Abstract
It is proposed that post-harvest longevity and appearance of salad crops is closely linked to pre-harvest leaf morphology (cell and leaf size) and biophysical structure (leaf strength). Transgenic lettuce plants (Lactuca sativa cv. Valeria) were produced in which the production of the cell wall-modifying enzyme xyloglucan endotransglucosylase/hydrolase (XTH) was down-regulated by antisense inhibition. Independently transformed lines were shown to have multiple members of the LsXTH gene family down-regulated in mature leaves of 6-week-old plants and during the course of shelf life. Consequently, xyloglucan endotransglucosylase (XET) enzyme activity and action were down-regulated in the cell walls of these leaves and it was established that leaf area and fresh weight were decreased while leaf strength was increased in the transgenic lines. Membrane permeability was reduced towards the end of shelf life in the transgenic lines relative to the controls and bacteria were evident inside the leaves of control plants only. Most importantly, an extended shelf-life of transgenic lines was observed relative to the non-transgenic control plants. These data illustrate the potential for engineering cell wall traits for improving quality and longevity of salad crops using either genetic modification directly, or by using markers associated with XTH genes to inform a commercial breeding programme.
Keywords: Anti-sense, cell wall properties, lettuce, shelf life, XET, XTH
Introduction
Over the last 10 years the way in which salad crops are grown and packaged worldwide has changed such that the current market favours pre-packed whole and cut leafy salads with a variety of colours, textures, and flavours mixed together in a single bag. Pre-packed baby leaf salad accounted for over £400 million of UK business in 2006 and this figure is rising currently by more than 10% each year. The processing required to package salad crops results in physiological damage to the leaf lamina that results in water logging and bruising, both of which can be caused by cell wall damage (Clarkson et al., 2003; Newman et al., 2005). At present, retailers offer a guaranteed shelf life of no more than 5 or 6 d to the UK consumer, which typically follows a 1–4 d harvest–transport–package procedure during which time physiological damage can occur in the leaf. Improved cell wall strength and shelf life in lettuce leaf crops has already been achieved by altering the time of harvest to late evening when the cells are turgid, and can withstand processing more effectively due to accumulated photoassimilates (Clarkson, 2004; Clarkson et al., 2005). Here a genetic approach to altered cell wall characteristics was sought through manipulation of cell wall extensibility and cell expansion. It is hypothesized that a more robust leaf, able to withstand processing more effectively, would have a combination of small cells, high solute concentration, and stronger cell walls (Clarkson et al., 2003).
Plant cell wall loosening and extension represents a balance between wall synthesis, turgor pressure, and wall extensibility (Pilling and Höfte, 2003). The complexities of co-ordinated wall extensibility and growth are still being unravelled, but it has been demonstrated that xyloglucan endotransglucosylase/hydrolases (XTHs) are of major importance in the control of this process. These proteins exhibit one or both of two enzymic activities: xyloglucan endotransglucosylase (XET) and xyloglucan endohydrolase (XEH) (Rose et al., 2002). XET activity cleaves xyloglucan (a polysaccharide thought to form molecular ‘tethers’ in the cell wall) and rejoins the ends of the polymer to form chains of different lengths. This may result in cell wall loosening or cell wall strengthening depending on the molecular size, location, and age of the participating xyloglucan chains (Thompson and Fry, 2001). XTH genes form a large, multigene family consisting of at least 33 members in Arabidopsis (Rose et al., 2002). Many have very distinct and highly responsive transcriptional patterns which correlate well with physiological events (Nishitani, 2005). Measurable XET enzyme activity (in vitro) and action (in vivo) are also strongly controlled by developmental and environmental stimuli (Fry, 2004). Cell wall loosening is essential for developmental processes such as leaf expansion and the XET-action of XTHs enables turgor-driven expansion to occur with minimal weakening (Palmer and Davies, 1996). Cell wall strengthening and increased cell wall biogenesis occur as a result of mechanical stimulation of the plant via touch-induced up-regulation of genes such as TCH4 (AtXTH22) in Arabidopsis (Xu et al., 1995).
A number of XTH genes were targeted in the current study by applying antisense repression in transgenic lettuce (Lactuca sativa cv. Valeria, a variety of Lollo Rosso) using a lettuce XTH gene fragment encoding a region of high homology to other family members. In Arabidopsis, the orthologous gene (AtXTH16) is up-regulated in mature leaves, so it is hypothesized that in knock-down plants of the lettuce homologue LsXTH16, leaf cell expansion would be altered, increasing cell wall strength and hence generate a stronger leaf phenotype. When producing the transgenic plants it was hypothesized that, because the region used for antisense was highly conserved in several XTH genes, more than one XTH gene could be silenced. This hypothesis was upheld and in this paper it is shown that several LsXTH transcripts were down-regulated in three independently transformed lines of transgenic lettuce and that the resultant phenotypes were smaller and stronger leaves with an improved shelf-life. Our findings indicate that knocking down/out multiple members of this multigene family is a viable way of producing an improved baby leaf salad crop and ways in which these findings might be integrated into a lettuce breeding programme are discussed.
Materials and methods
Cloning of LsXTH and transformation of lettuce
A partial cDNA fragment encoding a conserved region of the XTH gene family was amplified from Lactuca sativa cv. Valeria using degenerate RT-PCR (primer sequences XETf2: ATGGGATCCGAYGARATHGAYTTYGARTT and XETr2: CATGAGCTCGTRCARTARTTRTADATCAT). The fragment was sequenced and cloned into the SLJ732 vector in antisense orientation under the control of a cauliflower mosaic virus 35S promoter (Clarkson, 2004). Generation of transgenic lettuce plants (cv. Valeria) was achieved by Agrobacterium tumefaciens-mediated transformation according to the method of Curtis et al. (1994). Results described in this paper were derived from the T3 generation of three independently transformed homozygous lines, following initial measurements on T2 (Clarkson et al., 2004). Non-transformed plants were used as controls. The known sequence of the XTH fragment was extended using 3′ and 5′ RACE (SMART RACE cDNA amplification kit, BD Biosciences, Oxford, UK) according to the manufacturer's instructions and aligned to the Arabidopsis XTH gene family where it was found to correspond most closely to AtXTH16, hence its classification as LsXTH16 throughout this paper. The nucleotide sequence of the XTH was entered into GenBank for public access. Accession numbers LsXTH16: AJ577755 and R31: DQ217595.
Plant growth
Seeds were sown directly onto F2 compost (Levington) and plants were maintained under long days (16/8 h photoperiod) at 25 °C, 60 μmol m−2 s−1, white fluorescent light. Plants were harvested at a commercial stage 6 weeks after sowing, and leaves designated 1 (first emerged, approximately 10 cm long) through to 6 (most recently emerged, approximately 4 cm long) at this stage.
Quantitative RT-PCR
Total RNA was extracted from young (leaf 6) and middle (leaf 3) and old (leaf 1) leaves from three different plants from each transgenic line and non-transformed plants at day 0, 6, and 12 of shelf life. The RNA was quantified and DNase-treated before using 5 μg total RNA in a cDNA synthesis reaction. 1/20 of each cDNA reaction was used as a template per PCR reaction. Cycle number was optimized for each primer pair using a pooled sample of the cDNAs and final reactions were performed at a number of cycles when the product concentration was in linear proportion to the cycle number. Three technical replicate reactions were performed for each of the three biological replicates from each stage/line. Primers used were as follows: LsXTH16F: ATGCAACTCAAACTAGTACC; LsXTH16R: TCCAGTCGGTCTTCACACG; LsXTH5F: TTGACCCCACCAAAGCTTAC LsXTH5R: GACCAATCGGTCTTCTCCAA; LsXTH8F: CAGTCGATGGTTGTCAATGG; LsXTH8R: ATCCGAACCCTAATCCCAAG; LsXTH28F: GAAGAGGTTTGATCCGGTGA; LsXTH28R: ACGCTCTCCCCTCCACTATT; LsXTH30F: TTTGAGCCTTCCAAAGCCTA; LsXTH30R: CACCATTGGTAGCCCAAGTT. Actin control primers were LsACTINF: AGAGCTGAGGGCTAGGGTTC; LsACTINR: GATCCAAACGGAGGATAGCA.
Physiology
Leaf and cell area were measured using ImageJ (NIH Image, National Institute of Health, USA) from scaled scanned whole leaves and.tiff images used for the localization of XET action, respectively, as described previously (Street et al., 2006). Fresh weights of whole leaves were recorded immediately after harvest. Breakstrength was measured using an Instron customized materials-testing instrument (Instron, High Wycombe, UK) with Bluehill software from the same company with the gauge length set to a standard 10 mm and the load set to 0 N at the start of each experiment. Leaves were stored in 100% methanol at harvest and then washed for 15 min in dH2O before measurement, following preliminary analysis which showed this to be adequate for full rehydration (Zhang, 2006). Ten replicate strips (15 mm×5 mm) from each line were cut from leaves parallel and adjacent to the midrib and mounted in the Instron before stretching the material continuously until it fractured. The maximum load was recorded in Newtons. Conductivity measurements were performed according to the method of Wagstaff et al. (2007) and six replicate leaves were used from each line on each day post-processing. Measurements were taken on fresh leaves, which were subsequently frozen at –20 °C and then remeasured to obtain the maximum leakage value. Samples were then dried to completion so the final measurements were expressed as a percentage of the maximum leakage mg−1 dry weight.
XET activity
A radiochemical assay was performed as described using a 3H-labelled oligosaccharide (XLLGol) as acceptor substrate to which a portion of the donor substrate (xyloglucan polysaccharide) can be joined. Leaf tissue was ground in succinate buffer (300 mM succinic acid, 10 mM CaCl2, 10 mM ascorbic acid, 10% (v/v) glycerol in 0.5% (w/v) chlorobutanol; final pH 5.5 with NaOH) in a 1:3 ratio (tissue FW:buffer volume). The homogenate was centrifuged at 10 000 g for 4 min and the supernatant was transferred to a fresh tube and stored at –80 °C until required. Equal volumes of extract and assay solution [0.3% (w/v) tamarind-XG (polysaccharide), 10 mM CaCl2, 300 mM succinic acid pH 5.5 in 0.5% (w/v) chlorobutanol] were mixed together with [3H]XLLGol (oligosaccharide) at 300 000 cpm per 10 μl. After incubation for 60 min with tissue extracts from a known FW the reaction was stopped with formic acid and products were adsorbed onto 3MM paper (Whatman) and dried. Following overnight washing each replicate (three technical replicates of six biological replicates per leaf age per line) was assayed for H on a Beckman LS6500 scintillation-counter. Enzyme activity was calculated at H counts min−1 g−1 FW h−1 incubation.
XET action
XET action was localized using a 90 μM xyloglucan oligosaccharide– sulphorhodamine conjugate (XLLG–SR; a fluorescent acceptor substrate), which was vacuum infiltrated into 2 mm×2 mm leaf sections in 100 μl 25 mM MES buffer (pH 5.6). The tissue was incubated with the fluorescent substrate for 2 h at room temperature before transferring to 70% EtOH overnight at 4 °C. The tissue was given two more washes in 70% EtOH the next day before mounting in the same solution for microscopy on a Leica TCS CLSM (Leica Microsystems AG, Wetzlar, Germany). The Metamorph imaging system (Universal Imaging Corp., PA, USA) was used to compare signal intensity of XET action in the cell walls in three replicate images from each leaf age when the zoom and gain settings on the microscope were standardized.
Shelf life
Six-week-old plants from each line were bulk harvested and the leaves removed at the base of the stem using a sharp blade. They were then hand-sorted to remove any obviously damaged leaves prior to mock-processing (Clarkson, 2004; Zhang et al.., 2007; Wagstaff et al., 2007) by washing in 4.0 l of rapidly agitated water for 1 min. Leaves were then spun for 20 s to remove excess water and then packaged into zip-sealed polythene bags in 10 g aliquots before storage in the dark at 6 °C. Samples were examined daily for any sign of damage and the bag was discarded as soon as one leaf within it lost quality. Between 10 and 19 replicate bags were examined for each line.
Transmission electron microscopy
Tissue samples approximately 1 mm were excised from the centre of the leaf lamina (away from the midrib) at four post-harvest stages of Valeria and line 9–11. Leaves were chosen from the same stage of development. Samples were immediately fixed in 3% (v/v) glutaraldehyde, 4% (v/v) formaldehyde, 0.1 M PIPES buffer pH 7.2 and subsequently rinsed twice in buffer and then post-fixed in aqueous 1% (w/v) osmium. After aqueous washing (twice), tissue was dehydrated through a graded series of ethanol and embedded in Spurr's resin. Following polymerization, thin sections (c. 60 nm) were cut on an ultramicrotome, post-stained with uranyl acetate (ethanolic) and lead citrate, and viewed under a Hitachi H7000 transmission electron microscope (Hitachi, Wokingham, UK).
Statistical analyses
Statistics were used on the physiological data to determine if the measurements on each individual line were significantly different from the controls. A two-tailed heteroscedastic Student's t test was used in most cases, unless otherwise stated. One-way ANOVA/LSD post hoc was used for the XET activity data and also to examine the interaction between lines and controls for the fresh weight data in order to determine if the ages of the leaves under examination was also significantly different from each other.
Results
Cloning of novel XTH genes from lettuce
Using degenerate oligonucleotide primers to conserved XTH domains, two XTH sequences, LsXTH16 (Accession no. AJ577755) and R31 (Accession no. DQ217595), were identified from cDNA from young rapidly expanding leaves. Both were confirmed by BLAST alignment as XTH gene fragments but only LsXTH16 was taken forwards for further study. Alignment against other XTH sequences suggests that LsXTH16 encodes a class 2 XTH. RACE was used to extend the sequence of LsXTH16. The original LsXTH16 fragment was cloned in an antisense orientation into the binary vector SLJ732 (Jones et al., 1992), replacing the GUS marker gene. This vector uses the CaMV 35S promoter to drive expression. The region used for antisense included the catalytic centre and shared between 52% and 66% amino acid identity to other developmentally regulated XTH genes from Arabidopsis across the same region (data not shown). A total of 19 transgenic lettuce plants were produced (Clarkson, 2004); however, several failed to reach maturity or set seed, or had multiple insertions. From the remaining plants, three lines (7-25, 9-11, and 19-3, Clarkson, 2004) were selected as homozygous expressers of the transgene and were studied further in the T3 generation.
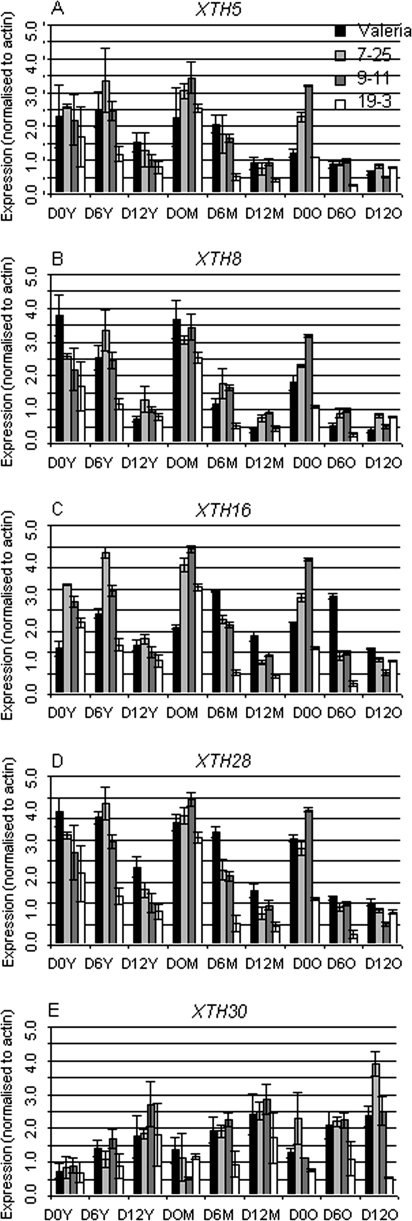
The expression of the endogenous LsXTH16 gene was investigated using real-time qPCR with one primer located in the endogenous gene outside the region used for antisense. In non-transformed plants LsXTH16 is usually slightly up-regulated in mature (6-week old) leaves compared to young rosette leaves, but in the antisense lines the expression was up-regulated at the time of harvest, but was down-regulated by half in leaves at the later stages of development during the course of shelf life (Fig. 1C).
Fig. 1.
Gene expression of LsXTH genes in non-transformed and transgenic lines. (A) LsXTH5, (B) LsXTH8, (C) LsXTH16, (D) LsXTH28, (E) LsXTH30 at three ages of leaf (old, first emerged, 6-week-old; middle, leaf 3; young, leaf 6) at day 0, day 6, and day 12 after harvest and subsequent storage in bags at 4 °C. Bars are the mean of three biological replicates (within which are three technical replicates) of each stage and line. Expression of LsXTH genes is normalized to actin at each data point.
The expression of a number of other LsXTH gene family members available in the Lactuca database was investigated using semi-quantitative PCR. LsXTH5, LsXTH8, and LsXTH28 all showed down-regulation during shelf life in control plants (Fig. 1C, B, D) with LsXTH5 and LsXTH8 also showing developmental down-regulation in the oldest leaves examined. LsXTH5 and LsXTH8 were down-regulated in line 19-3 during the mid-point of shelf life (Fig. 1A, B), however, all the lines showed down-regulation of LsXTH8 and LsXTH28 in young leaves at the time of harvest (Fig. 1B, D). There was an effect of shelf life in all the transgenic lines when examining the expression of LsXTH28 with the major impact occurring at the end of shelf life in young leaves and from day 6 of shelf life in middle-aged leaves (Fig. 1D). The expression of LsXTH30 in control plants was different from the other family members examined in that it showed increased expression in both development and during shelf life (Fig. 1E). The lines showed mixed effects, with 7-25 increasing in mature leaves whereas 9-11 and 19-3 showed down-regulation at different stages.
Growth physiology of transgenic lettuce
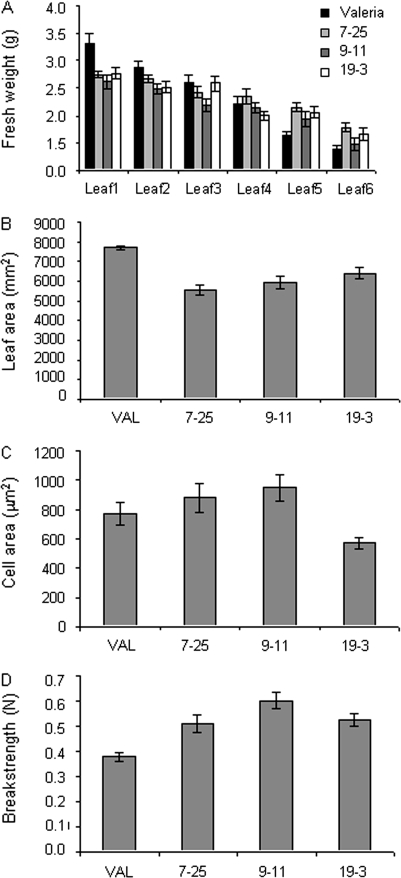
Analysis of fresh weight (Fig. 2A) showed an interesting pattern in relation to leaf expansion in the transgenic lines with a more rapid gain in fresh weight of younger leaves than in non-transformed plants, but an arrest of growth as leaves became older (leaf 3 on a 6-week-old plant) with the result that fresh weight was stable in the transgenics after this point, whereas the controls continued to gain weight. A two-way ANOVA showed a significant interaction (P <0.0001) between line and leaf age indicating that the reduced duration of leaf growth was correlated with the silencing caused by the antisense transcript. The remaining analyses were conducted on the 6-week-old mature leaf as this stage was when the transgene was having a physiological effect on development. Analysis of leaf area on a 6-week-old mature leaf (i.e. at point of harvest) using a two-tailed heteroscedastic Student's t test showed that leaf area was significantly reduced in all three lines (0.01 > P > 0.0001) compared with the controls (Fig. 2B). However, the two-tailed heteroscedastic Student's t test showed that cell area was only significantly different (smaller) (P=0.03) than the controls in line 19-3 (Fig. 2C). Leaf strength was also characterized in the oldest leaf of a 6-week-old plant using an Instron (Fig. 2D). All transgenic lines required a significantly greater force to break a uniform strip of tissue taken from the leaf lamina than an equivalent strip from leaves of non-transformed plants (P <0.001), indicating that the transgenic leaves were possibly more robust than their non-transformed counterparts.
Fig. 2.
Physiological traits of non-transformed and transgenic leaves. (A) Fresh weight of leaves at the time of harvest from 6-week-old plants, one from each line. Leaf 1 is the oldest, leaf 6 is the most recently emerged, n=12. (B) Leaf area of leaf 1 at time of harvest, n=10. (C) Cell area of leaf 1 at time of harvest (n > 12 taken from three biologically independent samples). (D) Breakstrength (force required to snap tissue) measured using an Instron using strips (15 mm×5 mm in a parallel orientation to the midrib) cut from leaf 1 at time of harvest (6 weeks) (n=10). In all cases bars=sem.
XET enzyme activity in vitro and action in vivo
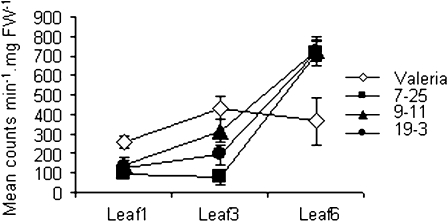
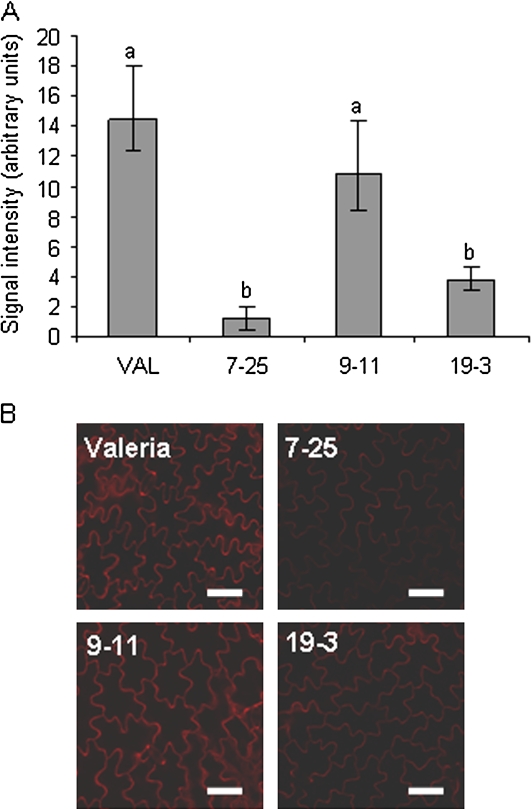
The XET activity of the total extractable XTHs was measured by radiochemical assay at three leaf ages (Fig. 3) and was found to be elevated in young leaves (leaf 6) in transgenic plants compared with the controls, but was reduced in all the transgenic lines in the oldest leaf of a 6-week-old plant (leaf 1). The differences in the oldest leaf were significant (0.01 < P < 0.05, two-tailed heteroscedastic t test) in two of the transgenic lines (7-25 and 19-3), indicating that altering LsXTH transcription had a direct effect on post-translational activity in these lines. An experiment to localize XET action (i.e. to detect the co-localization of endogenous XET activity with endogenous donor-substrate xyloglucan in vivo), using as an acceptor substrate, a low-molecular-weight xyloglucan-oligosaccharide–sulphorhodamine conjugate (XLLG–SR), revealed much-reduced signal intensity in the three lines that had also shown reduced extractable XET activity in the radiochemical assay (Fig. 4A). ANOVA followed by a post hoc LSD test showed that lines 7-25 and 19-3 were significantly different from Valeria (P=0.014 and P=0.0223, respectively). The associated confocal laser scanning microscopy (CLSM) images indicated that the XET action was indeed detectable in the cell wall only (Fig. 4B) and that 7-25 and 19-3 showed less fluorescence than the equivalent non-transformed leaf.
Fig. 3.
Extractable XET enzyme activity assayed in vitro. A radiochemical assay was used to test extracts from leaves 1 (first emerged), 3, and 6 (youngest) from 6-week-old control and transgenic lines using tamarind xyloglucan as a donor substrate and [3H]XLLGol as acceptor. Values are the mean of six biological replicates from each leaf age. Bars=sem.
Fig. 4.
Localization of XET action in vivo within the oldest leaves of 6-week-old control and antisense plants. Leaves were infiltrated with a fluorescently labelled low-molecular-weight acceptor substrate (XGO–SR) that is utilized by endogenous XTHs acting on high-molecular-weight endogenous xyloglucans as donor substrate. (A) Quantification of fluorescence in cell walls using three biological replicates and a standardized gain and zoom on the CLSM. Different letters indicate lines significantly different from each other (ANOVA/LSD post hoc, P <0.05). Bars=range. (B) CLSM images of representative samples used for quantifying XET action in leaf 1 of control and transgenic lines. Bar=20 μm.
Shelf-life of transgenic lettuce lines is extended
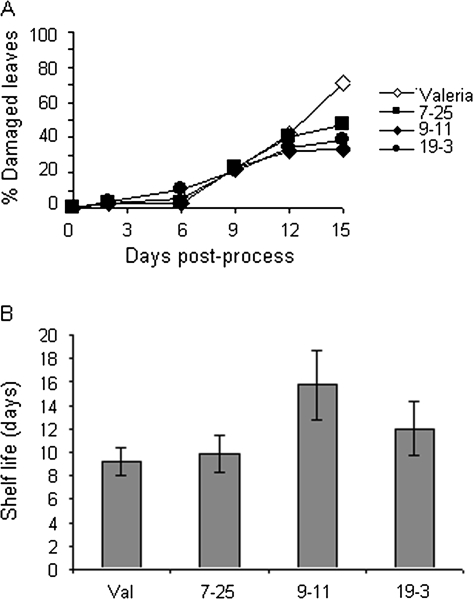
Six-week-old plants were harvested and subjected to mock-processing in the laboratory before storage in bags at 6 °C. The appearance of leaves from each line was scored over a 15 d period (Fig. 5A) and the control line was clearly worst at the end of the shelf life, with line 9-11 having the best visual score. Bags were rejected when one or more leaves showed signs of bruising, water-logging or browning. The mean shelf life for one of the antisense lines was significantly increased by 6 d (line 9-11; P=0.03) compared with control plants, giving a total post-harvest life of 15 d (Fig. 5B). This represents an important increase in scheduling flexibility for those managing stock control and ultimately a better quality product for the consumer.
Fig. 5.
Improved shelf life of transgenic lettuce. Six-week-old plants were harvested, washed, dried, and packed in a replication of the commercial process. A minimum of eight replicate 10 g were packed from each line. (A) Incidence of leaf damage (bruising, waterlogging, breakage) during shelf life. (B) Shelf life of leaves from each line. Samples were rejected from storage at 6 °C (average retailer's chilled cabinet temperature) when one or more leaves in the bag showed signs of bruising, waterlogging or browning. Bars=sem.
Ultrastructure and membrane integrity of line 9-11
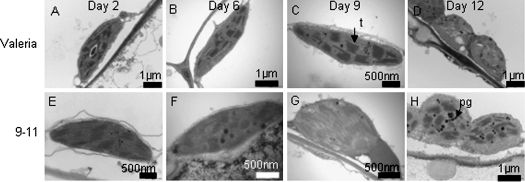
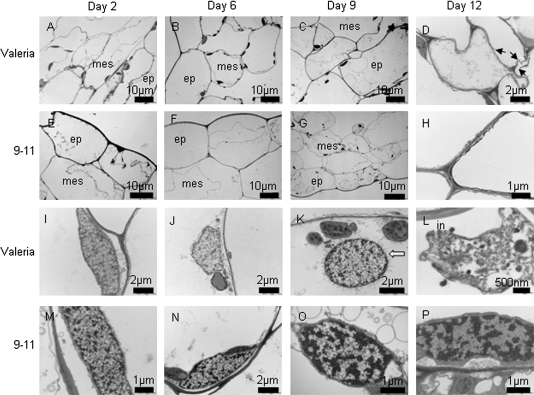
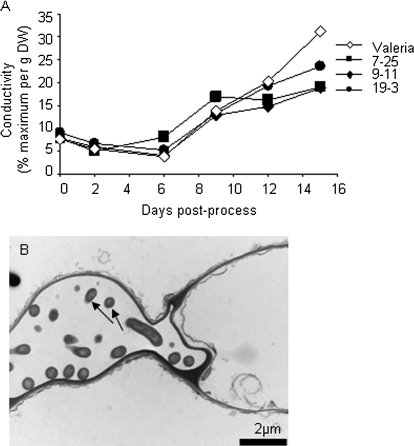
Having identified a transgenic line with increased shelf life, further examination of this line was made at the ultrastructural level in order to establish the parameters affecting its longevity. The plastids of line 9-11 showed very little difference from the controls since both retained thylakoid integrity (Fig. 6) during senescence until the last stage of shelf life examined (Fig. 6D, H). Plastoglobuli accumulated in both the control and 9-11, possibly at a slower rate in the transgenic lines although this would need further quantification using more numerous sections from different plants. Cell wall ultrastructure was similar in both the control and 9-11 (Fig. 7A–H) with wall thickening and plasmolysis occurring as previously reported (Wagstaff et al., 2007). However, there was a marked change in membrane structure with line 9-11 showing a thicker membrane that remained intact at day 12 (Fig. 7H) whereas the control line showed distinct fractures at multiple points in the membrane (Fig. 7D) in line with previous observations of lettuce at this stage of shelf life. This improvement in membrane integrity may also be the reason for the improved nuclear appearance in 9-11 during shelf life (Fig. 7M-P) where chromatin was maintained at a high density and the margins of the nucleus appeared unaltered over shelf life. However, the control line showed a dispersal of chromatin at day 12 and signs of invagination and distortion of the nuclear margin (Fig. 7L). Examination of membrane leakage in all the lines showed that 9-11 and 7-25 were significantly reduced compared with Valeria by day 12 of shelf life, and all the transgenic lines were reduced by day 15 (Fig. 8A). During the ultrastructural examination, bacteria were evident inside the mesophyll cells of Valerion on day 12 on numerous occasions (example in Fig. 8B) but this phenomenon was never observed in line 9-11.
Fig. 6.
Plastid ultrastructure. Transmission electron microscopy of plastids within mesophyll cells of Valeria (A–D) and line 9-11 (E–H) at days 2, 6, 9, and 12 of shelf life storage at 6 °C. Leaves are of comparable age. Thylakoid stacks (t) are visible throughout, only losing integrity at day 12 of shelf life. Electron dense plastoglobuli (pg) are visible as black spheres.
Fig. 7.
Cell wall and membrane structure. Transmission electron microscopy of cells in epidermal (ep) and mesophyll (mes) layers of Valeria (A–D) and line 9-11 (E–H) at day 2, 6, 9, and 12 of shelf life storage at 6 °C. Leaves are of comparable age. Fractured membranes (arrows) are evident at day 12 in Valeria, but not in 9-11. Nuclei are shown in Valeria (I–L) and 9-11 (M–P). Chromatin are the more electron dense regions of each nucleus. Nuclear invagination (in) and loss of nuclear membrane integrity (white arrow) are evident in Valeria.
Fig. 8.
Membrane leakage of transgenic lines and Valeria. (A) Conductivity measurements (in μS) were taken throughout the shelf life of fresh leaves that were subsequently frozen in order to obtain a percentage of maximum leakage and then dried so final values were given as a percentage maximum g−1 DW. (B) Bacteria (arrowed) were evident inside mesophyll cells of Valeria on numerous occasions but were never observed in 9-11.
Discussion
These results demonstrate that genetic modification is a viable means of changing cell wall properties with a direct consequence for post-harvest leaf longevity and therefore shelf life in lettuce. From our studies it appears that several XTH family members were knocked down during the course of shelf life in the transgenic lines, with line 19-3 showing the most obvious differences in expression. Cell area was also reduced in line 19-3, although all the transgenic lines had a reduced leaf area but an increased fresh leaf weight. This implies that the leaves of transgenic lines were thicker, a phenotype together with small leaves and small cells that we hypothesized contributes to improved shelf life (Clarkson et al., 2003). Some of the observed expression and phenotypic traits in the antisense lines were unexpected, for example, the up-regulation of the endogenous genes (LsXTH16) in young and middle leaves and during the early stages of shelf life, possibly as a consequence of the knock-down of related XTH genes leading to compensatory XTH expression at certain development and environmental stages. There are undoubtedly more XTH genes in lettuce than it has been possible to examine, since the gene family in Arabidopsis consists of 33 family members (Rose et al., 2002), some of which may contribute to shelf life and/or be altered by the antisense insertion. The number of XTH genes present is unknown, but only nine are listed in the Compositae database (annotated according to the closest homology to Arabidopsis XTH genes) and expression was examined in those for which it was possible to design unique PCR primers that produced a single product. Some of the other unidentified or uncharacterized XTH genes may map to regions of the genome that co-locate with physiological QTL for shelf life, identified by Zhang et al. (2007). The relationship between reduced gene expression, XET activity, and leaf phenotype is rather complex. For example, line 9-11 showed the most significant increase in shelf life, but lower reductions in XTH transcript abundance than some of the other lines, intermediate decreases in extractable activity and almost no decrease in in vivo XET activity. It is hypothesized that this is because the extended shelf life phenotype is a result of the impact of all the changes brought about by the antisense construct, whereas it has only been possible to measure some members of the gene family directly. It was also not possible to ascertain any secondary effects of the transgene, such as the impact on cytoskeletal structures underlying the cell wall.
XTH has been shown to be involved in cell wall loosening (Fry et al., 1992; Nishitani and Tominaga, 1992) and it was demonstrated here that, by down-regulating several members of this gene family through targeting a conserved region, it was possible to reduce leaf expansion and increase leaf strength. Previous studies have shown that xyloglucan binds non-covalently to cellulose and suggested that it is responsible for the major tension-bearing structure in the plant cell wall (Fry, 1989; McCann et al., 1990), tethering neighbouring cellulose microfibrils. Xyloglucan metabolizing enzymes are therefore key components for the control of cell wall extensibility and strength as they can cleave the tethers that hold the cellulose microfibrils together (Fry, 1989; Vissenberg et al., 2005). It has been previously shown (Xu et al., 1996) that expression of several XTH family members is developmentally and/or environmentally regulated in dicotyledonous plants and, more recently, (Yokoyama et al., 2004), that monocotyledonous plants also have a diverse XTH gene family with members involved in the response to several developmental and environmental cues. Often several XTH genes have their expression up-or down-regulated simultaneously (Nishitani, 2005), reflected in the present study where LsXTH30 was up-regulated and LsXTH5, LsXTH8, and LsXTH28 were down-regulated in the same tissues, presumably because some of these family members are involved in wall loosening and others in tethering of the microfibrils. Recent studies (Dimmer et al., 2004), have revealed that several related XTH genes in sugar beet are developmentally expressed during the formation of vascular tissue and environmentally stimulated in response to pathogen attack or wounding. Therefore, it comes as no surprise to find that a subset of the gene family is involved in leaf expansion and several family members have to be knocked down in order to obtain a physiological effect. It has been shown recently (Matsui et al., 2005) that a loss-of-function mutation in a single XTH gene in Arabidopsis can produce a noticeable phenotype, in this case affecting xylem development. However, the general dearth of published work citing the effect of engineering just one member of a cell wall gene family, such as XTH or expansin, together with unpublished findings that single gene antisense knockouts of XTH genes do not give a phenotype (SC Fry, personal communication) suggests that single-target transgenic approaches do not produce plants with a measurable phenotype, presumably because of gene redundancy amongst family members causing compensation for the missing transcript.
These findings provide evidence that XTHs have a wall-loosening role, in agreement with the finding that XET activity (Pritchard et al., 1993) and action (Vissenberg et al., 2000) appear in zones of cell elongation in roots. In the present study, high XET activity and action were detected in leaf cell walls that are undergoing expansion, but not in antisense lines where expansion has been arrested. Although previous studies (McQueen-Mason et al., 1993) reported that XET activity was not essential for in vitro elongation (creep) of heat-denatured hypocotyl cell walls, it would appear that it is an integral part of normal plant cell extension and the consequences of reducing it have biophysical and physiological effects and effects on the underlying membrane structure of the cell. In control Valeria plants, the membranes of the plasmalemma were observed to fragment by day 12, and the nuclear membrane also showed signs of disruption and invagination, correlating with a loss of chromatin density as previously shown in commercial cultivars of lettuce (Wagstaff et al., 2007). Loss of membrane integrity in the controls, as observed by electron microscopy and inferred from increased conductivity, was correlated with the incidence of bacterial infection, leaf damage, and loss of shelf life, but the relationship linking cell wall biology with membrane structure is yet to be fully explored although the wall is now thought to be a continuum of the membrane and cytoskeleton (Baluska et al., 2003). It is hypothesized that either the presence of an intact and strong cell wall inherently protects the underlying membrane from the environment, as shown by the need for cell wall-membrane adhesion for effective protection against fungal pathogens (Mellersh and Heath, 2001), or that there is cross-talk between the two structures as reported by Humphrey et al. (2007), but these theories require rigorous testing.
The results presented in this paper show that modifying cell wall properties can have a direct benefit for post-harvest quality. The modification of cell wall proteins such as expansins (Brummell et al., 1999) and galactosidases (Smith et al., 2002) has already been established as a means of regulating fruit ripening and softening in tomato, but to our knowledge this is the first report of XTH modification being used to improve post-harvest quality of a leafy crop. Since post-harvest quality relies on the integration of many stimuli including ageing, wounding, and temperature stress, it is possible that engineering other XTH genes responding to these specific stresses could have an additionally beneficial effect. A number of allelic lines could be integrated into breeding populations using novel techniques such as TILLING (Henikoff et al., 2004), or by using marker-assisted breeding to identify lines with endogenously reduced XET activity. Our work highlights the potential for cell wall modification using a multi-target approach to improve post-harvest crop quality and longevity that is likely to have wide significance for many leafy crops.
Acknowledgments
We thank Jonathan Jones for the gift of the SLJ732 vector. This research was supported by a BBSRC IPA grant (BBS/B/08574) to GT, MSD, and GJJC with financial support from Vitacress Salads Ltd. GJJC conceptualized and initiated this research during a studentship funded by Vitacress Salads Ltd. FZ was supported by a BBSRC industrial CASE (Co-operative Award in Science and Engineering BBS/B/08574) studentship. SCF thanks the BBSRC for financial support. We thank Dr Matt Cuttle for assistance with CLSM analysis and Dr Anton Page for assistance with sample preparation for TEM.
References
- Baluska F, Samaj J, Wojtaszek P, Volkmann D, Menzel D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiology. 2003;133:482–491. doi: 10.1104/pp.103.027250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. The Plant Cell. 1999;11:2203–2216. doi: 10.1105/tpc.11.11.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson GJJ. Southampton: University of Southampton; 2004. The diagnosis and manipulation of baby salad leaf processability. PhD thesis. [Google Scholar]
- Clarkson GJJ, O'Byrne EE, Rothwell SD, Taylor G. Identifying traits to improve postharvest processability in baby leaf salad. Postharvest Biology and Technology. 2003;30:287–298. [Google Scholar]
- Clarkson GJJ, Rothwell SD, Taylor G. End of day harvest extends shelf life. Hortscience. 2005;40:1431–1435. [Google Scholar]
- Curtis IS, Power JB, Blackhall NW, de Laat AMM, Davey MR. Genotype-independent transformations of lettuce using Agrobacterium tumefaciens. Journal of Experimantal Botany. 1994;45:1441–1449. [Google Scholar]
- Dimmer E, Roden L, Cai D, Kingsnorth C, Mutasa-Göttgens E. Transgenic analysis of sugar beet xyloglucan endotransglucosylase/hydrolase Bv-XTH1 and BvXTH2 promoters reveals overlapping tissue-specific and wound inducible expression profiles. Plant Biotechnology Journal. 2004;2:127–139. doi: 10.1046/j.1467-7652.2004.00056.x. [DOI] [PubMed] [Google Scholar]
- Fry SC. Cellulases, hemicelluloses and auxin-stimulated growth: a possible relationship. Physiologia Plantarum. 1989;75:532–536. [Google Scholar]
- Fry SC. Primary cell wall metabolism: tracking the careers of wall polymers in living plant cells. New Phytologist. 2004;161:641–675. doi: 10.1111/j.1469-8137.2004.00980.x. [DOI] [PubMed] [Google Scholar]
- Fry SC, Smith RC, Renwick KF, Martin DJ, Hodge SK, Matthews KJ. Xyloglucan endotransglycosylase, a new wall-loosening enzyme activity from plants. Biochemical Journal. 1992;282:821–828. doi: 10.1042/bj2820821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Till BJ, Comai L. TILLING: traditional mutagenesis meets functional genomics. Plant Physiology. 2004;135:630–636. doi: 10.1104/pp.104.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey TV, Bonetta DT, Goring DR. Sentinels at the wall: cell wall receptors and sensors. New Phytologist. 2007;176:7–21. doi: 10.1111/j.1469-8137.2007.02192.x. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Shulumukov L, Carland F, Engllish J, Scofield SR, Bishop GJ, Harrison K. Effective vectors for transformation, expression of heterologous genes, and assaying transposon excision in transgenic plants. Transgenic Research. 1992;1:285–297. doi: 10.1007/BF02525170. [DOI] [PubMed] [Google Scholar]
- Matsui A, Yokoyama R, Seki M, Ito T, Shinozaki K, Takahashi T, Komeda Y, Nishitani K. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. The Plant Journal. 2005;42:525–534. doi: 10.1111/j.1365-313X.2005.02395.x. [DOI] [PubMed] [Google Scholar]
- McCann MC, Wells B, Roberts K. Direct visualization of cross-links in the primary plant-cell wall. Journal of Cell Science. 1990;96:323–334. [Google Scholar]
- McQueen-Mason SJ, Fry SC, Durachko DM, Cosgrove DJ. The relationship between xyloglucan endotransglycosylase and in vitro cell wall extension in cucumber hypocotyls. Planta. 1993;190:327–331. doi: 10.1007/BF00196961. [DOI] [PubMed] [Google Scholar]
- Mellersh DG, Heath MC. Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration. The Plant Cell. 2001;13:413–424. doi: 10.1105/tpc.13.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JM, Hilton HW, Clifford SC, Smith AC. The mechanical properties of lettuce: a comparison of some agronomic and postharvest effects. Journal of Materials Science. 2005;40:1101–1104. [Google Scholar]
- Nishitani K. Division of roles among members of the XTH gene family in plants. Plant Biosystems. 2005;139:98–101. [Google Scholar]
- Nishitani K, Tominaga T. Endo-xyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. Journal of Biological Chemistry. 1992;267:21058–21064. [PubMed] [Google Scholar]
- Palmer SJ, Davies WJ. An analysis of relative elemental growth rate, epidermal cell size and xyloglucan endotransglycosylase activity through the growing zone of ageing maize leaves. Journal of Experimental Botany. 1996;47:339–347. [Google Scholar]
- Pilling E, Höfte H. Feedback from the wall. Current Opinions in Plant Biology. 2003;6:611–616. doi: 10.1016/j.pbi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Pritchard J, Hetherington PR, Fry SC, Tomos AD. Xyloglucan endotransglycosylase activity, microfibril orientation and the profiles of cell wall properties along growing regions of maize roots. Journal of Experimantal Botany. 1993;44:1281–1289. [Google Scholar]
- Rose JKC, Braam J, Fry SC, Nishitani K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant and Cell Physiology. 2002;43:1421–1435. doi: 10.1093/pcp/pcf171. [DOI] [PubMed] [Google Scholar]
- Smith DL, Abbott JA, Gross KC. Down-regulation of tomato beta-galactosidase 4 results in decreased fruit softening. Plant Physiology. 2002;129:1755–1762. doi: 10.1104/pp.011025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street NR, Skogstrom O, Sjodin A, Tucker J, Rodriguez-Acosta M, Nilsson P, Jansson S, Taylor G. The genetics and genomics of drought response in Populus. The Plant Journal. 2006;48:321–341. doi: 10.1111/j.1365-313X.2006.02864.x. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Fry SC. Restructuring of wall-bound xyloglucan by transglycosylation in living plant cells. The Plant Journal. 2001;26:23–34. doi: 10.1046/j.1365-313x.2001.01005.x. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Fry SC, Pauly M, Höfte H, Verbelen J-P. XTH acts at the microfibril–matrix interface during cell elongation. Journal of Experimantal Botany. 2005;56:673–683. doi: 10.1093/jxb/eri048. [DOI] [PubMed] [Google Scholar]
- Vissenberg K, Martinez-Vilchez IM, Verbelen J-P, Miller JG, Fry SC. In vivo co-localization of xyloglucan endotransglycosylase activity and its donor substrate in the elongation zone of Arabidopsis roots. The Plant Cell. 2000;12:1229–1238. doi: 10.1105/tpc.12.7.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff C, Clarkson GJJ, Rothwell SD, Page A, Taylor G, Dixon MS. Characterization of cell death in bagged baby salad leaves. Postharvest Biology and Technology. 2007;46:150–159. [Google Scholar]
- Xu W, Purugganan MM, Polisensky DH, Antosiewicz DM, Fry SC, Braam J. Arabidopsis TCH4, regulated by hormones and the environment, encodes a xyloglucan endotransglycosylase. The Plant Cell. 1995;7:1555–1567. doi: 10.1105/tpc.7.10.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Campbell P, Vargheese AK, Braam J. The Arabidopsis XET-related gene family: environmental and hormonal regulation of expression. The Plant Journal. 1996;9:879–889. doi: 10.1046/j.1365-313x.1996.9060879.x. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, Rose JKC, Nishitani K. A surprising diversity and abundance of xyloglucan endotransglucosylase/hydrolases in rice. Classification and expression analysis. Plant Physiology. 2004;134:1088–1099. doi: 10.1104/pp.103.035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FZ. University of Southampton; 2006. The genetic basis of salad leaf processability. PhD thesis. [Google Scholar]
- Zhang FZ, Wagstaff C, Rae AM, et al. QTL for shelf life in lettuce co-locate with those for leaf biophysical properties but not for leaf developmental traits. Journal of Experimental Botany. 2007;58:1433–1449. doi: 10.1093/jxb/erm006. [DOI] [PubMed] [Google Scholar]