Abstract
Plant cell walls and their polymers are regulated during plant development, but the specific roles of their molecular components are still unclear, as well as the functional meaning of wall changes in different cell types and processes. In this work the in situ analysis of the distribution of different cell wall components was performed during two developmental programmes, gametophytic pollen development, which is a differentiation process, and stress-induced pollen embryogenesis, which involves proliferation followed by differentiation processes. The changes in cell wall polymers were compared with a system of plant cell proliferation and differentiation, the root apical meristem. The analysis was also carried out during the first stages of zygotic embryogenesis. Specific antibodies recognizing the major cell wall polymers, xyloglucan (XG) and the rhamnogalacturonan II (RGII) pectin domain, and antibodies against high- and low-methyl-esterified pectins were used for both dot-blot and immunolocalization with light and electron microscopy. The results showed differences in the distribution pattern of these molecular complexes, as well as in the proportion of esterified and non-esterified pectins in the two pollen developmental pathways. Highly esterified pectins were characteristics of proliferation, whereas high levels of the non-esterified pectins, XG and RGII were abundant in walls of differentiating cells. Distribution patterns similar to those of pollen embryos were found in zygotic embryos. The wall changes reported are characteristic of proliferation and differentiation events as markers of these processes that take place during pollen development and embryogenesis.
Keywords: Capsicum annuum L., cell wall, pectins, pepper, pollen embryogenesis, xyloglucan
Introduction
The plant cell wall is an intricate structure which is involved in the determination of cell size and shape, growth and development, intercellular communication, and interaction with the environment. The primary cell wall is largely composed of polysaccharides (cellulose, hemicellulose, and pectins), enzymes, and structural proteins, which change their composition during cell growth and the various differentiation stages. Furthermore, several cell wall polymers are regulated during this development (Knox, 2008). Despite the extensive knowledge of the chemistry of isolated cell wall polymers and the increasing identification of gene families involved in their synthesis and modification (Knox, 2008), little is still known about the presence of polymers in the cell walls and their changes in specific cell types during plant growth and development.
The structural stiffness and resistance of the cell wall depends on the integrity of the grid formed by the interlinked cellulose and glucans. Xyloglucan (XG), a hemicellulose, constitutes up to about 30% by weight of primary cell walls. The modification of XG catalysed by enzymes is a key process that leads to expansion during cellular growth (Talbott and Ray, 1992).
Pectins are the major matrix components of dicotyledonous cell walls. The term pectin refers to a complex and heterogeneous family of polysaccharides in which galacturonic acid (GalA) and rhamnose (Rha) are the common components (Willats et al., 2001a). GalA is present in the two major structural features that form the backbone of three polysaccharide domains, which are thought to be found in all pectin species: homogalacturonan (HGA), rhamnogalacturonan-I (RG-I), and rhamnogalacturonan-II (RG-II) (O'Neil et al., 1990; Albertsheim et al., 1996; Mohnen, 1999). Pectins are polymerized and methyl-esterified in the Golgi, and secreted into the wall as highly methyl-esterified forms. Subsequently, they can be modified by pectin methylesterases, which catalyse the demethylesterification of homogalacturonans releasing acidic pectins and methanol.
The relationship between the esterified and the non-esterified pectins, and their distribution in the plant cell walls is the result of different processes (Goldberg et al., 1986; Dolan et al., 1997; Ermel et al., 2000; Hasegawa et al., 2000; Guillemin et al., 2005; Domozych et al., 2006). It has been proposed that they also contribute to cell cohesion and have an important role to play in the mechanical features and texture of plant cells (Ryden et al., 2003; Baluška et al., 2005). Pectins are involved in the formation of the pollen grain walls, especially the primexine (Hess and Frosch, 1994; Majewska-Sawka and Rodríguez-García, 2006) and the intine (Heslop-Harrison and Heslop-Harrison, 1980; Cresti et al., 1983; Bedinger, 1992; van Aelst and van Went, 1992; Geitmann et al., 1995; Li et al., 1995), with massive accumulation appearing in the aperture zone (Taylor and Hepler, 1997). In pollen, changes in the distribution of the pectins have been reported in previous works on young pro-embryos generated from microspores of Quercus suber L. (Ramírez et al., 2004) and Citrus clementina (Ramírez et al., 2003b).
After stress treatment, the in vitro-cultured pollen changes its normal gametophytic developmental pathway towards embryogenesis (Vicente et al., 1991), producing multicellular embryos from which, finally, haploid and double haploid plants develop (for a review see Chupeau et al., 1998). The search for molecular and cellular markers during the early stages of microspore embryogenesis constitutes an important goal in the identification of cells committed to the embryogenesis developmental programme as opposed to those cells which are non-responsive to the embryogenic pathway. They are also important tools for monitoring the metabolic processes involved in induction. Many of the molecular markers of somatic embryogenesis and organogenesis have been found in cell walls (Fortes et al., 2002). Changes in various cell activities and in the structural organization of subcellular compartments have been reported as accompanying the cell reprogramming process in some herbaceous and woody species (Testillano et al., 2000, 2002, 2005; Coronado et al., 2002; Bárány et al., 2005; Seguí-Simarro et al., 2005; Solís et al., 2008).
In Capsicum annuum, pollen embryogenesis has been analysed and changes in the cellular organization were reported through pollen-derived embryo development, the sequence of structural events being related to the activation of proliferation at the very early stages, and to differentiation occurring at the later stages (Bárány et al., 2005). After the inductive treatment, several rounds of divisions of the microspore produce multicellular pro-embryos, which are confined by the special pollen wall, the exine, at the early stages. Subsequent breakdown of the exine permits the growth of the multicellular pro-embryos formed by typical proliferating cells. Later on, differentiation of specific cell types occur at the embryo periphery, this process gradually progressing until giving rise to typical globular embryos which develop similar to zygotic embryos (Bárány et al., 2005).
In the present work, structural changes during the formation, growth, and maturation of cell walls were analysed using four antibodies for recognizing major cell wall epitopes: JIM5, JIM7 (Knox et al., 1990), anti-RGII (Matoh et al., 1998), and anti-XG (Lynch and Staehelin, 1992) antibodies specific for low- and high-esterified pectins, B-rhamnogalacturonan II complexes, and xyloglucans, respectively. They were used to identify the cell wall changes in two different developmental programmes of the pollen grain: gametophytic development, a differentiating cell process, and the pollen reprogramming to embryogenesis (Vicente et al., 1991), which involves firstly proliferation followed by differentiation. The analysis was also carried out during the first stages of zygotic embryogenesis and compared with pollen embryogenesis. Moreover, a comparison with the root apical meristem, a well-characterized system (De la Torre and González-Fernández, 1979; De la Torre et al., 1985) allowed us to associate defined cell wall changes with proliferation and differentiation events, and to extend the findings to different developmental processes.
Materials and methods
Plant material
Seeds of Capsicum annuum L., var. Yolo Wonder B (Ramiro Arnedo, SA, Calahorra, La Rioja, Spain) were germinated in soil and the plants grown in a growth chamber (MLR 350H, SANYO) at 25 °C, 80% humidity, and a photoperiod of 16 h of light. Flower buds of different sizes were selected, and anthers were excised and used for either microscopic analysis of normal gametophytic development or in vitro culture. Some ovaries were also excised from open flowers for microscopical study of zygotic embryos. Bulbs of Allium cepa L. were germinated in water under standard conditions, and, after 2 d, root segments corresponding to the meristematic and elongation zones were selected.
In vitro anther culture for microspore embryogenesis induction
Capsicum annuum anther culture was performed essentially as described in previous work (Bárány et al., 2005). Only buds from the first blossoms were used for the cultures. The pollen development stage was determined by DAPI staining on anther squashes. Anthers with blue-coloured tips from buds with similar petal and sepal lengths, corresponding to the late vacuolated microspore as the most responsive stage (González-Melendi et al., 1995) were selected for the cultures. The buds were surface-sterilized with 5% calcium hypochlorite. Fifteen anthers were plated on each 5 cm diameter Petri dish containing Cp induction medium (Dumas de Vaulx et al., 1981) supplemented with 0.01 mg l−1 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin. After 8 d at 35 °C in darkness, the cultures were transferred to 25 °C with a photoperiod of 12 h of light at 500 μmol photons m−2 s−1. After 12 d of induction in Cp medium, anthers were transferred to R1 medium (Dumas de Vaulx et al., 1981) supplemented with 0. 1 mg l−1 of kinetin.
Antibodies
The antibodies used in this study were the JIM7 and JIM5 rat monoclonal antibodies and the anti-XG, and anti-RGII rabbit polyclonal antibodies. Primary references for the generation of these antibodies and epitopes are shown in Table 1.
Table 1.
Antibodies used to map the distribution of cell wall epitopes
| Antibodies | Antigen/epitope | References |
| JIM5 | Partially methyl-esterified HG epitope: unesterified and partially esterified residues (up to 40%) | Knox et al., 1990 |
| JIM7 | Partially methyl-esterified HG epitope: methyl-esterified residues (up to 80%) | Knox et al., 1990 |
| Anti-RGII | Rhamnogalacturonan II pectin domain dimer cross-linked with boron | Matoh et al., 1998 |
| Anti-XG | Xyloglucan | Lynch and Staehelin, 1992 |
Immuno-dot-blot assay
Two different materials were used for the study. Capsicum annuum pollen grains were collected from flower bud anthers and Allium cepa roots were germinated from bulbs.
In order to obtain the pollen grains, the anthers were squashed with a plastic plunger in an Eppendorf tube containing TRIS-buffered saline (TBS, 0.14 M NaCl, 2.7 mM KCI, 24.8 mM TRIS base, pH 7.2). The anther debris was filtered with a 40 μm (pore size) nylon mesh, which retained the anther wall fraction, thereby allowing us to obtain the pollen fraction. The resulting suspension was centrifuged three times at 1000 rpm for 5 min to wash the pollen pellet.
The germinated roots 30–40 mm in length were obtained after 2 d at room temperature in water. For the immuno-dot assay, samples of the meristematic zone were obtained by dissecting 3–4 mm long segments from the root tip. Segments obtained by dissecting the root 5–8 mm from the tip were collected for the elongation zone.
The onion root segments and the pollen pellet, obtained as described previously, were homogenized with the plastic plunger on ice in a Eppendorf tube in 100 μl of buffer containing 50 mM TRIS-HCl pH 7.2, 50 mM trans-1,2-diaminocyclohexane-N,N,N′N′-tetraacetic acid (CDTA), and 25 mM dithiothreitol, and centrifuged at 7000 rpm for 10 min at 4 °C. The concentration of the resulting supernatants was determined and all samples were adjusted to a concentration of 1 mg ml−1. For immuno-dot-blot assays, 5 μl aliquots of adjusted supernatants were applied to nitrocellulose membrane (Millipore; Bedford, MA) and left to dry for 1 h. The membrane was incubated overnight at room temperature, with the JIM5, JIM7, anti-XG, and anti-RGII antibodies diluted 1:100 in the blocking buffer (2% powdered skimmed milk containing 0.05% Tween-20 in PBS), washed, and incubated for 1 h with alkaline phosphatase-conjugated anti-rat (for JIM5 or JIM7) and anti-rabbit (for anti-XG or anti-RGII) antibodies diluted 1:1000 in the blocking solution. Finally, the epitopes recognized by the antibodies were revealed by treatment with a nitroblue tetrazolium, bromo-chloroindolyl-phosphate (NBT-BCIP) mixture.
Immunofluorescence and confocal microscopy
The root tips of Allium cepa L. were carefully excised and fixed in 4% (w/v) formaldehyde in PBS, pH 7.3 overnight at 4 °C. Then the samples were washed three times for 10 min in PBS, 30 μm vibratome (Formely Lancer) sections were obtained and placed onto 3-aminopropyltrietoxysilane–coated slides and stored at –20 °C until they were used. Then they were washed in PBS and dehydrated through a methanol series (30%, 50%, 70%, and 100% methanol in water) for 5 min in each solution, and rehydrated with the inverse methanol series (100%, 70%, 50%, and 30% methanol in water) for another 5 min. After a wash in PBS for 5 min, the sections were incubated with 5% bovine serum albumin (BSA) in PBS for 5 min and then with JIM5 or JIM7 antibodies (applied undiluted) or anti-XG antibody (diluted 1/100 in 0.1% Tween-20) or anti-RGII antibody (diluted 1/50 in 1% of BSA) for 1 h at room temperature. After washing in PBS three times, 5 min for each wash, the signal was revealed with either ALEXA 488 (green fluorescence) or 546 (red fluorescence)-conjugated anti-rabbit or anti-rat antibodies (Molecular Probes, Eugene, Oregon, USA), diluted 1:25 in PBS for 45 min in the dark. After washing in PBS, the sections were counterstained with DAPI, mounted in Mowiol and observed first under a Zeiss Axioplan fluorescence microscope equipped with a CCD camera (CH 250/A, Photometrics). Confocal optical sections were collected using Leica TCS SP2 confocal microscope. Controls were performed by replacing the first antibody with PBS.
Sample cryo-processing and electron microscopy
The samples were processed at low temperature for further immunogold labelling. The onion roots and anthers at different developmental stages throughout gametogenesis, and at different time points during the anther culture, along with macroscopic embryos emerging from the anthers were fixed overnight at 4 °C in 4% formaldehyde in PBS, pH 7.3. Then they were washed in PBS and dehydrated through a methanol series (30%, 50%, 70%, and 100%), with a progressive lowering of temperature from 4 °C to −30 °C. The specimens were infiltrated with mixtures of methanol/Lowicryl K4M (in a series 2:1, 1:1, and 1:2) at −30 °C, embedded in pure resin at the same temperature, and polymerized under UV light at −30 °C in a Leica AFS device, as described previously (Testillano et al., 1995). Semi-thin (1 μm) and ultra-thin (100 nm) sections were obtained and used for immunogold labelling and light and electron microscopy observations.
Immunogold labelling and silver-enhancement for immunolocalization by light microscopy
Lowicryl semi-thin sections were incubated with 5% bovine serum albumin (BSA) in PBS for 5 min and then in JIM5 or JIM7 antibodies (applied undiluted) or in anti-XG antibodies (diluted 1≠100 in 0.1% Tween-20) or anti-RGII antibodies (diluted 1/50 in 1% BSA) for 1 h at room temperature. After washing with PBS, the sections were incubated with anti-rat (to detect JIM5 and JIM7 labelling) or anti-rabbit (for anti-XG and anti-RGII detection) secondary antibodies conjugated with 10 nm gold particles (BioCell, Cardiff, UK), diluted 1/25 in PBS for 45 min. Then the sections were washed in PBS and in distilled water for 5 min and revealed with a silver enhancing kit (British Biocell, Cardiff UK). Finally, the samples were mounted in Mowiol to be observed by a Leitz microscope under phase contrast (for structure observation) and bright field (for silver precipitate observation) and photographed by a digital camera Olympus DC10.
Immunogold labelling for immunolocalization at electron microscopy
Immunogold labelling was performed essentially as previously described by Coronado et al. (2002, 2007). Grids carrying ultra-thin sections from cryo-processed samples were floated on drops of distilled water, PBS, and 5% BSA in PBS. They were then incubated with JIM5 or JIM7 antibodies (applied undiluted) or anti-XG antibodies (diluted 1/100 in 0.1% Tween-20) or anti-RGII antibodies (diluted 1:50 in 1% BSA) for 1 h at room temperature. After washing with PBS, the sections were incubated with anti-rat (to detect JIM5 and JIM7 labelling) or anti-rabbit (for anti-XG and anti-RGII detection) secondary antibodies conjugated with 10 nm gold particles (BioCell, Cardiff, UK), diluted 1/25 in PBS for 45 min. Then the grids were washed in PBS, rinsed in distilled water, and air-dried. Finally, the grids were counterstained with 5% uranyl acetate and 1% lead citrate (Reynolds, 1963) and observed in a JEOL 1010 electron microscope at 80 kV. For ultrastructure analysis some sections, without immunogold, were stained and observed in the EM.
Quantitative analysis of gold labelling
The quantitative analysis of the immunogold labelling was performed essentially as described previously by Coronado et al. (2007). The density of gold labelling, expressed as the number of gold particles μm−2, was determined in the cell wall by counting the number of gold particles over the cell wall compartment. The number of gold particles was counted on randomly chosen electron micrographs obtained from three different labelling experiments for each sample, and three different samples were obtained for each plant system. The density of gold labelling was expressed as the average number of gold particles μm−2 ± standard error. The areas were estimated by a point-counting procedure, using a 15×15 mm square grid. The minimum sample size (MSS) was determined by the progressive mean technique. All the quantitative analyses were carried out using a BASIC programme developed by Dr J Renau-Piqueras (La Fe Hospital Research Centre, Valencia, Spain) and the results represented in histograms. The results were statistically tested with a Student's t test where α=0.05.
Results
Immuno-dot-blot assays to analyse changes in the occurrence of wall components in proliferating and differentiating root tip cells and during pollen development
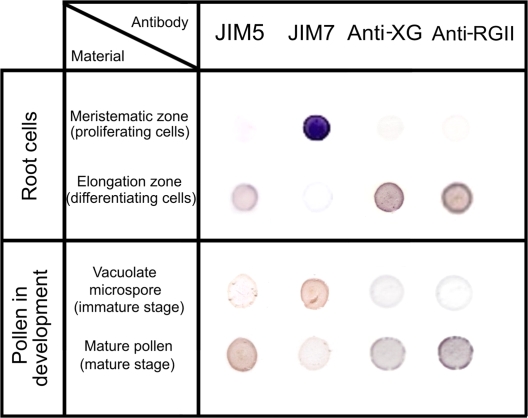
A well-known model of proliferation and differentiation, namely, the root apical meristems of Allium cepa L. were used as they are readily available; and their cell cycle has been characterized in detail (De la Torre and González-Fernández, 1979; De la Torre et al., 1985). An immuno-dot-blot assay was carried out with the antibodies JIM5, JIM7, anti-XG, and anti-RGII to determine the differences in the presence of these antigens in the two cell types of the root apex (Fig. 1), in the cells in active proliferation of the meristematic region, and in the differentiating cells in the elongating region. The dot-blot analysis was also performed on the pollen grains, at two stages during gametophytic development: the immature stage of the vacuolated microspore (which is a key phase for embryogenesis induction), and the late stage of the mature pollen grain (Fig. 1). In the root meristematic cells, a very weak signal, hardly detectable by this technique, was observed with the JIM5, anti-XG, and anti-RGII antibodies whereas a very strong signal was detected with the JIM7 antibody. On the other hand, an intense signal was observed on more differentiated root cells with the JIM5, anti-XG, and anti-RGII antibodies, and an almost non-detectable signal with the JIM7 antibody (Fig. 1).
Fig. 1.
Immuno-dot-blot of cell wall antigens in proliferating and differentiating cells of the root apical meristems of Allium cepa L. and in pollen at two developmental stages, vacuolated microspore and mature pollen of Capsicum annuum L. With the JIM5, anti-XG, and anti-RGII antibodies, dark positive signals appeared in the differentiated zone of the root, as well as in the mature pollen stage. With the antibody JIM7, an intense signal was detected in the proliferating (meristematic) root cells and in the vacuolated microspore, immature stage. (This figure is available in colour at JXB online.)
The results obtained in the two stages of pollen development showed that the immature vacuolated microspore displayed a similar pattern of dot-blot signals for the four antibodies as the one of the meristematic root cells, whereas the mature pollen showed a similar proportion of wall components to the one of the differentiating root cells of the elongation zone (Fig. 1). The controls carried out without primary antibodies showed no signals. These results suggested that the high presence of JIM5, anti-XG, and anti-RG II antigens were related to differentiation, whereas the high JIM7 antibody signal was associated with proliferation events.
In situ analysis of cell wall components in a model system of cells in proliferation and differentiation: the root apical meristem
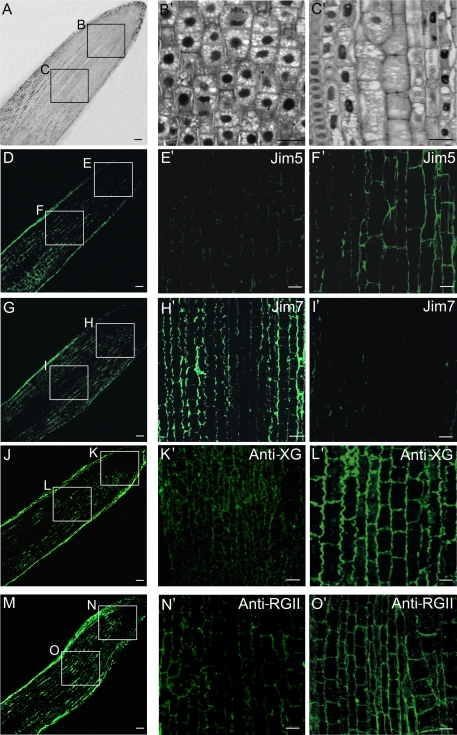
The meristematic and elongation regions were identified on Lowicryl semi-thin sections. Figure 2A shows the two areas of the root observed at the cellular level using an optical microscope. The proliferating cells (square B in Fig. 2A and B′) appeared as small isodiametric cells with thin walls, large nuclei, and small vacuoles. The differentiating cells (square C in Fig. 2A and C′) showed elongated and bigger shapes, thick cell walls, elongated and smaller nuclei, and bigger vacuoles.
Fig. 2.
Immunofluorescence of cell wall antigens on proliferating and differentiating cells of the root apical meristems of Allium cepa L. (A) General view of the root tip and location of the proliferating (square B) and differentiating (square C) regions. (B′, C′) Structural organization of proliferating (B′) and differentiating (C′) root cells, Lowicryl semi-thin sections, toluidine blue staining, observed under bright field light microscopy. (D–O′) Immunofluorescence on vibratome sections, images of confocal laser scanning microscopy (CLSM) to localize JIM5 (non-esterified pectins), JIM7 (esterified pectins), anti-XG (xyloglucan), and anti-RG II (rhamnogalacturonan II) antigens in proliferating (E′, H′, K′, N′) and differentiating (F′, I′, L′, O′) root tip cells. Bars: (A, D, G, J, M) 80 μm; (B′, C′, E′, F′, H′, I′, K′, L′, N′, O′) 10 μm.
Immunofluorescence assays with the antibodies JIM5, JIM7, anti-XG, and anti-RGII were carried out (Fig. 2D, G, J, M) and analysed by confocal microscopy, providing signals of different intensities in the cell walls; whereas the immunoreaction with other cellular components was negative. In the meristematic area, a weak fluorescence signal was observed in cell walls with JIM5 (Fig. 2E′), anti-XG (Fig. 2K′) and anti-RGII (Fig. 2N′) antibodies; whereas a stronger signal was observed in the same proliferating cells with the JIM7 antibody (Fig. 2H′). On the other hand, in the elongating cells a stronger immunofluorescence signal was observed with JIM5 (Fig. 2F′), anti-XG (Fig. 2L′), and anti-RGII (Fig. 2O′) antibodies in the walls; while a weak fluorescence signal was observed with the JIM7 antibody (Fig. 2I′). The controls carried out without the first antibody did not show any signal. These patterns of distribution clearly matched the results of the dot-blot assays reported above, with small differences in the intensity of the signals obtained with each antibody using both techniques, due to the inherent characteristics of each detection method which provided different sensitivities for antibody performance both on section and membrane.
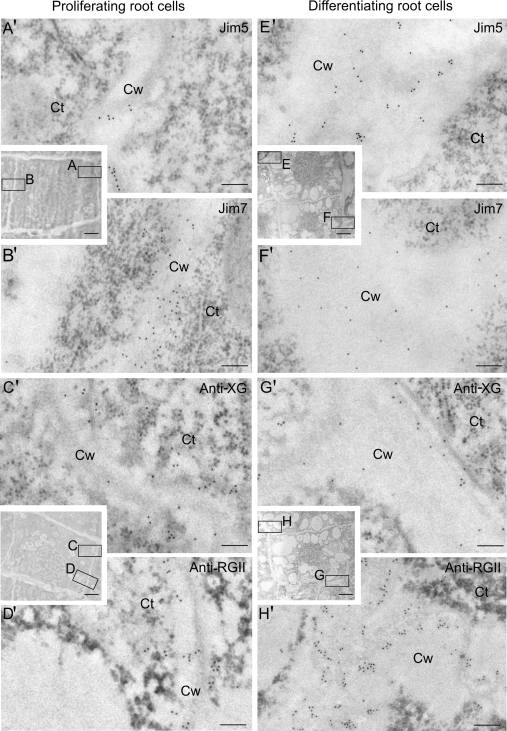
Immunogold labelling revealed that, in the newly-formed walls of the meristematic cells (Fig. 3A, B, C, D), the gold particles conjugated to the JIM5 antibody were very scarce and could be found in the darker central area of the cell wall, the middle lamella (Fig. 3A′); whereas JIM7 gold-labelling was more abundant and localized over a wider area of the cell wall (Fig. 3B′). Gold labelling with anti-XG and anti-RGII antibodies was scarce and mainly observed in the central area of the cell wall (Fig. 3C′, D′), similar to the pattern of labelling with JIM5. In the more developed walls of differentiating root cells (Fig. 3E, F, G, H), the gold particles localizing JIM5 were more abundant and located in the middle lamella of the cell wall, which was thicker than in the newly-formed walls (Fig. 3E′), whereas the particles with JIM7 were observed throughout the entire thickness of the cell wall (Fig. 3F′), although JIM7 labelling was lower in these walls. XG immunogold labelling was located in the darker areas of the wall (Fig. 3G′), while RGII labelling was located throughout the whole thickness of the cell wall and appeared more intense (Fig. 3H′).
Fig. 3.
Immunogold labelling of cell wall antigens on proliferating (A–D, A′–D′) and differentiating (E–H, E′–H′) cells of the root apical meristems of Allium cepa L. Electron micrographs of Lowicryl ultrathin sections. Inserts: low magnification micrographs showing the structural organization of the proliferating and differentiating cells of the root apex. Location and distribution at the ultrastructural level of JIM5, JIM7, anti-XG, and anti-RGII antigens in the newly-formed walls (squares A, B, C, D) of the proliferating area (A′, B′, C′, D′) and in the more developed walls (squares E, F, G, H) of the differentiating cells (E′, F′, G′, H′) of the elongation area. Ct, cytoplasm; Cw, cell wall. Bars: (A′, B′, C′, D′, E′, F′, G′, H′) 200 nm; inserts: 5 μm.
In situ analysis of cell wall components and quantification of the labelling pattern during the gametophytic development of pollen as a differentiation system
Due to the unspecific autofluorescence of the pollen external wall, the exine, immunolocalization at the optical microscopy level could not be carried out by fluorescence, so immunogold labelling followed by silver enhancement (IG+SE) was used instead.
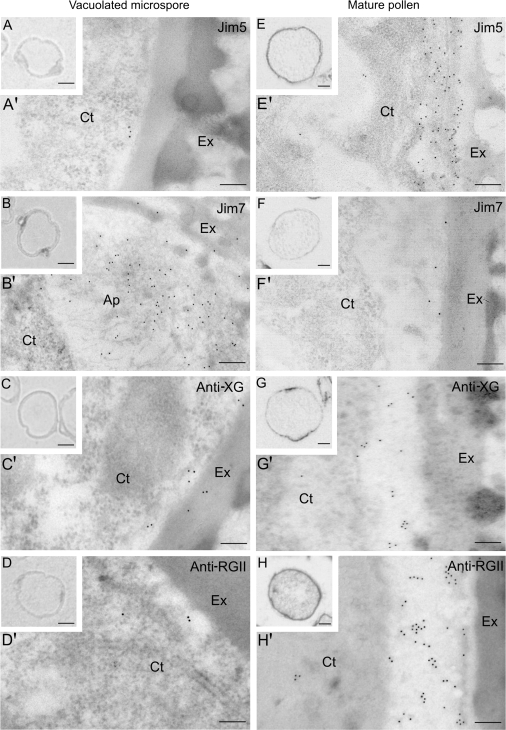
In the vacuolated microspore stage, the IG+SE technique provided a positive signal, a dark silver precipitate, associated with the JIM7 antibody, mainly on the aperture regions of the microspore wall (Fig. 4B), whereas no signal was detected with the antibodies JIM5 (Fig. 4A), anti-XG (Fig. 4C), and anti-RGII (Fig. 4D). At the mature pollen stage, positive signals with different intensities were observed in the inner pollen wall with the JIM5, anti-XG, and anti-RGII antibodies (Fig. 4E, G, H) and almost no signal was found with the JIM7 antibody (Fig. 4F). The controls carried out without primary antibodies showed no significant signal.
Fig. 4.
Immunolocalization by light (LM) and electron microscopy (TEM) of cell wall antigens on pollen of Capsicum annuum L. in two developmental stages: vacuolated microspore (A–D, A′–D′) and mature pollen (E–H, E′–H′). Location and distribution of the antigens of JIM5, JIM7, anti-XG, and anti-RGII antibodies, at the cellular (A–H) and ultrastructural (A′–H′) levels. (A–H) Semi-thin Lowicryl sections after an immunogold plus silver enhancement technique observed under bright field LM showing the immunoreaction as dark deposits. (A′–H′) Immunogold labelling on ultra-thin Lowicryl sections at TEM. Ct, cytoplasm; Ex, exine; V, vacuole. Bars: (A′–H′) 200 nm; inserts: 10 μm.
Immunogold labelling at the ultrastructural level showed in the vacuolated microspore a very low amount of gold particles with JIM5 (Fig. 4A′), anti-XG (Fig. 4C′), and anti-RGII (Fig. 4D′) antibodies, but in the mature bicellular stage the immunogold labelling densities provided by these three antibodies (Fig. 4E′, G′, H′), were higher in the inner wall, which appeared thicker at this mature pollen stage. With the JIM7 antibody, labelling was higher in the vacuolated microspore stage, gold particles appearing as a linear array of particles along the inner wall, and in the apertures (Fig. 4B′) where labelling was more intense. JIM7 labelling greatly decreased in the mature pollen stage (Fig. 4F′). Regarding the subcellular distribution of the signals, they were detected in all cases in the inner wall, the intine, which is located below the exine and was more developed and thicker in the mature pollen than in the vacuolated microspore.
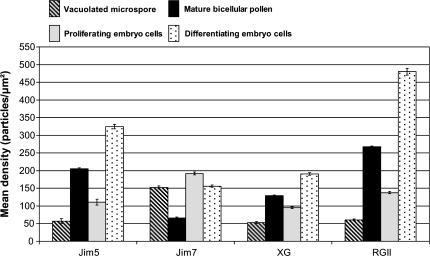
Quantitative analysis of the immunogold labelling density (Fig. 8) assessed the statistical relevance of the differences found using the immunoassays. In the vacuolated microspore stage, low densities of labelling were found with JIM5 (57.20 particles μm−2), anti-XG (53.23 particles μm−2), and anti-RGII (60.58 particles μm−2) antibodies; whereas higher and significantly different labelling densities were found with the JIM7 antibody (152.51 particles μm−2). By contrast, at the mature pollen stage, high labelling densities were quantified with JIM5 (205.13 particles μm−2), anti-XG (129.01 particles μm−2), and anti-RGII (268.07 particles μm−2) antibodies, while a low labelling density with significant differences was observed with JIM7 antibody (65.66 particles μm−2).
Fig. 8.
Quantification of immunogold labelling of cell wall antigens during pollen development and in pollen-derived embryos of Capsicum annuum L. The histogram shows the labelling density as the number of gold particles per area unit (square micrometre). Striped and black bars correspond to pollen development: striped bars correspond to vacuolated microspores, black bars to mature pollen. Grey and dotted bars correspond to pollen-derived embryos: grey bars correspond to proliferating cells of early pro-embryos, dotted bars to differentiating cells of mature embryos.
In situ analysis of cell wall components and quantification of the labelling pattern during pollen embryogenesis as a cell reprogramming system
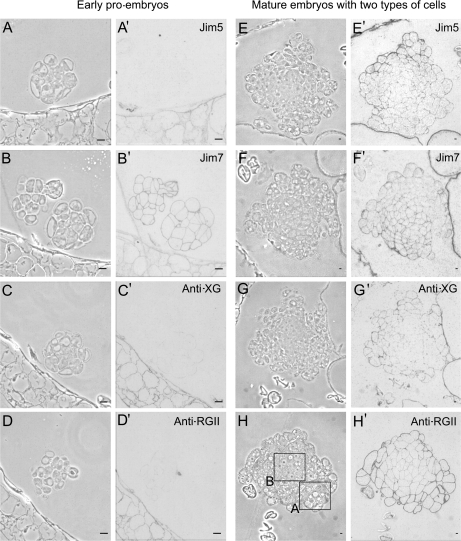
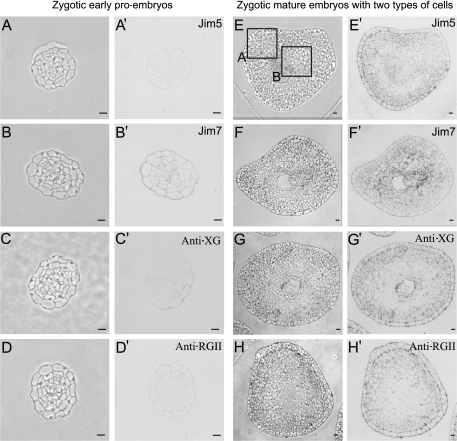
After the induction of pollen embryogenesis, the microspores started to divide producing multicellular structures or early pro-embryos, initially surrounded by the microspore wall, the exine, with disappeared later (Fig. 5A–D). The early pro-embryos displayed 10–15 polygonal cells with dense cytoplasms, large nuclei, and thin walls which showed positive immunolabelling with the JIM7 antibody (Fig. 5B′); whereas no signal was observed with the JIM5 (Fig. 5A′), anti-XG (Fig. 5C′), and anti-RGII antibodies (Fig. 5D′). The cell walls of the differentiated somatic cells of the anther exhibited positive signals with all antibodies (Fig. 5A′, B′, C′, D′). At late developmental stages, mature multicellular embryos were formed and exhibited two types of cells (Fig. 5E–H): an interior area of more dense cells with small vacuoles (Fig. 5H, square B), i.e. typical proliferating cells similar to the early pro-embryo cells, and an outer area containing large cells with big vacuoles (Fig. 5H, square A). A stronger signal was detected in the walls of the outer differentiating cells of the mature embryos with JIM5, anti-XG, and anti-RGII antibodies (Fig. 5E′, G′, H′); contrasting with a very weak to no signal in the walls of the inner proliferating cells (Fig. 5E′, G′, H′). Labelling with the antibody JIM7 was mainly detected in the walls of the interior area cells (Fig. 5F′). In the controls carried out without the primary antibodies, no signal was detected.
Fig. 5.
Immunolocalization of cell wall antigens on pollen-derived embryos of Capsicum annuum L. during two developmental stages: early pro-embryos (A–D, A′–D′) and more developed embryos with two cell types (E–H, E′–H′). Immunogold labelling and amplification with silver enhancement with antibodies JIM5, JIM7, anti-XG, and anti-RGII observed under phase contrast (A–H), to visualize the general structure, and bright field (A′–H′) to visualize the immunoreaction. The two cell types of the more developed embryos are indicated by the squares in (H); differentiating cells in square A, and proliferating cells in square B. Bars: (A, B, C, D) 10 μm; (E, F, G, H) 50 μm.
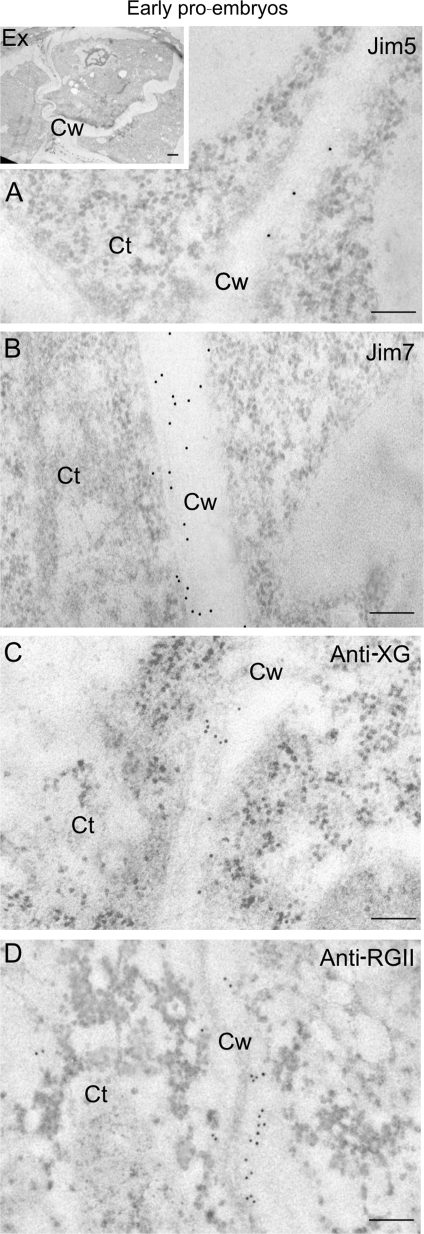
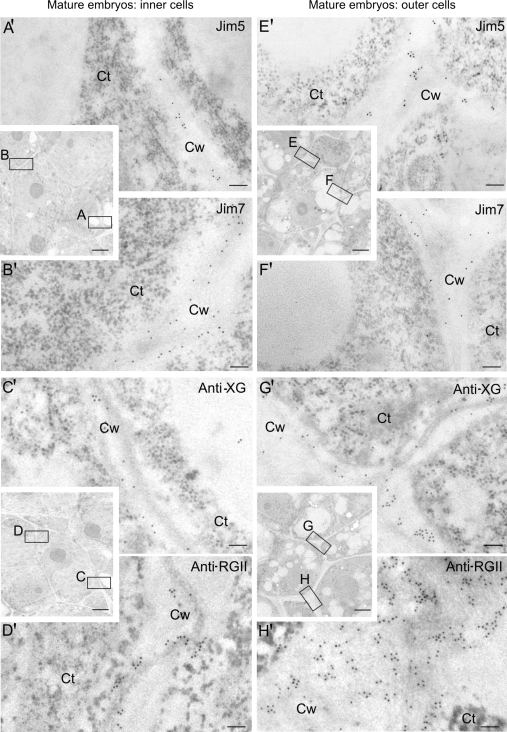
At the ultrastructural level, the pattern of immunogold labelling of the four antibodies was very similar in the newly-formed walls of early pro-embryos (Fig. 6A, B, C, D) and in the inner cells of the mature embryos (Fig. 7A, B, C, D), both cells with the typical organization of proliferating cells; they exhibited a low labelling density with the JIM5, anti-XG, and anti-RGII antibodies (Figs 6A, C, D, 7A′, C′, D′), while a stronger signal was detected with the same antibodies in the more developed walls of the outer cells of the mature embryos (Fig. 7E′, G′, H′), cells with the structural features of differentiating cells. The labelling density with the JIM7 antibody was higher in the newly formed walls of early pro-embryos and the inner cells of mature embryos (Figs 6B, 7B′) compared with that of the more developed walls of the outer area cells (Fig. 7F′). Gold particles were mainly observed in the middle lamella of the cell wall, which appeared as just a thin band in the central area of the wall in the newly formed cell walls (Figs 6, 7A′, B′, C′, D′); while being thicker in the differentiating cells of the mature embryo (Fig. 7E′, F′, G′, H′).
Fig. 6.
Immunogold labelling of cell wall antigens in early pollen-derived proembryos of Capsicum annuum L. Electron micrographs of Lowicryl ultra-thin sections. Insert: low magnification micrograph showing proliferating cells of an early proembryo still surrounded by the special pollen wall, the exine (Ex). (A, B, C, D) High magnification micrographs showing the immunogold labelling with JIM5 (A), JIM7 (B), anti-XG (C), and anti-RGII (D) in the cell walls (Cw). Ct, cytoplasm. Bars: (A, B, C, D) 200 nm; insert: 1 μm.
Fig. 7.
Immunogold labelling of cell wall antigens in the two cell types of late-mature pollen-derived embryos of Capsicum annuum L. Electron micrographs of Lowicryl ultrathin sections. Inserts: low magnification micrographs showing the structural organization of the cells of the inner and outer areas of the mature embryo. Location and distribution at the ultrastructural level of JIM5, JIM7, anti-XG, and anti-RGII antigens in the newly-formed walls (squares A, B, C, D) of the inner proliferating cells (A′, B′, C′, D′) and in the more developed walls (squares E, F, G, H) of the outer differentiating cells (E′, F′, G′, H′) of the mature embryo. Ct, cytoplasm; Cw, cell wall. Bars: (A′-H′) 200 nm; inserts: 1 μm.
The quantitative analysis of the immunogold labelling density showed significant differences in labelling densities of the four antigens between the proliferating cells of early pro-embryos and the differentiating cells of the mature embryos (Fig. 8). In the newly formed walls of the proliferating embryo cells high labelling density with JIM7 (192.03 particles μm−2) was observed; whereas the labelling densities detected with JIM5 (111.25 particles μm−2), anti-XG (94.93 particles μm−2), and anti-RGII (137.70 particles μm−2) were significantly lower. In the more developed walls of the differentiating cells of the mature embryos, higher labelling densities with JIM5 (324.84 particles μm−2), anti-XG (190.56 particles μm−2), and anti-RGII (479.93 particles μm−2) antibodies, and much lower density corresponding to the JIM7 antibody (156.02 particles μm−2) were found (Fig. 8).
The comparison among labelling densities of the differentiated wall of the mature pollen and the newly formed walls of the proliferating cells of pollen embryos (in Fig. 8 compare the second and third bars of each antigen) revealed opposite patterns of distribution of the four antigens in the two pollen developmental programmes: for JIM5, anti-XG, and anti-RGII, the labelling densities were high in mature pollen walls and lower in proliferating cell walls of embryos, whereas for JIM7, the labelling density was low in mature pollen and much higher in embryo proliferating cells. On the other hand, the pattern of distribution of the labelling densities of the four antigens was similar between the differentiated wall of the mature pollen and the differentiating cells of the embryo (in Fig. 8 compare the second and fourth bars of each antigen).
In situ analysis of cell wall components at early stages of the zygotic embryogenesis
Two developmental stages of zygotic embryos, similar to those analysed in the pollen embryogenesis, were selected and studied. The early zygotic pro-embryos and the mature zygotic embryos with two different areas were identified by the starch detected in the cells of the outer area (Bárány et al., 2005). The early zygotic pro-embryos could be identified by their polygonal cells (Fig. 9A) and the absence of starch (data not shown); whereas the mature multicellular embryos showed two areas (Fig. 9E): the inner area with smaller, polygonal cells (Fig. 9E, square B); and the outer area with large cells with big vacuoles (Fig. 9E, square A) containing a high amount of starch (data not shown).
Fig. 9.
Immunolocalization of cell wall antigens on zygotic embryos of Capsicum annuum L. in two developmental stages: early pro-embryos (A–D, A′–D′) and mature embryos with two cell types (E–H, E′–H′). Immunogold labelling and amplification with silver enhancement with antibodies JIM5, JIM7, anti-XG, and anti-RGII observed under phase contrast (A–H), to visualize the general structure, and bright field (A′–H′) to visualize the immunoreaction. The two cell types of the mature embryos are indicated by the squares in (E): differentiating cells in square A, and proliferating cells in square B. Bars: (A, B, C, D) 10 μm; (E, F, G, H) 50 μm.
The presence and relative proportion of immunolabelling of the four wall antigens found in the two types of zygotic embryo cells followed a pattern of distribution similar to that found in the two types of pollen embryo cells (compare Fig. 5 with Fig. 9). A positive JIM7 antibody signal was detected in the cell walls of early zygotic embryos (Fig. 9B′), whereas no signal was detected with JIM5 (Fig. 9A′), anti-XG (Fig. 9C′), and anti-RGII (Fig. 9D′) antibodies. In the mature zygotic embryos with the two cell types described, signals with JIM5 (Fig. 9E′), anti-XG (Fig. 9G′), and anti-RGII (Fig. 9H′) antibodies were detected in the walls of the outer area cells, whereas very low or no signals were observed in the inner area (Fig. 9E′, G′, H′). In comparison, the signal with the JIM7 antibody was mostly detected in the inner cells of the mature zygotic embryos (Fig. 9F′).
Discussion
The cell wall components change with proliferation and differentiation processes
Different reports have studied cell walls, establishing schematic models of the cell wall structure and organization of polysaccharide components, but, nevertheless, these general models cannot be applied to all primary cell walls or to the diverse plant cell developmental processes (Knox, 2008). For this reason, reports on the development-specific or cell-type-specific wall configurations of polymers are of high interest for understanding the functional meaning of cell wall changes. To accomplish this task, immunocytochemical techniques, by using the wide range of antibodies developed against cell wall epitopes, rank among the best methods for analysing the diversity of in situ cell wall macromolecular configurations that occur during plant development (Knox, 2008). In the present work, these in situ techniques have been applied to specific developmental processes in which proliferation and differentiation events take place. Results showed differences in the distribution pattern of major cell wall molecular complexes, as well as in the proportion of esterified and non-esterified pectins in the two pollen developmental pathways (gametophytic development and pollen embryogenesis), and in the two regions of the root apex (meristematic and elongation regions). Highly esterified pectins were characteristic of proliferation, whereas high levels of non-esterified pectins, XG, and RGII were abundant in the walls of differentiating cells.
There are different studies on antigen distribution detected by the antibodies JIM5 and JIM7 in several plant tissues and organs, mainly at the light microscopy level (Goldberg et al., 1986; Dolan et al., 1997; Guglielmino et al., 1997; Hasegawa et al., 2000). Evidence has been provided indicating that the de-esterification of pectins by methyl esterases is involved in specific developmental processes (Goldberg et al., 1986; Dolan et al., 1997; Hasegawa et al., 2000), and specifically in the ratio of esterified to de-esterified pectins, and their distribution in the cell wall (Goldberg et al., 1986; Guglielmino et al., 1997; Hasegawa et al., 2000; Willats et al., 2001a, b). Differences in the proportion of esterified pectins between microspores and microspore-derived embryos were reported in tree species, such as Quercus suber L., Citrus clementina, and Olea europaea L. (Ramírez et al., 2003a, b; Solís et al., 2008), as well as some preliminary results on different cell types of the root meristem of Allium cepa L. (Ramírez et al., 2001, 2003a). In very young pollen-derived embryos of herbaceous species (pepper and tobacco), a high level of esterified pectins was observed in the cell walls (Bárány et al., 2006). The results reported here in the four systems studied, using an in situ localization approach at both light and electron microscopy levels, allowed a quantitative analysis of the labelling signals, and revealed that, in walls of proliferating cells, the levels of esterified pectins were higher than in the differentiating cells, which showed cell walls rich in de-esterified pectins.
Other cell wall domains, such as the RGII and the XG have been studied less, perhaps due to the limited set of tests applicable to them. A few reports demonstrated the presence of xyloglucan in developmental processes in Arabidopsis thaliana (Otegui and Staehelin, 2000; Freshour et al., 2003). Xyloglucan has been detected using anti-XG antibodies during endosperm development (Otegui and Staehelin, 2000); and also in other systems such as tamarind and nasturtium seeds (Marcus et al., 2008). In this report, immunofluorescence experiments with the LM15 monoclonal antibody against a specific xyloglucan epitope were performed, and revealed XG to be present extensively in the walls of tobacco and pea stems after the enzymatic removal of pectic HG, whereas in non-digested sections some cells were not labelled, which suggested that XG epitopes might be masked by other cell wall components (Marcus et al., 2008). In the present work, with a different anti-XG antibody, XG was immunodetected in all cell types studied, but with different labelling intensity levels. Even though some masking of XG epitopes could not be completely excluded, since the same immunoassay procedure was used in all cell types, the results permitted a comparison to be made of the relative proportions of XG among cell types and was found to be higher in more differentiated cell walls than in the newly formed walls of proliferating cells. The RGII domain is a highly conserved structure of pectins that can be cross-linked by borate in cell walls (Willats et al., 2001a). The boron cross-linked RGII domains of pectins are critical, not only for cell wall integrity but also for plant cell growth (O'Neill et al., 2001). The RGII antibody has been reported to have labelled cell walls of maize root apices by using an immunofluorescence approach (Baluška et al., 2002). The results obtained in the present work revealed that both XG and RGII increased with cell differentiation in the plant developmental processes analysed.
The cell wall components are markers of the microspore reprogramming towards embryogenesis
The comparative analysis performed here of different cell wall polymers during in vivo gametophytic development and the early pro-embryos formed after stress-induced microspore embryogenesis has permitted the identification of differential features, specific to the reprogramming process towards embryogenesis. One of these defined features is a high level of esterified pectins in the early embryogenic stages. Immunocytochemical studies carried out in mature and germinated pollen of other species, showed a labelling density for de-esterified pectins that was much higher than that for esterified pectins, indicating a low degree of esterification of pectins in mature pollen grains (Stepka et al., 2000; Suárez-Cervera et al., 2002). Furthermore, in the present work, during the vacuolated microspore stage in C. annuum, a considerable amount of esterified pectins was detected in the aperture areas; regions where the synthesis of a new wall takes place (Taylor and Hepler, 1997). This evidence was consistent with the abundance of esterified pectins in the newly-formed walls of proliferating cells of both young pollen-derived embryos and root tip meristematic cells, thus providing experimental support indicating that a high proportion of esterified pectins in walls is not only a marker for proliferation events, but also an early marker of microspore reprogramming to embryogenesis.
By contrast, the differentiated cells of the systems studied displayed a high proportion of de-esterified pectins, as well as an increase in the presence of RGII and XG, due to being the peripheral layers of the late pollen-derived embryos and the mature pollen. A recent paper reported modifications in the cell walls of young microspore embryos, in olive (Solís et al., 2008). This change was based on a differential reactivity of Calcofluor White staining, a cytochemical method preferential for cellulosic components (Galbraith, 1981), thus providing evidence that the structure, arrangement and/or amount of cellulosic or hemicellulosic components of these cell walls were modified after embryogenic induction (Solís et al., 2008). Our present results which showed changes in the anti-XG labelling densities after pollen embryogenic induction also support the fact that modifications not only in the pectin domains but also in the cellulosic and hemicellulosic polymers accompanies the microspore reprogramming process.
Taking all these results into account, high levels of de-esterified pectins, XG, and RGII can be considered markers of the pollen gametophytic pathway, as a differentiation system, whereas the esterified pectins can be used as an early marker of the pollen embryogenesis pathway, identifying induced cells and young pollen-derived embryos. Moreover, the results suggest that the antibody JIM7 could be used as a convenient test to detect the reprogramming state of the microspore, and the antibodies JIM5, anti-XG, and anti-RGII to detect the differentiation process of pollen. Based on the results presented above, the approach used in this work constitutes a convenient tool to monitor the embryogenesis induction process of pollen and to differentiate the responsive microspores in the very early stages.
The reported cell wall changes occurring during pollen reprogramming to embryogenesis also take place during zygotic embryogenesis
There are only a few studies comparing the development of embryos from pollen with those from the zygote (Yeung et al., 1996; Testillano et al., 2002; Bárány et al., 2005). The results obtained in pepper indicated that, in the initial stage of microspore embryogenesis, the formation of multicellular pro-embryos did not follow a constant and defined division pattern, as happens in zygotic embryogenesis (Raghavan, 2000). However, in the later stages, such as the differentiation of the protodermis in the globular embryos and the ulterior development of the heart and torpedo embryos, the pattern is very similar to the sequence of events that take place during the development of a zygotic embryo (Bárány et al., 2005). A similar structural organization pattern has also been reported for pollen-derived embryos and zygotic embryos, in the globular stage in a tree species (Bueno et al., 2003).
Up to the time of this present study, there have been no reports on the comparative analysis of cell wall components during the two types of embryogenesis, derived from both the microspore and the zygote. The results showed the same pattern of distribution for the cell wall antigens at similar developmental stages in both embryogenic programmes. A high level of esterified pectins and very low or the absence of de-esterified pectins, RGII, and XG was observed in early zygotic embryos and pollen-derived embryos. At later stages, positive signals of de-esterified pectins, RGII, and XG were indeed detected in the peripheral differentiating cells of both zygotic and pollen-derived embryos, whereas cells of the inner area appeared rich in esterified pectins. These results indicated a common pattern of cell wall structural organization for the two developmental embryogenesis processes in their initial stages, an observation not previously documented, giving additional support to the idea that microspore-derived embryo development mimics zygotic embryogenesis.
Defined distribution patterns of cell wall components are characteristics of proliferating and differentiating cells in the four plant developmental systems analysed: root apical meristem, pollen maturation, pollen embryogenesis, and zygotic embryogenesis
Root tip development follows a similar pattern in most species and the walls of cells in proliferation from those in differentiation could be clearly distinguished. The identification of the distribution patterns of wall components for well-characterized proliferating and differentiating cells, patterns that were not previously defined, supported the distribution patterns of wall components in pollen development, pollen embryogenesis, and zygotic embryogenesis.
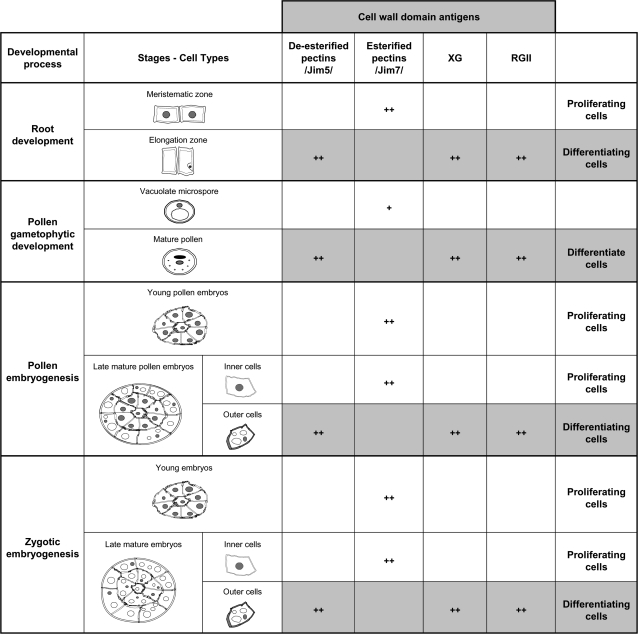
The results reported here showed variations in the presence and distribution of the XG and RGII molecular complexes, as well as changes in the esterification levels of pectins in defined cell types and at specific developmental stages. A summary of the results is presented in Fig. 10, which shows that, in all the systems studied, the distribution pattern of the four cell wall antigens analysed is the same for the proliferating cells (high levels of esterified pectins, low levels of XG and RGII), as well as for the differentiating cells (high levels of de-esterified pectins, XG, and RGII). The same modifications of pectins occurred in the four systems analysed, indicating a common feature of pectin de-esterification dynamics associated with cell differentiation during the plant developmental processes. Moreover, the increase in XG and RGII found in differentiating cells of all the plant systems analysed indicated that there is a link between the abundance of XG and RGII and the progressive development of the wall during differentiation.
Fig. 10.
Overview and summary of the occurrence of cell wall antigens during different developmental processes and cell types. (+) The presence of the antigen; (++) an abundance of antigens. Grey boxes indicate a common distribution pattern of cell wall antigens in all differentiating cell types of the various plant systems, whereas white boxes indicate a different distribution pattern which is common to the proliferating cells in all systems and developmental processes studied.
The comparison of the findings on the cell wall components in the two pollen developmental pathways, gametophytic and embryogenic, and in the zygotic embryogenesis with the root apical meristems, permitted the common distribution patterns for proliferation and differentiation to be identified. These patterns for the wall polymers can therefore be considered as markers of proliferation and differentiation processes that take place during the four plant developmental systems chosen for this study, namely: root apical meristem, pollen development, pollen embryogenesis, and zygotic embryogenesis.
Acknowledgments
This work was supported by projects granted by the Spanish Ministry of Science and Innovation, MICINN, BFU2008-00203 and AGL2008-04255. Thanks are due to Dr F Baluška (University of Bonn, Germany) and Professor A Staehlein (University of Colorado at Boulder, USA) for kindly providing us with the anti-RGII and anti-XG antibodies, respectively. We also wish to thank Professor Keith Roberts and the John Innes Centre (UK) for providing us with the JIM5 and JIM7 antibodies.
References
- Albertsheim P, Darvill AG, O'Neill MA, Schols HA, Voragen AGJ. An hypothesis; the same six polysaccharides are components of the primary cell walls of all higher plants. In: Viser J, Voragen AGJ, editors. Pectins and pectinases. Amsterdam: Elsevier Science BV; 1996. pp. 47–55. [Google Scholar]
- Baluška F, Hlavacka A, Šamaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, Volkmann D. F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from Brefeldin A-induced compartments. Plant Physiology. 2002;130:422–431. doi: 10.1104/pp.007526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluška F, Volkmann D, Menzel D. Plant synapses: actin-based adhesion domains for cell-to-cell communication. Trends in Plant Science. 2005;10:106–111. doi: 10.1016/j.tplants.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Bárány I, González-Melendi P, Coronado MJ, Ramírez C, Testillano PS, Risueño MC. Plant cell wall components rearrange during cell reprogramming to embryogenesis. In: Iijima S, editor. Electron microscopy 2006. Sapporo, Japan: Microscopy Society of Japan; 2006. 245. [Google Scholar]
- Bárány I, González-Melendi P, Fadón B, Mitykó J, Risueño M, Testillano PS. Microspore-derived embryogenesis in pepper (Capsicum annuum L.): subcellular rearrangements through development. Biology of the Cell. 2005;97:709–722. doi: 10.1042/BC20040142. [DOI] [PubMed] [Google Scholar]
- Bedinger PA. The remarkable biology of pollen. The Plant Cell. 1992;4:879–887. doi: 10.1105/tpc.4.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno MA, Gómez A, Sepúlveda F, Seguí JM, Testillano PS, Manzanera JA, Risueño MC. Microspore-derived embryos from Quercus suber anthers mimic zygotic embryos and mantain haploidy in long-term anther culture. Journal of Plant Physiology. 2003;160:953–960. doi: 10.1078/0176-1617-00800. [DOI] [PubMed] [Google Scholar]
- Chupeau Y, Caboche M, Henry Y. Androgenesis and haploid plants. Berlin, Heidelberg: Springer-Verlag; 1998. [Google Scholar]
- Cresti M, Ciampolini F, Sarfatti G. Ultrastructural features of Malus communis L. mature pollen. In: Mulcahy DL, Ottaviano E, editors. Pollen: biology and implications for plant breeding. New York: Elsevier; 1983. pp. 165–172. [Google Scholar]
- Coronado MJ, González-Melendi P, Seguí JM, Ramírez C, Bárány I, Testillano PS, Risueño MC. MAPKs entry into the nucleus at specific interchromatin domains in plant differentiation and proliferation processes. Journal of Structural Biology. 2002;140:200–213. doi: 10.1016/s1047-8477(02)00542-7. [DOI] [PubMed] [Google Scholar]
- Coronado MJ, Testillano PS, Wilson C, Vicente O, Heberle-Bors E, Risueño MC. The in situ molecular identification of the Ntf4-MAP kinase expression sites in maturing and germinating pollen. Biology of the Cell. 2007;99:209–221. doi: 10.1042/BC20060076. [DOI] [PubMed] [Google Scholar]
- De la Torre C, González-Fernández A. Cell cycle mapping by irradiating cells with bromosubstituted DNA segments. Photochemistry and Photobiology. 1979;29:977–981. [Google Scholar]
- De la Torre C, Sans J, Aller P, González-Fernández A. Replication time of the genome portions involved in a G2 transition points in meristems. European Journal of Cell Biology. 1985;37:216–129. [Google Scholar]
- Domozych DS, Serfis A, Kiemle SN, Gretz MR. The structure and biochemistry of charophycean cell walls. I. Pectins of Penium margaritaceum. Protoplasma. 2006;183:615–632. doi: 10.1007/s00709-006-0197-8. [DOI] [PubMed] [Google Scholar]
- Dolan L, Linstead PJ, Roberts K. Developmental regulation of pectic polysaccharides in the root meristem of Arabidopsis. Journal of Experimental Botany. 1997;48:713–720. [Google Scholar]
- Dumas de Vaulx R, Chambonnet D, Pochard E. Culture in vitro d'anthères de piment (Capsicum annuum L.): amèlioration des taux d'obtenction de plantes chez différents génotypes par des traitments à +35 °C. Agronomie. 1981;1:859–864. [Google Scholar]
- Ermel FF, Follet-Gueye ML, Cibert C, Vian B, Morvan C, Catesson AM, Goldberg R. Differential localization of arabinan and galactan side chains of rhamnogalacturonan 1 in cambial derivatives. Planta. 2000;210:732–740. doi: 10.1007/s004250050674. [DOI] [PubMed] [Google Scholar]
- Fortes AM, Testillano PS, Risueño MC, Pais MS. Studies on callose and cutin during the expression of competence and determination for organogenic nodule formation from internodes of Humulus lupulus var. Nugget. Physiologia Plantarum. 2002;116:113–120. doi: 10.1034/j.1399-3054.2002.1160114.x. [DOI] [PubMed] [Google Scholar]
- Freshour G, Bonin CP, Reiter WD, Albersheim P, Darvill AG, Hahn MG. Distribution of fucose-containing xyloglucans in cell walls of the mur1 mutant of Arabidopsis. Plant Physiology. 2003;131:1602–1612. doi: 10.1104/pp.102.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith DW. Microfluorimetric quantitation of cellulose biosynthesis by plant protoplasts using Calcofluor white. Physiologia Plantarum. 1981;53:111–116. [Google Scholar]
- Geitmann A, Li YQ, Cresti M. Ultrastructural inmunolocalization of periodic pectin deposition in the cell wall of Nicotiana tabacum pollen tubes. Protoplasma. 1995;187:172–181. [Google Scholar]
- Goldberg R, Morvan C, Roland JC. Composition, properties and localisation of pectins in young and mature cells of the mung bean hypocotyl. Plant and Cell Physiology. 1986;27:419–427. [Google Scholar]
- González-Melendi P, Testillano PS, Ahmadian P, Fadón B, Vicente O, Risueño MC. In situ characterization of the late vacuolate microspore as a convenient stage to induce embryogenesis in Capsicum. Protoplasma. 1995;187:60–71. [Google Scholar]
- Guglielmino N, Liberman M, Catesson AM, Mareck A, Prat R, Mutaftschiev S, Goldberg R. Pectin methylesterases from poplar cambium and inner bark: localization, properties and seasonal changes. Planta. 1997;202:70–75. doi: 10.1007/s004250050104. [DOI] [PubMed] [Google Scholar]
- Guillemin F, Guillon F, Bonnin E, Devaux MF, Chevalier T, Knox JP, Liners F, Thibault JF. Distribution of pectic epitopes in walls of the sugar beet root. Planta. 2005;222:355–371. doi: 10.1007/s00425-005-1535-3. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Nakamura S, Uheda E, Nakamura N. Immunolocalization and possible roles of pectins during pollen growth and callose plug formation in angiosperms. Grana. 2000;39:46–55. [Google Scholar]
- Heslop-Harrison J, Heslop-Harrison Y. Cytochemistry and function of the Zwischenkörper in grass pollen. Pollen Spores. 1980;22:5–10. [Google Scholar]
- Hess MW, Frosch A. Subunits of forming pollen exine and Ubisch bodies as seen freeze substituted Ledebouria socialis Roth (Hyacinthaceae) Protoplasma. 1994;182:10–14. [Google Scholar]
- Knox JP. Revealing the structural and functional diversity of plant cell walls. Current Opinion in Plant Biology. 2008;11:308–313. doi: 10.1016/j.pbi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Knox JP, Linstead PJ, King J, Cooper C, Roberts K. Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta. 1990;181:512–521. doi: 10.1007/BF00193004. [DOI] [PubMed] [Google Scholar]
- Li YQ, Faleri C, Geitmann A, Zhang HQ, Cresti M. Immunogold localization of arabinogalactan proteins, unersterified and esterified pectins in pollen grains and pollen tubes of Nicotiana tabacum L. Protoplasma. 1995;189:26–36. [Google Scholar]
- Lynch MA, Staehelin LA. Domains-specific and cell type-specific localization of two types of cell wall matrix polysaccharides in the clover root tip. Journal of Cell Biology. 1992;118:467–479. doi: 10.1083/jcb.118.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska-Sawka A, Rodríguez-García MI. Immunodetection of pectin and arabinogalactan protein epitopes during pollen exine formation of Beta vulgaris L. Protoplasma. 2006;228:41–47. doi: 10.1007/s00709-006-0160-8. [DOI] [PubMed] [Google Scholar]
- Marcus SE, Verhertbruggen Y, Hervé C, Ordaz-Ortiz JJ, Farkas V, Pedersen HL, Willats WG, Knox JP. Pectic homogalacturonan masks abundant sets of xyloglucan epitopes in plant cell walls. BMC Plant Biology. 2008;8:60. doi: 10.1186/1471-2229-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T, Takahashi M, Takabe K, Kobayashi M. Inmunochemistry of rhamnogalacturonan II in cell walls of higher plants. Plant and Cell Physiology. 1998;39:483–491. [Google Scholar]
- Mohnen D. Biosyntesis of pectins and galactomannans. In: Pinto BM, editor. Comprehensive natural products chemistry. Vol. 3. Amsterdam: Elsevier; 1999. pp. 497–527. [Google Scholar]
- O'Neil MA, Albertsheim P, Darwill A. The pectic polysaccharides of primary cell walls. In: Dey PM, editor. Methods in plant biochemistry. Vol. 2. London: Academic Press; 1990. pp. 425–441. [Google Scholar]
- O'Neil MA, Eberthard S, Albertsheim P, Darvill AG. Requirement of borate cross-linking of cell wall rhamnogalactorunan II for Arabidopsis growth. Science. 2001;294:846–849. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- Otegui M, Staehelin LA. Syncytial-type cell plates: a novel kind of cell plate involved in endosperm cellularization of Arabidopsis. The Plant Cell. 2000;12:933–947. doi: 10.1105/tpc.12.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V. Developmental biology of flowering plants. Springer-Verlag; 2000. [Google Scholar]
- Ramírez C, Testillano PS, Risueño MC. Newly formed cell walls exhibit a high expression of esterified pectins in onion root meristems. In: Abe J, editor. Roots: the dynamic interface between plants and the Earth. 2001. Proceeedings of the 6th Symposium of the International Society of Root Research (ISRR), Nagoya, Japan: Japanese Society for Root Research Publishing, 218–219. [Google Scholar]
- Ramírez C, Testillano PS, Risueño MC. Differential pectin distribution during cell wall formation in root meristems. In: Mistrik I, editor. Plant development and adaptation to stress. 2003a. Proceedings of the 6th International Symposium on Structure and Function of Roots. Bratislava: Slovak Academy of Sciences 27. [Google Scholar]
- Ramírez C, Chiancone B, Testillano PS, Garcia-Fojeda B, Germaná MA, Risueño MC. First embryogenic stages of Citrus microspore-derived embryos. Acta Biologica Cracoviensia. 2003b;45:53–58. [Google Scholar]
- Ramírez C, Testillano PS, Pintos B, Moreno-Risueño MA, Bueno MA, Risueño MC. Changes in pectins and MAPKs related to cell development during early microspore embryogenesis in Quercus suber L. European Journal of Cell Biology. 2004;83:213–225. doi: 10.1078/0171-9335-00368. [DOI] [PubMed] [Google Scholar]
- Reynolds ES. The use of lead citrate at high pH as an electron opaque stain in electron microscopy. Journal of Cell Biology. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryden P, Sugimoto-Shirasu K, Smith AC, Findlay K, Reiter WD, McCann MC. Tensible properties of Arabidopsis cell walls depend on both a xyloglucan cross-linked microfibrillar network and rhamnogalacturonan II-borate complexes. Plant Physiology. 2003;132:1033–1040. doi: 10.1104/pp.103.021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguí-Simarro JM, Testillano PS, Jouannic S, Henry Y, Risueño MC. Mitogen-activated protein kinases are developmentally regulated during stress-induced microspore embryogenesis in Brassica napus L. Histochemistry and Cell Biology. 2005;123:541–551. doi: 10.1007/s00418-004-0749-y. [DOI] [PubMed] [Google Scholar]
- Solís MT, Pintos B, Prado MJ, Bueno MA, Raška I, Risueño MC, Testillano PS. Early markers of in vitro microspore reprogramming to embryogenesis in olive (Olea europaea L.) Plant Science. 2008;174:597–605. [Google Scholar]
- Stepka M, Ciampolini F, Charzynska M, Cresti M. Localization of pectins in the pollen tube wall of Ornithogalum virens L. Does the pattern of pectin distribution depend on the growth rate of the pollen tube? Planta. 2000;210:630–635. doi: 10.1007/s004250050053. [DOI] [PubMed] [Google Scholar]
- Suárez-Cervera M, Arcalís E, Le Thomas A, Seoane-Camba J. Pectin distribution pattern in the apertural intine of Euphorbia peplus L. (Euphorbiaceae) pollen. Sexual Plant Reproduction. 2002;14:291–298. [Google Scholar]
- Talbott LD, Ray PM. Changes in molecular size of previously and newly synthesized pea cell wall matrix polysaccharides. Effect of auxin and turgor. Plant Physiology. 1992;98:369–379. doi: 10.1104/pp.98.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor LP, Hepler PK. Pollen gemination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:461–491. doi: 10.1146/annurev.arplant.48.1.461. [DOI] [PubMed] [Google Scholar]
- Testillano PS, Coronado MJ, Seguí JM, Domenech J, González-Melendi P, Raka I, Risueño MC. Defined nuclear changes accompany the reprogramming of the microspore to embryogenesis. Journal of Structural Biology. 2000;129:223–232. doi: 10.1006/jsbi.2000.4249. [DOI] [PubMed] [Google Scholar]
- Testillano PS, González-Melendi P, Ahmadian P, Fadón B, Risueño MC. The immunolocalization of nuclear antigens during the pollen developmental program and the induction of pollen embryogenesis. Experimental Cell Research. 1995;221:41–54. doi: 10.1006/excr.1995.1350. [DOI] [PubMed] [Google Scholar]
- Testillano PS, González-Melendi P, Coronado MJ, Seguí-Simarro JM, Moreno MA, Risueño MC. Differentiating plant cells switched to proliferation remodel the functional organization of nuclear domains. Cytogenetic Genome Research. 2005;109:166–174. doi: 10.1159/000082396. [DOI] [PubMed] [Google Scholar]
- Testillano PS, Ramírez C, Domenech J, Coronado MJ, Vergne P, Matthys-Rochon E, Risueño MC. Young microspore-derived maize embryos show two domains with defined features also present in zygotic embryogenesis. International Journal of Developmental Biology. 2002;46:1035–1047. [PubMed] [Google Scholar]
- Van Aelst AC, van Went J. Ultrastructural immunolocalization of pectin and glycoproteins in Arabidopsis thaliana pollen grains. Protoplasma. 1992;168:14–19. [Google Scholar]
- Vicente O, Moreno RM, Heberle-Bors E. Pollen cultures as a tool to study plant development. Cell Biology Reviews. 1991;25:295–306. [PubMed] [Google Scholar]
- Willats WGT, Mcartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Molecular Biology. 2001a;47:9–27. [PubMed] [Google Scholar]
- Willats WGT, Orfila C, Limberg G, et al. Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Journal of Biological Chemistry. 2001b;276:19404–19413. doi: 10.1074/jbc.M011242200. [DOI] [PubMed] [Google Scholar]
- Yeung EC, Rahman MH, Thorpe TA. Comparative development of zygotic and microspore-derived embryos in Brassica napus L. cv. Topas. I. Histodifferentiation. International Journal of Plant Science. 1996;157:27–39. [Google Scholar]