shortly before the stabilized water content in both seasons and all grain positions. Averaged across the three cases studied here, grain length reached its final value at 244 °Cd while water content levelled off at 307 °Cd (Table 3). Similar results were observed in grain positions 1 and 4 in season 2 (data not shown). To illustrate the importance of grain length for grain weight determination, a regression analysis was performed between final grain weight and stabilized grain length and a close association was found (Fig. 2).
Fig. 2.
Relationship between grain weight and stabilized grain length of grain position 2 in season 1 and positions 1–4 in growing season 2.
Expansin transcript accumulation in developing grains
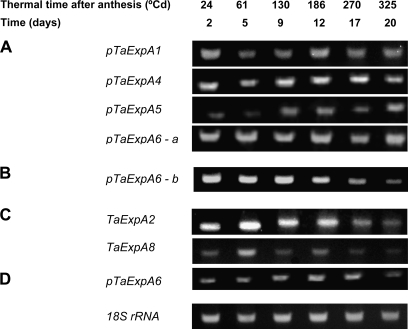
To identify expansin transcripts present in developing wheat grains, PCR amplification was carried out using degenerate PCR primers, designed to amplify all α-expansin transcripts present in the pericarp of wheat grains. We cloned and sequenced 24 PCR products from three independent RT-PCR experiments. The alignment and BLASTX searches of these sequences revealed five unique sequences (Table 1) that showed high homology to α-expansins and a number of sequences with higher homology to TaExpA6 but with sufficient differences in sequence between them to suggest they are homologous to each other.
Three subgroups were distinguished from this pool of sequences, these were called pTaExpA6 (a group with higher homology to TaExpA6; Table 1), pTaExpA6-a and pTaExpA6-b. The homology of TaExpA6 with pTaExpA6-a was 88% and with pTaExpA6-b 91%. pTaExpA6-a showed 88% homology with pTaExpA6-b. From the homologue expansin sequences, only TaExpA4 has been mapped to chromosome 3A of the wheat hexaploid genome (Qi et al., 2004). Sequences were named putative (p) when protein sequence showed less than 95% identity with the α-expansin gene family reported in wheat). Amplification was possible for each one of the three subgroups of TaExpA6 and for other five unique sequences cloned (Fig. 3). The eight α-expansins transcripts (115–220 bp) identified were expressed in all the stages of grain development that were evaluated (24–325 °Cd) in season 1. Four expression patterns of these α-expansins during grain development were identified (Fig. 3). Expansin genes pTaExpA1, pTaExpA4, pTaExpA5, and pTaExpA6-a showed variable expression profiles between 24 °Cd to 325 °Cd after anthesis (2–20 DAA) with no clear peak of transcript abundance at one specific grain development stage (Fig. 3A). pTaExpA6-b transcripts decreased progressively from 24 °Cd to 325 °Cd (2–20 DAA; Fig. 3B). Two expansin genes, TaExpA2 and pTaExpA8, were most highly expressed at 61 °Cd from anthesis (5 DAA) then declining sharply to 325 °Cd (20 DAA; Fig. 3C). pTaExpA6 sequence reached a peak of expression at 186 °Cd from anthesis (12 DAA), also declining at 325 °Cd (20 DAA) of grain development stage (Fig. 3D). pTaExpA6 and subgroups a and b showed different expression patterns in season 1 (Fig. 3).
Fig. 3.
Expression profile of fragments encoding expansins sequences of wheat grain pericarps. Four expression patterns were identified: A. Variable expression (no single peak was identified); B. Expression decrease gradually; C. Peak to 61°Cd after anthesis; D. Peak to 186 °Cd after anthesis. Total RNA (2 ng) from grain position 2 collected between 24 °Cd and 325 °Cd after anthesis (2–20 DAA) in growing season 1 was used as a template for RT-PCR analysis by gene-specific primers. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
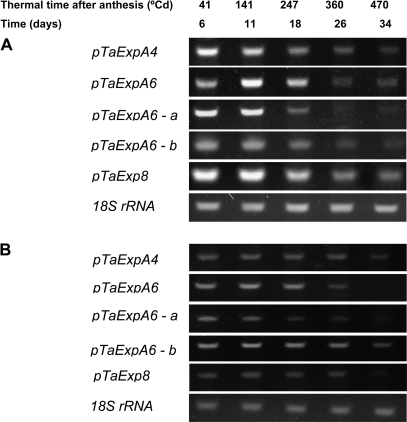
Considering the types of expression profiles detected in season 1 for the eight α-expansins transcripts, five representative sequences (pTaExpA4, pTaExpA6, pTaExpA6-a, pTaExpA6-b, and pTaExpA8) were selected to be analysed in season 2 (Fig. 4). An extended grain development period was tested on this season (41–470 °Cd, matched to 3–32 DAA) corresponding to 67% of the grain-filling. As longer intervals of time were analysed, stronger differences in expression levels were visualized at each moment of grain development. In grain position 2 (Fig. 4A), most of the expansin genes (pTaExpA4, pTaExpA6-a, pTaExpA6-b, and pTaExpA8) showed similar expression at 41 °Cd and 141 °Cd after anthesis (6 DAA and 11 DAA). pTaExpA6 showed a peak of expression at 141 °Cd after anthesis. A reduction in transcript levels was found from 247 °Cd after anthesis (18 DAA). The three consensus sequences that encode homeologues of TaExpA6 showed the strongest reduction of transcript expression from 247 °Cd after anthesis according with the stabilization of grain enlargement. In addition, grain position 3 from the spike rachis was evaluated at season 2 (Fig. 4). The same patterns of expression were found in grain 3 for the five α-expansin transcripts (Fig. 4B), but a late decrease in transcripts was detected relative to grain 2.
Fig. 4.
Expression profile of fragments encoding expansin sequences of wheat grain pericarp. Total RNA (2 ng) from grain positions 2 (A) and 3 (B) collected between 41 °Cd and 470 °Cd after anthesis (6–34 DAA) in growing season 2 was used as a template for RT-PCR analysis by primers. The PCR products were separated on a 1% agarose gel and stained with ethidium bromide.
In both crop seasons the time of high abundance of most of the expansin transcripts matched with the length increase and incoming water content on grain (Table 3; Fig. 1). For example, final grain length (FGL) and stabilized grain water content (SGWC) were reached at 222 °Cd and 275 °Cd in season 1 (Table 3) while the decline of some expansin transcripts (pTaExpA2, pTaexpA6, pTaexpA6-b, and pTaExpA8) were evident from 270 °Cd from anthesis. In season 2, FGL and SGWC were reached at 246 °Cd and 319 °Cd in grain 2 while the abundance of all transcripts tested was sharply decreased from 360 °Cd after anthesis.
In situ localization of the expansins transcripts in developing grains
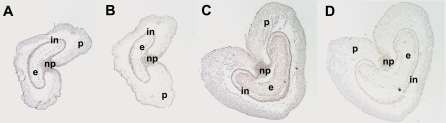
In order to establish the localization of the expansin genes expressed within the anatomical structures of the grain, in situ hybridization (ISH) of pTaExpA6 in grain sections was performed. Although hybridization of pTaExpA6 showed expression of the gene in all the tissues of the grain, at 93 °Cd the transcript accumulated preferentially in the pericarp (Fig. 5A). At 187 °Cd (10 DAA) the expression of this transcript was stronger in the pericarp and endosperm tissues, showing a higher signal intensity in the endosperm (Fig. 5C). A weak signal for pTaExpA6 detected in the endosperm of 93 °Cd grains may be related to scarce cell wall development of this tissue at this stage (Philippe et al., 2006). This result is in agreement with RT-PCR expression analysis that showed a peak of expression in whole grains for pTaExpA6 around 180 °Cd after anthesis (Figs 3D, 4A, B), when high ISH signal was detected in both the pericarp and the endosperm.
Fig. 5.
In situ localization of expansin mRNA on transverse sections of wheat grains (grain position 2) at 5 (A, B) and 10 (C, D) DAA. (A, C) Hybridization with a gene-specific TaExpA6 antisense probe. (B, D) Hybridization with a sense probe. In (A) and (C), arrows identify cells showing hybridization signals. For grains, the following cell types are annotated: pericarp (p), endosperm (e), nucellar projection (np), integuments (in).
Discussion
In the present study, stable water content and grain length were identified as the most important physiological traits best correlated with final grain weight in wheat. Although crop seasons and grain positions generated a wide range of final grain weight (32–56 mg) our data supported the association between grain weight and grain volume at harvest (r2=0.94, P <0.01) reported previously (Dunstone and Evans, 1974; Millet and Pinthus, 1984; Saini and Westgate, 2000). Moreover, the dynamic of grain water content (especially SGWC), evaluated only in specific grains (grain position 2 in season 1 and grain positions 2 and 3 in season 2), showed a strong association with final grain weight (r2=0.88, P <0.01). These results underline the importance of water dynamics on grain weight determination reported previously (Chanda and Singh, 1998; Saini and Westgate, 2000). Less information is available regarding the relationship between grain dimension dynamics and grain weight/size. Rogers and Quatrano (1983), plotted various traits of grain growth and showed that 90% of grain length is attained early in grain development (7 DAA under their conditions), but no associations with water content, volume or final grain weight were made. In the present study, grain length was the trait that showed the best correlation with final grain weight (r2=0.98; P <0.01) and grain volume (r2=0.94, P <0.01). Under high temperatures after anthesis, Tashiro and Wardlaw (1990) showed that both grain weight and length were the most affected traits, supporting the importance of grain length in wheat.
In our experiments, grain length, water content, volume, and weight increased 2.5, 7, 10, and 25 times during grain growth, respectively. All of these traits showed the highest rate of increase during the first third of the grain growth period (Fig. 1), especially grain length and water content, and they reached the maximum value at this time (250–300 °Cd). It is of note that grain length showed a close association with grain weight (Fig. 2). Although this association does not imply a cause–effect relationship, the data presented in this study suggest that stabilized grain length may be critical for grain weight determination. During the initial phase of grain growth, maternal tissues (the pericarp of the grains) undergo a remarkable expansion as it is the main component of grain at this time (Drea et al., 2005). Grain length seems to be the trait more specifically associated with pericarp expansion as the fast endosperm expansion on grain starts when the maximum grain length is close to being reached (Phillipe et al., 2006). This correlation supports a proposed hypothesis stating that grain weight determination is driven by pericarp growth (Calderini et al., 1999a, b; Calderini and Reynolds, 2000).
Having established the importance of grain enlargement early in the grain development in the determination of grain weight, it was decided to explore possible molecular drivers of this physiological process. The approach used was expression analysis of target genes by semi-quantitative reverse-transcription PCR complemented with in situ hybridization to define the cellular specificity of the expression patterns.
In a bottom-up analysis of the expression of 7835 genes in developing wheat caryopses by cDNA arrays, Laudencia-Chingcuanco et al. (2007) identified 2237 genes that were differentially expressed during grain development. Wan et al. (2008) analysed 55 052 transcripts of developing cariopses from hexaploid wheat using Affimetrix wheat GeneChip® oligonucleotide arrays and found that 14 550 of these genes showed significant different regulation between 6 DAA and 42 DAA. Six α-expansins were found in the last microarray study. Three sequences homologous to them were also detected in our experiment (pTaExpA5, pTaExpA6, and pTaExpA8) but no information is available about the other three expansins (pTaExpA1, TaExpA2, and pTaExpA4) found in the present study. Different expression patterns were observed between our study and the microarray data used by Wan et al. (2008). In their study, TaExpA8 showed higher expression beyond 20 DAA while, in the present study, this transcript declined. These differences can be explained by the differences in the sequences between the expansin genes considered in each work (Table 1). Moreover, even the three sequences with homology to TaExpA6 found in our study (pTaExpA6, pTaExpA6-a, and pTaExpA6-b) showed different expression patterns during grain development.
The hexaploid nature of wheat (genes located in three homeologous loci) increases the number of transcripts with little difference between them, raising the overall likelihood of cross-hybridization on cDNA microarrays (Drea et al., 2005). In addition, although gene expression analysis by microarrays offers a broad insight into gene expression, RT-PCR allows a more accurate determination of expression patterns for small groups of genes (Drea et al., 2005). Therefore, an alternative strategy was used (a top-down approach) identifying target genes associated with important traits involved in grain growth. In this way, expansins were identified as one of the key factors in the determination of cell wall extensibility and cell length in plants (McQueen-Mason et al., 1992). Integrating the ecophysiological level of analysis with knowledge at a molecular level, we focused our study on the expression of expansins during the first half of the grain-filling period. Using the grain pericarp as the source of RNA, five unique expansin sequences and three consensus sequences were isolated whose expression profiles were analysed between 24–325 °Cd after anthesis in season 1 (54% of the grain-filling and 100% of the grain-length periods). Five of these sequences were further analysed in season 2 but in a more extended grain development period (41–470 °Cd after antesis) corresponding to 67% of the grain-filling and 100% of the grain-length periods. Differences in the expression levels throughout the development stages were more evident in season 2, because samples were evaluated over longer intervals of time in this experiment. The six development stages analysed in season 1 matched almost completely with the three first stages in season 2, when less variation of transcript accumulation was detected. These moments corresponded to the linear increase phase of grain length. At 360 °Cd and 470 °Cd in season 2, grain length was already stabilized.
As shown in Fig. 4, from 360 °Cd onwards the expression of the five expansins analysed decreased sharply. Conversely, the highest expression was found before 247 °Cd, when a high rate of increase in grain length and water accumulation occurs (Fig. 1). pTaExpA6 showed a peak of expression at 141 °Cd after anthesis when the higher grain length rate is reached (Figs 1, 4). A previous study reported eight wheat α-expansin genes expressed in developing grains (2–12 d after pollination, DAP) (Lin et al., 2005). Five sequences with high identity to those expansin genes were also evaluated in the present study. Expression patterns were different between studies. pTaExpA1 and pTaExpA4 showed significant but almost unchanged expression up to 325 °Cd after anthesis (Fig. 3A) in the present study, while Lin et al. (2005) reported a peak at 6 DAP and high expression at 2 DAP and 12 DAP for TaExpA1 and TaExpA4, respectively. TaExpA2 and pTaExpA8 showed expression peaks at early developmental stages (5–12 DAA and 5 DAA, respectively, Fig. 3) in our study, while Lin et al. (2005) showed a peak of expression at 8 DAP in the first case and a peak of expression at 12 DAP in the second. Interestingly, in spite of the different genotypes and growth conditions (greenhouse in Lin et al., 2005 and field conditions in our study) the common transcripts evaluated in these studies showed expression at the early developmental stages when the grain is actively growing, but no previous information is available about expression levels of these expansins when the maximum grain length has already been reached. Liu et al. (2007) reported high expression of α-expansins in the rapidly elongating tissues of anther filaments (TaExpA1 and TaExpA2) and stem internodes (TaExpA3) in wheat, but no grain tissues were evaluated in their study. Overall, these results support an association between the expression levels of pTaExpA1, pTaExpA4, TaExpA2, and pTaExpA8 and fast growth of the wheat grain taking place at the early developmental stages.
Lin et al. (2005) analysed the expression of TaExpA6 showing peaks of expression at 2 DAP and 12 DAP. In our study, pTaExpA6 pTaExpA6-a, and pTaExpA6-b showed divergent expression profiles in seasons 1 and 2, although the peaks of expression were found before the stabilized grain length was reached in all cases. These results might support the view that they correspond to different groups of expansins with specific roles in growing grains. Although cross-hybridization, especially between homologous sequences to TaExpA6 may be not discounted, in the present study the particular temporal expression during wheat grain development of the eight transcripts tested support the suggestion of specific roles for each family member during grain growth. Similar results were also observed in tomato (Brummell et al., 1999) and strawberry fruits (Harrison et al., 2001) where five and six expansin genes, respectively, each exhibit a unique expression pattern during fruit development. This behaviour is characteristic of the multigene families where different members may play a unique developmental or tissue-specific role which could be necessary for plant growth and development (Sampedro and Cosgrove, 2005).
Increased expression of particular expansin genes could reflect specific interactions between the protein and cell wall composition, as the substrate changes throughout development of most of the tissues. For example, a different composition of the endosperm cell walls was found in wheat (Philippe et al., 2006) and barley (Coles, 1979) at different development stages. In these cereals β-(1→3)(1→4) glucans, were most abundant at the early development stages (90 °Cd in wheat, 17 DAA in barley). As such glucans are thought to be key polysaccharides in cell wall extension in grasses (Carpita and Gibeaut, 1993) and the high expression of some expansins playing a specific role in the disruption of hydrogen bonds may be expected at this time.
Grain position in the spikelet did not affect the expression of expansins (Fig. 4). This result is consistent with the small difference in final grain weight in these grains (grain 3 was 10% lighter than grain 2). Expansin transcripts were also detected slightly later in G3, probably associated with the delay in floret fertilization of distal grains (G3) relative to basal grains (G2).
In situ hybridization was performed to complement the RT-PCR expression analysis and to provide information on the localization of expansin transcripts in maternal or endosperm tissues that follow distinct but co-ordinated developmental programmes in wheat grains (Drea et al., 2005). TaExpA6 was localized in the pericarp at 93 °Cd (5 DAA). However, a higher abundance of TaExpA6 transcripts was detected in the pericarp and endosperm at 187 °Cd (10 DAA) close to the peak expression detected by RT-PCR. These results show that this expansin does not have a specific expression in grain tissues at the beginning of grain growth, when both pericarp and endosperm are actively growing. The high sequence similarity between the three pTaExpA6 genes means that the signal in these images is likely to be the sum of the transcript levels of all three sequences. Similar results have been found in rice (Cho and Kende, 1998) were expansin transcripts by in situ hybridization were predominantly localized in the expansion and differentiation tissues of plants. As mentioned for RT-PCR analysis, the temporal/tissue-specific expression could be associated with different components of the cell walls during different developmental stages in the endosperm and pericarp of wheat grains (Coles, 1979; Philippe et al., 2006)
In this study, stabilized water content and grain length have been identified as the main components that are probably involved in grain weight determination, based on their association with final grain weight and early determination. Furthermore, a close association was found between grain growth dynamics and the expression of five putative expansin genes. The spatiotemporal expression of the expansins cloned from wheat grains in the present study represents a solid basis for further genetic studies to establish a link between expansin expression, grain size, and grain yield in wheat.
Acknowledgments
We especially thank Jorge Perez, Ahmed Hasan, Magda Lobnik, and the personnel of the experimental station of the Universidad Austral de Chile for technical assistance. This study was funded by the Chilean Technical and Scientific Research Council (CONICYT), Project FONDECYT 1040125 and the International Joint Project with Chile 2006/R2, and by Royal Society, UK (http://www.royalsoc.ac.uk/) competitive grants. XC Lizana held a postgraduate scholarship from CONICYT.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asana RD, Williams RF. The effect of temperature stress on grain development in wheat. Australian Journal of Agricultural Research. 1965;16:1–13. [Google Scholar]
- Berger F, Grini PE, Schnittger A. Endosperm: an integrator of seed growth and development. Current Opinion in Plant Biology. 2006;9:664–670. doi: 10.1016/j.pbi.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Borrás L, Slafer GA, Otegui ME. Seed dry weight response to source–sink manipulation in wheat, maize and soybean: a quantitative reappraisal. Field Crops Research. 2004;86:131–146. [Google Scholar]
- Brocklehurst PA. Factors controlling grain weight in wheat. Nature. 1977;266:348–349. [Google Scholar]
- Brummell DA, Harpster MH, Dunsmuir P. Differential expression of expansin gene family members during growth and ripening of tomato fruit. Plant Molecular Biology. 1999;39:161–169. doi: 10.1023/a:1006130018931. [DOI] [PubMed] [Google Scholar]
- Calderini DF, Abeledo LG, Savin R, Slafer GA. Effect of temperature and carpel size during pre-anthesis on potential grain weight in wheat. Journal of Agricultural Science (Cambridge) 1999a;132:453–459. [Google Scholar]
- Calderini DF, Abeledo LG, Savin R, Slafer GA. Final grain weight in wheat as affected by short periods of high temperature during pre- and post-anthesis under field conditions. Australian Journal of Plant Physiology. 1999b;26:453–458. [Google Scholar]
- Calderini DF, Abeledo LG, Slafer GA. Physiological maturity in wheat based on kernel water and dry mater. Agronomy Journal. 2000;92:895–901. [Google Scholar]
- Calderini DF, Miralles DJ, Sadras VO. Appearance and growth of individual leaves as affected by semidwarfism in isogenic lines of wheat. Annals of Botany. 1996;77:583–589. [Google Scholar]
- Calderini DF, Ortiz-Monasterio I. Grain position affects grain macronutrient and micronutrient concentrations in wheat. Crop Science. 2003;43:141–151. [Google Scholar]
- Calderini DF, Reynolds MP. Changes in grain weight as a consequence of de-graining treatments at pre- and post-anthesis in synthetic hexaploid lines of wheat. Australian Journal of Plant Physiology. 2000;27:183–191. [Google Scholar]
- Calderini DF, Riegel R, McQueen-Mason SJ. Frontis Workshop: Gene, Plant, Crop Relation. 2006. Expansin expression in growing grains. An alternative approach for studying grain weight determination in wheat. Wageningen University, Netherlands. pp. 54. [Google Scholar]
- Calderini DF, Savin R, Abeledo LG, Reynolds MP, Slafer GA. The importance of the period immediately preceding anthesis for grain weight determination in wheat. Euphytica. 2001;119:199–204. [Google Scholar]
- Cantagallo JE, Medan D, Hall AJ. Grain number in sunflower as affected by shading during floret growth, anthesis and grain setting. Field Crops Research. 2004;85:191–202. [Google Scholar]
- Carpita NC, Gibeaut DM. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal. 1993;3:1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Chanda SV, Singh YD. Cell enlargement as an important factor in controlling grain weight in wheat. Journal of Agronomy and Crop Science. 1998;181:223–228. [Google Scholar]
- Cho H, Kende H. Tissue localization of expansins in deepwater rice. The Plant Journal. 1998;15:805–812. doi: 10.1046/j.1365-313x.1998.00258.x. [DOI] [PubMed] [Google Scholar]
- Coen ES, Romero JM, Doyle S, Elliot R, Murphy G, Carpenter R. Floricaula: a homeotic gene required for flower development in Antirrhinum majus. Cell. 1990;63:1311–1322. doi: 10.1016/0092-8674(90)90426-f. [DOI] [PubMed] [Google Scholar]
- Coles G. Relationship of mixed-link beta-glucan accumulation to accumulation of free sugars and other glucans in the developing barley endosperm. Carlsberg Research Communications. 1979;44:439–453. [Google Scholar]
- Cosgrove DJ. Relaxation in a high-stress environment: the molecular bases of extensible cell walls and cell enlargement. The Plant Cell. 1997;9:1031–1041. doi: 10.1105/tpc.9.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000;407:321–326. doi: 10.1038/35030000. [DOI] [PubMed] [Google Scholar]
- Coventry SJ, Barr AR, Eglinton JK, McDonald GK. The determinants and genome locations influencing grain weight and size in barley (Hordeum vulgare L.) Australian Journal of Agricultural Research. 2003;54:1103–1115. [Google Scholar]
- Drea S, Leader DJ, Arnold BC, Shaw P, Dolan L, Doonana JH. Systematic spatial analysis of gene expression during wheat caryopsis development. The Plant Cell. 2005;17:2172–2185. doi: 10.1105/tpc.105.034058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunstone RL, Evans LT. Role of changes in cell size in the evolution of wheat. Austalian Journal of Plant Physiology. 1974;1:157–165. [Google Scholar]
- Fobert PR, Coen ES, Murphy G, Doonan JH. Patterns of cell division revealed by transcriptional regulations of genes during the cell cycle in plants. EMBO Journal. 1994;13:616–624. doi: 10.1002/j.1460-2075.1994.tb06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambín B, Borrás L, Otegui M. Kernel water relations and duration of grain-filling in maize temperate hybrids. Field Crops Research. 2007a;101:1–9. [Google Scholar]
- Gambín B, Borrás L, Otegui M. Plasticity of sorghum kernel weight to increase assimilate availability. Field Crops Research. 2007b;100:272–284. [Google Scholar]
- Granville WA. Calculo diferencial e integral. 1952 México DF. Editorial Unión Tipográfica Editorial Hispano-Americana. [Google Scholar]
- Hay RKM, Kirby EJM. Convergence and synchrony: a review of the co-ordination of development in wheat. Australian Journal of Agricultural Research. 1991;42:661–700. [Google Scholar]
- Jackson DP. In situ hybridization in plants. In: Bowles DJ, Gurr SJ, McPhereson M, editors. Molecular plant pathology: a practical approach. Oxford, UK: Oxford University Press; 1992. pp. 163–174. [Google Scholar]
- Jandel . Table curve v. 3.0. User's manual for version 3.0 AISN software. Corte Madera, CA: Jandel Scientific; 1991. [Google Scholar]
- Jones L, McQueen-Mason SJ. A role for expansins in dehydratation and rehydration of the resurrection plant Craterostigma plantagineum. FEBS Letters. 2004;559:61–65. doi: 10.1016/S0014-5793(04)00023-7. [DOI] [PubMed] [Google Scholar]
- Kong L, Ohm HW, Anderson JM. Expression analysis of defense-related genes in wheat in response to infection by Fusarium graminearum. Genome. 2007;50:1038–1048. doi: 10.1139/g07-085. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Niklas KJ. The epidermal-growth-control theory of stem elongation: an old and a new perspective. Journal of Plant Physiology. 2007;164:1395–1409. doi: 10.1016/j.jplph.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Laudencia-Chingcuanco DL, Stamova BS, You FM, Lazo GR, Beckles DM, Anderson OD. Transcriptional profiling of wheat caryopsis development using cDNA microarrays. Plant Molecular Biology. 2007;63:651–668. doi: 10.1007/s11103-006-9114-y. [DOI] [PubMed] [Google Scholar]
- Li Y, McQueen-Mason SJ. Expansins and cell growth. Current Opinion in Plant Biology. 2003;6:603–610. doi: 10.1016/j.pbi.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Lin Z, Ni Z, Zhang Y, Yao Y, Wu H, Sun Q. Isolation and characterization of 18 genes encoding α- and β-expansins in wheat (Triticum aestivum L.) Molecular Genetics and Genomics. 2005;274:548–556. doi: 10.1007/s00438-005-0029-0. [DOI] [PubMed] [Google Scholar]
- Lindstöm LI, Pellegrini CN, Aguirrezabal LAN, Hernandez LF. Growth and development of sunflower fruits under shade during pre- and early post-anthesis period. Field Crops Research. 2006;96:151–159. [Google Scholar]
- Liu Y, Liu D, Zhang H, Gao H, Guo X, Wang D, Zhang X, Zhang A. The α- and β-expansin and xyloglucan endotransglucosylase/hydrolase gene families of wheat: molecular cloning, gene expression, and EST data mining. Genomics. 2007;90:516–529. doi: 10.1016/j.ygeno.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Lopes MA, Larkins BA. Endosperm origin, development and function. The Plant Cell. 1993;5:1383–1399. doi: 10.1105/tpc.5.10.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DR, Mares AJH, Moss B, Allison FW. Effects of grain shape and size on milling yields in wheat. II. Experimental studies. Australian Journal of Agricultural Research. 1986;37:331–342. [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. Disruption of hydrogen bonding between plant cell wall polymers by proteins that induce wall extension. Proceedings of the National Academy of Sciences, USA. 1994;91:6574–6578. doi: 10.1073/pnas.91.14.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Cosgrove DJ. Expansin mode of action on cell wall (analysis of wall hydrolysis, stress relaxation and binding) Plant Physiology. 1995;107:87–100. doi: 10.1104/pp.107.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. The Plant Cell. 1992;4:1425–1433. doi: 10.1105/tpc.4.11.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet E. Relationships between grain weight and the size of floret cavity in the wheat spike. Annals of Botany. 1986;58:417–423. [Google Scholar]
- Millet E, Pinthus MJ. The association between grain volume and grain weight in wheat. Journal of Cereal Science. 1984;2:31–35. [Google Scholar]
- Miralles DJ, Calderini DF, Pomar K, D'Ambrogio A. Dwarfing genes and cell dimensions in different organs of wheat. Journal of Experimental Botany. 1998;49:1119–1127. [Google Scholar]
- Pepler S, Gooding MJ, Ellis RH. Modeling simultaneously water content and dry matter accumulation of wheat grains. Field Crops Research. 2006;95:49–63. [Google Scholar]
- Philippe S, Robert P, Barron CC, Saulnier L, Guillon F. Deposition of cell wall polysaccharides in wheat endosperm during grain development: Fourier Transform-Infrared Microspectroscopy study. Journal of Agricultural and Food Chemistry. 2006;54:2303–2308. doi: 10.1021/jf052922x. [DOI] [PubMed] [Google Scholar]
- Pikunov N. In: Cálculo diferencial e integral. Montaner y Simón SA, editor. Barcelona: España; 1978. [Google Scholar]
- Qi LL, Echalier B, Chao S, et al. A chromosome bin map of 16,000 expressed sequence tag loci and distri-bution of genes among the three genomes of polyploidy wheat. Genetics. 2004;168:701–712. doi: 10.1534/genetics.104.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RA, Lukacs Z. Seedling vigour in wheat: sources of variation for genetic and agronomic improvement. Australian Journal of Agricultural Research. 2002;53:41–50. [Google Scholar]
- Rogers SO, Quatrano RS. A quantitative analyis of some ultrastructural aspects of seed development of wheat cariopsis. American Journal of Botany. 1983;70:308–311. [Google Scholar]
- Rondanini DP, Savin R, Hall AJ. Estimation of physiological maturity in sunflower as a function of fruit water concentration. European Journal of Agronomy. 2007;26:295–1309. [Google Scholar]
- Saini HS, Westgate ME. Reproductive development in grain crops during drought. Advances in Agronomy. 2000;68:59–196. [Google Scholar]
- Sala RG, Westgate ME, Andrade FH. Source/sink ratio and the relationship between maximum water content, maximum volume, and final dry weight of maize kernels. Field Crops Research. 2007;101:19–25. [Google Scholar]
- Sampedro J, Cosgrove DJ. The expansin superfamily. Genome Biology. 2005;6:242. doi: 10.1186/gb-2005-6-12-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnyder H, Baum U. Growth of the grain of wheat (Triticum aestivum L.). The relationship between water content and dry matter accumulation, European Journal of Agronomy. 1992;1:51–57. [Google Scholar]
- Scott RW, Appleyard M, Fellowes G, Kirby EJM. Effect of genotype and position in the ears on carpel and grain growth and mature grain weight of spring barley. Journal of Agricultural Science (Cambridge) 1983;100:383–391. [Google Scholar]
- Slafer GA, Satorre EH, Andrade FH. Increases in grain yield in bread wheat from breeding and associated physiological changes. In: Slafer GA, editor. Genetic improvement of field crops. New York: Marcel Dekker; 1994. pp. 1–68. [Google Scholar]
- Sofield I, Evans LT, Cook MG, Wardlaw IF. Factors influencing the rate and duration of grain-filling in wheat. Australian Journal of Plant Physiology. 1977;4:785–1797. [Google Scholar]
- Tashiro T, Wardlaw IF. The effect of high temperature at different stages of ripening on grain set, grain weight and grain dimensions in the semi-dwarf wheat ‘Banks’. Annals of Botany. 1990;65:51–61. [Google Scholar]
- Ugarte C, Calderini DF, Slafer GA. Grain weight and grain number responsiveness to pre anthesis temperature in wheat, barley and triticale. Field Crops Research. 2007;100:240–1248. [Google Scholar]
- Wan Y, Poole RL, Huttly AK, et al. Transcriptome analysis of grain development in hexaploid wheat. Genomics. 2008;9:121–137. doi: 10.1186/1471-2164-9-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warringa J, De Viseer R, Kreuzer A. Seed weight in Lolium perenne as affected by interactions among seeds within the inflorescence. Annals of Botany. 1998;82:835–1841. [Google Scholar]
- Wiegand CL, Cuellar JA. Duration of grain-filling and kernel weight of wheat as affected by temperature. Crop Science. 1981;21:95–101. [Google Scholar]
- Yang Z, van Oosterom EJ, Jordan DR, Hammer GL. Pre-anthesis ovary development determines genotypic differences in potential kernel weight in sorghum. Journal of Experimental Botany. 2009;60:1399–1408. doi: 10.1093/jxb/erp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Research. 1974;14:415–1421. [Google Scholar]