Abstract
Aims:
To determine the outcome of colorectal liver metastasis (CRLM) patients based on tumour burden, represented by tumour number and size, and tumour biology as assessed by an inflammatory response to tumour (IRT) and margin positivity.
Methods:
Data were collated from CRLM patients undergoing resection from January 1993 to March 2007. Patients were divided into: low (≤3 metastases and/or ≤3 cm); moderate (4–7 metastases and/or >3–≤5 cm); and high (≥8 metastases and/or >5 cm) tumour burden.
Results:
Seven hundred and five patients underwent resection, of which 154 (21.8%), 262 (37.2%) and 289 (41.0%) patients were in the low, moderate and high tumour burden groups, respectively. The 5-year disease-free (P < 0.001) and overall (P < 0.001) survival were significantly different between the groups. IRT (P < 0.001), extent of resection (P < 0.001) and margin (P < 0.001) also differed between the groups.
Sub-group analysis revealed that IRT was the only adverse predictor for disease-free and overall survival in the low group. In the moderate group, IRT predicted poorer disease-free survival on multi-variate analysis. In the high group, R1 resection and transfusion were predictors of poorer disease-free survival and age ≥65 years, R1 resection and IRT were adverse predictors of overall survival.
Conclusion:
Resection margin influenced the outcome of patients with high tumour burden, hence the importance of achieving clear margins. IRT influenced the outcome of patients with less aggressive disease.
Keywords: hepatectomy, liver metastases, colorectal, survival, resection margin
Introduction
Hepatic resection is the only treatment modality associated with long-term survival in patients with colorectal liver metastases (CRLM). Despite variability in criteria for patient selection, 5-year survival rates have ranged consistently from 25% to 58% with most recent series now reporting in excess of 50%.1–5 In contrast, the median survival of patients with untreated disease ranges from 6–12 months,6,7 and the addition of optimal chemotherapy regimens only improves their median survival to approximately 20 months.8–10
In 1986, Ekberg et al. concluded that resection for CRLM was only indicated in patients with less than four liver metastases including bilobar cases, no evidence of extra-hepatic disease and when a resection margin of at least 10 mm could be achieved.11 With a better understanding of hepatic segmental anatomy, refined haemostatic techniques,12,13‘down-sizing’ chemotherapy and portal vein embolization,14 more patients are being subjected to hepatic resection, including patients considered unresectable previously. The benefits of surveillance after resection of CRLM have been exemplified by studies that have reported up to 40% survival at 5-years following repeat hepatic resection for recurrence of CRLM, with acceptable morbidity and mortality rates.15–18 Recent data have suggested that if lung metastases of colorectal origin are resectable, 5-year survival after thoracotomy is similar to that observed in patients following resection of CRLM.19,20 These results reflect a more aggressive approach being adopted towards the treatment of metastatic colorectal disease. Although the selection criteria for resection of CRLM have expanded over the past two decades, there is still no consensus as to specific selection criteria for surgical resection in these patients.
Several clinico-pathological features such as: size of the largest hepatic metastasis; number of hepatic metastases; distribution of hepatic tumours; extent of hepatic resection; and status of resection margin have been identified as prognostic factors.21–24 Recent published literature from this unit has shown a correlation between the presence of pre-operative systemic inflammation, represented by the expression of C-reactive protein (CRP)25 and an elevation in neutrophil to lymphocyte ratio (NLR),26 with poorer cancer-specific survival in patients with CRLM. This suggests the presence of a host's systemic inflammatory response to a tumour (IRT), and may play a significant role in determining the ‘aggressiveness’ of tumour biology and prognosis. Nevertheless, the interplay of tumour biology and surgical technique remains to be elucidated.
The aim of the present study was to analyse the impact of well-established prognostic factors on outcome in patients undergoing hepatic resection for CRLM based on the extent of tumour burden defined by tumour number and size. Further analysis was conducted in these groups to determine the impact of surgical technique, represented by resection margin, and tumour biology as reflected by IRT, on outcome.
Patients and methods
Patients
Patients with CRLM undergoing hepatic resection at the Hepatobiliary Unit, St James's University Hospital (SJUH), Leeds, United Kingdom, during the 14-year period from January 1993 to March 2007, were identified from the hepatobiliary database. Patients who had primary hepatic resection during the study period were included in the analysis, whereas those undergoing repeat resections were excluded.
Demographic data included patient age, gender, disease presentation and laboratory analyses (white cell counts with differentials and CRP). The white cell and differential counts as well as CRP were taken on the day prior to surgery with none of the patients showing clinical symptoms or signs of sepsis. The neutrophil to lymphocyte ratio (NLR) was calculated from the differential count by dividing the absolute neutrophil count by the absolute lymphocyte count. A NLR ≥5 was considered raised in accordance with published literature.27 The unit does not routinely measure CRP pre-operatively, and in patients with a pre-operative CRP measured, a CRP level >10 mg/l was considered elevated.25 Systemic IRT was defined as an elevation in pre-operative NLR and/or CRP above the normal reference ranges. Pre-operative radiological assessment included a computed tomography (CT) scan of the thorax, abdomen and pelvis and magnetic resonance imaging (MRI) of the liver.
A subgroup of patients received neo-adjuvant Oxaliplatin-based chemotherapy in order to ‘down-size’ disease before resection. These patients received six cycles of chemotherapy followed by re-assessment prior to resection. According to the unit's protocol, all patients received adjuvant chemotherapy comprising of a 24-week treatment with 5-fluorouracil and folinic acid, unless they had underwent chemotherapy adjuvant to bowel resection within 12 months of the primary hepatic resection.
Surgery
Parenchymal transection was performed using the Cavi-Pulse Ultrasonic Surgical Aspirator (CUSA, Model 200T; Valley Laboratory, Boulder, CO, USA). Intra-operative ultrasound was performed to confirm the findings of pre-operative imaging and to assist in surgical planning. The number of hepatic (Couinaud's) segments resected was determined by the procedure performed as stated in the Brisbane nomenclature.28 In cases where multiple resections were performed at a single setting, the most extensive resection was considered the main procedure, with others listed as additional hepatic procedures. In the present study, the extent of hepatic resection was classified into two groups: less than hemi-hepatectomy and hemi-hepatectomy or more. Transfusion of blood products (packed red cells or whole blood) during surgery or during subsequent in-hospital stay after surgery was also recorded. Blood transfusion was indicated in patients with a haemoglobin less than 8 g/dl and symptomatic patients with a haemoglobin of 8–10 g/dl.
Histology
Histopathological data regarding the resected specimen were collated. This included: tumour size in maximum diameter; tumour number; and status of resection margin. R0 resection was defined as no microscopic evidence of tumour at or within 1 mm of the margin. The extent of tumour burden in patients with CRLM was based on tumour number and size in surgical specimens, and patients were divided into three groups: low (≤3 metastases and/or ≤3 cm); moderate (4–7 metastases and/or >3–5 cm); and high (≥8 metastases and/or >5 cm) tumour burden.
Follow-up
Patients were followed up at specialist hepatobiliary clinics. After an initial post-operative review at 1 month, all patients were examined in the outpatient clinic at 3, 6, 12, 18 and 24 months and annually thereafter. At each clinic review, blood tests were performed for liver function tests and CEA levels. Patients underwent 3-monthly CT scans of the thorax, abdomen and pelvis during the first 2 post-operative years, followed by 6 monthly thereafter for 3 years (year 3–5). A CT scan was next performed annually at 7 to 10 years of follow-up. Liver MRI was used to define suspicious lesions demonstrated on CT or in cases of negative CT with rising tumour markers. Development of symptoms prompted an earlier review than scheduled. Recurrence was defined as the development of new liver metastases or metastases elsewhere on CT or MRI after resection. Overall and disease-free survival data were recorded.
Statistical analysis
Categorical data were presented as frequency and proportions (%) and were analysed using the Pearson's χ2 test. The Kaplan–Meier method was used to assess the survival and disease recurrence rate. Univariate analysis was performed to assess for a significant difference in clinico-pathological characteristics that influenced disease recurrence after curative resection. A multi-variate analysis was performed using Cox regression (Step-wise forward model) for variables significant on univariate analysis. All statistical analyses were performed using the SPSS for WindowsΣ version 15.0 (SPSS Inc., Chicago, IL, USA), and statistical significance was taken at the 5% level.
Results
Demographics
During the study period, 705 patients underwent primary hepatic resections for CRLM at SJUH, of which 434 (61.6%) patients developed recurrence and 363 (51.5%) patients died. The median follow-up period of the patients currently alive was 24 months (range: 6–168 months).
The median age at diagnosis was 63 years (range: 26–87 years). Synchronous colon and liver disease was present in 298 (42.3%) patients. Demographics and clinical factors are shown in Table 1.
Table 1.
Clinical, operative and pathological characteristics with respect to tumour burden
| Prognostic factors |
Tumour burden |
P-value | ||
|---|---|---|---|---|
| Low (n= 154) | Moderate (n= 262) | High (n= 289) | ||
| Age (years) | ||||
| <65 | 74 (48.1%) | 140 (53.4%) | 148 (51.2%) | 0.569 |
| ≥65 | 80 (51.9%) | 122 (46.6%) | 141 (48.8%) | |
| Gender | ||||
| Male | 92 (59.7%) | 167 (63.7%) | 187 (64.7%) | 0.575 |
| Female | 62 (40.3%) | 95 (36.3%) | 102 (35.3%) | |
| Presentation | ||||
| Synchronous | 60 (39.0%) | 99 (37.8%) | 139 (48.1%) | 0.164 |
| Metachronous | 94 (61.0%) | 163 (62.2%) | 150 (51.9%) | |
| IRT* | ||||
| Yes | 16 (14.0%) | 33 (16.3%) | 67 (29.5%) | <0.001 |
| No | 98 (86.0%) | 169 (83.7%) | 160 (70.5%) | |
| Extent of resection | ||||
| Less than hemi-hepatectomy | 102 (66.2%) | 100 (38.2%) | 61 (21.1%) | <0.001 |
| Hemi-hepatectomy or more | 52 (33.8%) | 162 (61.8%) | 228 (78.9%) | |
| Blood transfusion | 32 (20.8%) | 61 (23.3%) | 56 (19.4%) | 0.529 |
| Morbidity | 59 (38.3%) | 102 (38.9%) | 130 (45.0%) | 0.248 |
| Resection margin | ||||
| R1 (Involved) | 35 (22.7%) | 83 (31.7%) | 140 (48.4%) | <0.001 |
| R0 (≥1 mm) | 119 (77.3%) | 179 (68.3%) | 149 (51.6%) | |
IRT was available in 543 (77.0%) patients, with 114 (74.0%), 202 (77.1%) and 227 (78.5%) patients, respectively, in the low, moderate and high tumour burden groups.
Anatomically based resections were performed in 524 (74.3%) patients and 183 (26.0%) patients had a combination of an anatomical with a non-anatomical resection (Table 2). There were 25 (3.5%) post-operative deaths.
Table 2.
Operative details of patients in this study
| Operative data | |
|---|---|
| Major hepatic resection (Hemi-hepatectomy or more) | 442 (62.7%) |
| Left hemihepatectomy (resection of segments 2,3,4 +/− 1) | 20 (4.5%) |
| + Non-anatomical resection | 15 (3.4%) |
| Right hemihepatectomy (resection of segments 5,6,7,8 +/− 1) | 143 (32.4%) |
| + Non-anatomical resection | 69 (15.6%) |
| Left trisectionectomy (resection of segments 2,3,4,5,8 +/− 1) | 35 (7.9%) |
| + Non-anatomical resection | 14 (3.2%) |
| Right trisectionectomy (resection of segments 4,5,6,7,8 +/− 1) | 102 (23.0%) |
| + Non-anatomical resection | 44 (10.0%) |
| Minor hepatic resection (Less than hemi-hepatectomy) | 263 (37.3%) |
| Left lateral sectionectomy (resection of segments 2 and 3) | 34 (12.9%) |
| + Non-anatomical resection | 29 (11.0%) |
| + Right posterior sectionectomy | 4 (1.5%) |
| + Transverse hepatectomy | 1 (0.4%) |
| Right posterior sectionectomy (resection of segment 6 and 7) | 5 (1.9%) |
| + Non-anatomical resection | 6 (2.3%) |
| Transverse hepatectomy (resection of segment 4B, 5 and 6) | 2 (0.8%) |
| + Non-anatomical resection | 1 (0.4%) |
| Non-anatomical resection/Metastectomy | 181 (68.8%) |
| Total | 705 |
There were 456 (64.7%) patients with two or more tumours resected [median tumours resected per patient = 2 (range: 1–21)]. The majority of tumours were less than 50 mm in maximum diameter (n= 400, 56.7%).
Clinico-pathological factors and outcome based on tumour burden
Patients in the high tumour burden group were more likely to have a systemic IRT, have a hemi-hepatectomy or more and have tumour involvement at the resection margin (Table 1) than patients in either of the other groups.
The disease-free survival in the low, moderate and high tumour burden groups is shown in Fig. 1. There was also a significant difference in the 1-, 3- and 5-year overall survival in these three groups: 95.3%, 89.1% and 80.5%; 72.9%, 51.2% and 41.5%; and 50.8%, 32.4% and 27.0%, respectively (P < 0.001).
Figure 1.
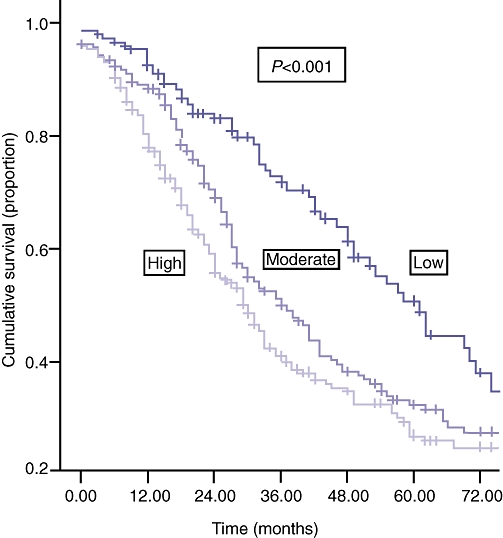
| Numbers at risk | |||||||
|---|---|---|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| Low | 153 | 130 | 90 | 64 | 47 | 33 | 18 |
| Moderate | 261 | 211 | 134 | 80 | 56 | 41 | 28 |
| High | 289 | 216 | 131 | 81 | 53 | 33 | 23 |
Predictors of disease-free and overall survival
Several clinical, operative and pathological factors affecting disease-free survival were identified on both univariate and multivariate analysis and are shown in Table 3. Similarly predictors of poorer overall survival are shown in Table 4.
Table 3.
Statistical analysis of prognostic factors with respect to disease-free survival
| Prognostic factors for disease-free survival | Univariate analysis | Multivariate analysis | Hazard Ratios | Confidence Interval |
|---|---|---|---|---|
| Age ≥65 years (n= 343, 48.7%) | 0.324 | NA | NA | NA |
| Female gender (n= 259, 36.7%) | 0.956 | NA | NA | NA |
| Synchronous presentation (n= 298, 42.3%) | 0.331 | NA | NA | NA |
| IRT (n= 116, 21.4%)* | <0.001 | <0.001 | 1.551 | 1.217–1.975 |
| Hemi-hepatectomy or more (n= 442, 62.7%) | 0.046 | 0.413 | 0.901 | 0.701–1.157 |
| Blood transfusion (n= 149, 21.1%) | 0.007 | 0.002 | 1.473 | 1.155–1.877 |
| High tumour burden (n= 289, 41%) | <0.001 | 0.023 | 1.205 | 1.026–1.416 |
| R1 margin (n= 258, 36.6%) | <0.001 | 0.001 | 1.487 | 1.185–1.865 |
NA, not applicable; IRT, inflammatory response to tumour.
IRT was available in 543 (77.0%) patients.
Table 4.
Statistical analysis of prognostic factors with respect to overall survival
| Prognostic factors for overall survival | Univariate analysis | Multivariate analysis | Hazard Ratios | Confidence Interval |
|---|---|---|---|---|
| Age ≥65 years (n= 343, 48.7%) | 0.104 | NA | NA | NA |
| Female gender (n= 259, 36.7%) | 0.948 | NA | NA | NA |
| Synchronous presentation (n= 298, 42.3%) | 0.306 | NA | NA | NA |
| IRT (n= 116, 21.4%)* | <0.001 | <0.001 | 1.707 | 1.309–2.277 |
| Hemi-hepatectomy or more (n= 442, 62.7%) | 0.024 | 0.733 | 0.951 | 0.714–1.267 |
| Blood transfusion (n= 149, 21.1%) | 0.508 | NA | NA | NA |
| High tumour burden (n= 289, 41%) | <0.001 | 0.005 | 1.303 | 1.084–1.568 |
| <0.001 | 0.011 | 1.408 | 1.082–1.833 | |
| R1 marginβ (n= 258, 36.6%) | <0.001 | 0.011 | 1.408 | 1.082–1.833 |
NA, not applicable; IRT, inflammatory response to tumour.
IRT was available in 543 (77.0%) patients.
Impact of systemic IRT and resection margin based on tumour burden
For patients in the low tumour burden group, raised pre-operative IRT was the only adverse predictor of disease-free [median (range) survival of no IRT was 48 (2–108) months vs. median (range) survival of IRT was 15 (3–72) months, P < 0.001] and overall survival (Fig. 2).
Figure 2.
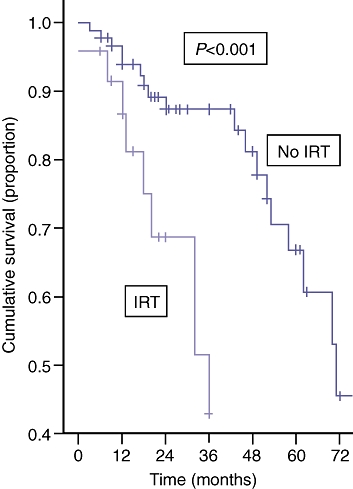
| Numbers at risk | |||||||
|---|---|---|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| IRT | 24 | 19 | 14 | 6 | 3 | 2 | 1 |
| No IRT | 89 | 74 | 60 | 35 | 26 | 18 | 6 |
With respect to the moderate tumour burden group, factors associated with poorer disease-free and overall survival are shown in Table 5 and Fig. 3, respectively.
Table 5.
Statistical analysis of prognostic factors influencing disease-free survival in patients with moderate tumour burden
| Prognostic factors (disease-free survival) n= 262 | Univariate analysis | Multivariate analysis | Hazard Ratios | Confidence Interval |
|---|---|---|---|---|
| Age ≥65 years (n= 122, 46.6%) | 0.142 | NA | NA | NA |
| Female gender (n= 95, 36.3%) | 0.311 | NA | NA | NA |
| Synchronous presentation (n= 99, 37.8%) | 0.251 | NA | NA | NA |
| IRT (n= 33, 16.3%)* | <0.001 | <0.001 | 2.023 | 1.347–3.037 |
| Hemi-hepatectomy or more (n= 162, 61.8%) | 0.437 | NA | NA | NA |
| Blood transfusion (n= 61, 23.3%) | 0.006 | 0.045 | 1.465 | 1.008–2.130 |
| R1 margin (n= 83, 31.7%) | 0.005 | 0.115 | 1.343 | 0.930–1.939 |
NA, not applicable; IRT, inflammatory response to tumour.
Figure 3.
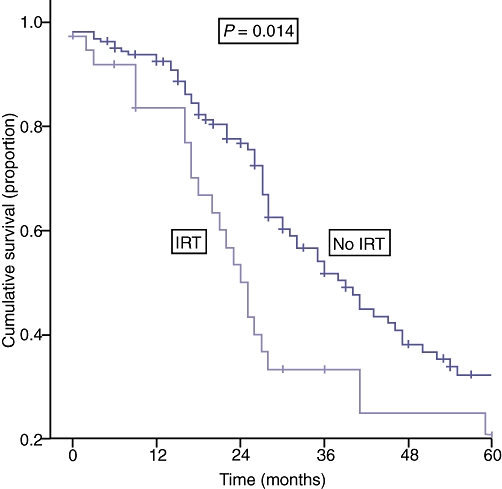
| Numbers at risk | ||||||
|---|---|---|---|---|---|---|
| Year | 0 | 1 | 2 | 3 | 4 | 5 |
| IRT | 38 | 28 | 16 | 9 | 6 | 5 |
| No IRT | 164 | 136 | 83 | 44 | 28 | 20 |
Prognostic factors for disease-free and overall survival in patients who fulfilled the criteria for high tumour burden are shown in Table 6a and b, respectively. The effect of pre-operative IRT and resection margin status on overall survival for those patients with high tumour burden are shown in Fig. 4a and b, respectively. Table 7 summarizes the independent clinico-pathological variables that significantly influenced outcome based on the extent of tumour burden.
Table 6.
Statistical analysis of prognostic factors influencing (a) disease-free and (b) overall survival in patients within the high tumour burden criteria
| (A) | ||||
|---|---|---|---|---|
| Prognostic factors for disease-free survival | Univariate analysis | Multivariate analysis | Hazard Ratios | Confidence Interval |
| n= 289 | ||||
| Age ≥65 years (n= 141, 48.8%) | 0.983 | NA | NA | NA |
| Female gender (n= 102, 35.3%) | 0.795 | NA | NA | NA |
| Synchronous presentation (n= 139, 48.1%) | 0.562 | NA | NA | NA |
| IRT (n= 67, 29.5%) | 0.257 | NA | NA | NA |
| Hemi-hepatectomy or more (n= 228, 78.9%) | 0.381 | NA | NA | NA |
| Blood transfusion (n= 56, 19.4%) | 0.004 | 0.002 | 1.710 | 1.211–2.413 |
| R1 margin (n= 140, 48.4%) | <0.001 | <0.001 | 1.653 | 1.239–2.205 |
| (B) | ||||
| Prognostic factors for overall survival | Univariate analysis | Multivariate analysis | Hazard Ratios | Confidence Interval |
| n = 289 | ||||
| Age ≥65 years (n= 141, 48.8%) | 0.018 | 0.002 | 0.558 | 0.387–0.804 |
| Female gender (n= 102, 35.3%) | 0.617 | NA | NA | NA |
| Synchronous presentation (n= 139, 48.1%) | 0.744 | NA | NA | NA |
| IRT (n= 67, 29.5%)* | 0.026 | 0.028 | 1.513 | 1.047–2.186 |
| Hemi-hepatectomy or more (n= 228, 78.9%) | 0.584 | NA | NA | NA |
| Blood transfusion (n= 56, 19.4%) | 0.361 | NA | NA | NA |
| R1 margin (n= 140, 48.4%) | <0.001 | 0.009 | 1.613 | 1.124–2.315 |
NA, not applicable; IRT, Inflammatory response to tumour.
Figure 4.
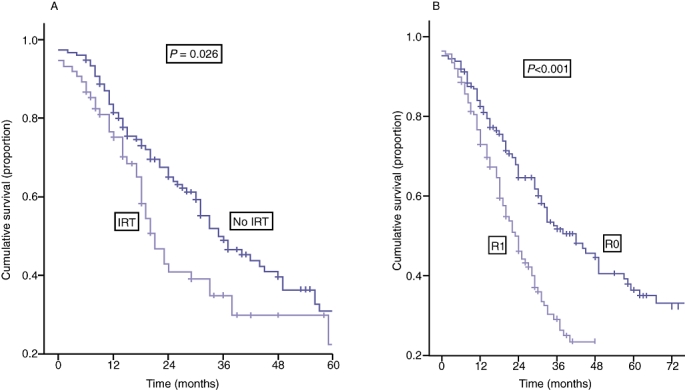
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Numbers at risk | |||||||
| Year | 0 | 1 | 2 | 3 | 4 | 5 | |
| IRT | 75 | 52 | 22 | 16 | 10 | 6 | |
| No IRT | 152 | 116 | 75 | 48 | 29 | 17 | |
| (B) | |||||||
| Numbers at risk | |||||||
| Year | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
| R0 | 149 | 114 | 79 | 55 | 38 | 26 | 19 |
| R1 | 140 | 102 | 47 | 26 | 15 | 7 | 4 |
Table 7.
Summary of independent clinico-pathological factors that significantly influenced disease-free and overall survival with respect to extent of tumour burden
| Prognostic factors | Tumour burden | ||
|---|---|---|---|
| Low | Moderate | High | |
| Disease-free survival | IRT | IRT | Blood transfusion |
| Blood transfusion | Resection margin | ||
| Overall survival | IRT | IRT | Age |
| IRT | |||
| Resection margin |
IRT, inflammatory response to tumour.
Discussion
Hepatic resection for CRLM has consistently achieved good long-term disease-free and overall survival based on absence of recurrence29,30 and is in stark contrast to the outcome of patients with unresectable disease.31 Although the selection criteria for resection have expanded over the past two decades, little exists by way of a consensus for selecting patients who would benefit most from surgery and adjuvant chemotherapy.
Patient selection for liver resection is currently based on resectability of all macroscopic disease with clear margins while leaving sufficient residual functioning liver volume, decisions dependent on results of cross-sectional imaging with CT and/or MRI. These criteria apply to solitary, multiple and bilobar disease as well as patients with extra-hepatic disease that is confined to the lungs, spleen or adrenal glands.32 The selection of patients with CRLM for resection with curative intent based on these guidelines differ significantly compared with the one proposed by Ekberg et al.11 which is a reflection of the accumulating evidence supporting the survival benefit from resection as a result of decreased peri-operative risk and the improvement in accuracy of imaging.
Nevertheless, despite numerous studies documenting the importance of prognostic factors including: patient demographics; tumour characteristics; operative factors;22–24,33,34 and more recently tumour-host biology35,36 in relation to disease recurrence and survival after resection of CRLM, these prognostic indicators remain inconsistent. This had lead to various authors developing scoring systems to refine candidacy for selection and categorize patients for clinical management. However, Zakaria et al.37 recently concluded that the application of risk scoring systems had limited clinical value after analysis of their own scoring system and other published scoring systems on their dataset.
The fact that prognostic factors such as tumour number and size have been inconsistently identified suggests that these factors do influence the outcome of patients depending on their tumour burden at presentation but may not be the only factors worthy of consideration. Selection criteria based on tumour number and size is an appropriate reflection of disease burden but does not necessarily take into account the disease biology. Hence, the aim of this study was to determine the outcome of patients based on the extent of disease burden that consisted of tumour number and size, but which also took into account the effect of tumour biology by considering the tumour's systemic IRT and the impact of surgical technique as represented by resection margin status.
The data from the current study were extracted from a large, single-institution experience. The primary finding of this study was that patients in the high tumour burden group (higher tumour number and larger size) had a significantly higher rate of R1 resections and were more likely to express a systemic IRT. The association of the expression of a systemic IRT with a greater tumour burden is likely to be a reflection of more aggressive disease. This may also account for the higher R1 margin present in this group as the greater the number of metastases, the more difficult it is to obtain a R0 resection.
With respect to outcome, the current study observed that systemic IRT, blood transfusion requirements, tumour burden and resection margin status were all independent predictors of disease-free survival. Similarly, the presence of a systemic IRT, extensive tumour burden and R1 resection margin were associated with a poorer overall survival. The findings in the present study are in agreement with the current literature, as discussed below.
Both Rosen et al.38 and Kooby and colleagues39 observed that patients who did not receive a blood transfusion had a significantly better outcome as assessed by univariate analysis.38 However, on multi-variate analysis incorporating other significant clinico-pathological co-variates, blood transfusion failed to reach significance in either study. In this series, blood transfusion requirement was an independent predictor of tumour recurrence, but not overall survival. One suggested underlying mechanism of the effect of blood transfusion on survival is the alteration in the immune function of the recipient leading to immuno-suppression;40,41 an environment that may promote cancer recurrence.
There is increasing evidence correlating tumour biology and the patient's systemic IRT with prognosis. The current series showed that there were significantly more patients with disease recurrence and adverse survival that exhibited an IRT. This suggests that CRLM patients that express an IRT may have an ‘aggressive’ tumour biology profile, and thus, are more likely to develop tumour recurrence. Nevertheless, the association between inflammation, CRP and NLR with poor prognosis is complex42 and remains to be elucidated. A possible explanation is that a systemic IRT may be indicative of a favourable environment that includes pro-angiogenic factors such as vascular endothelial growth factor (VEGF), for the development of metastases.43 Alternatively, systemic inflammation may reflect a poorer host immune response to tumour, which is lymphocyte dependent. This may lead to lymphocytopaenia and a weak infiltration of lymphocytes at the periphery of the tumour,35 thereby worsening their prognosis.
The role of margin status as a predictor of recurrence after resection for CRLM is controversial. Recently, Bodingbauer et al. observed that resection margin and size of margin width did not correlate significantly with survival after resection for CRLM.44 In a series of 1019 patients, Are and co-investigators demonstrated that a resection margin >1 cm was an independent predictor of survival after resection for CRLM.45 However, Figueras et al. showed that a margin width <1 cm in patients who underwent resection for CRLM did not significantly influence recurrent disease in a cohort of 609 patients.46 In the present series, a clear resection margin, defined as no microscopic evidence of tumour at or within 1 mm of the margin, was an independent predictor of both disease-free and overall survival.
The impact of systemic IRT and resection margin on outcome after resection of CRLM merits further discussion. On sub-group analysis, systemic IRT was an adverse predictor for patients with low and moderate tumour burden. Hence, in these groups, other clinico-pathological factors exert less influence with respect to survival. As a result of the differences in results observed with respect to resection margin between published studies, it may be that only a selected group of patients undergoing resection for CRLM are influenced by a clear margin. In the present series, sub-group analysis showed that only patients with high tumour burden benefited from a R0 margin. This could be due to the fact that these patients have an aggressive tumour profile and it is crucial that complete tumour clearance is obtained. Hence, for patients who fulfil the high tumour burden criteria, ‘down-sizing’ chemotherapy should certainly be considered prior to resection to aid in achieving a clear resection margin. Nevertheless, with the increased use of chemotherapy, there is an increase in prevalence of patients undergoing hepatic resection with a background of chemotherapy-related injury, such as steato-hepatitis47 and sinusoidal obstruction syndrome.48 In such cases, the quality, rather than quantity, of the remnant liver becomes an important issue to consider prior to extensive resection. For patients with less aggressive tumour burden, other clinic-pathological factors are significantly more likely to influence outcome. In this favourable group of patients, adjuvant chemotherapy is likely to treat residual disease, in particular cases of R1 resection. Further understanding is required in the area of systemic IRT and oncological outcomes which may lead to the development of pre-operative therapeutic targets that influence the expression of tumour-related inflammatory responses and this may improve survival outcomes after resection for CRLM.
In conclusion, the extent of tumour burden based on tumour number and size significantly influenced the outcome after hepatic resection for CRLM. By extending the criteria for surgery, resection margin influenced the outcome of patients in the high tumour burden group, hence the importance of achieving good clearance in these patients. Systemic IRT tend to influence the outcome of patients with less aggressive disease.
Acknowledgments
The authors thank Thomas Fitzgerald for maintaining the hepatobiliary database and providing the data set used in this study.
Conflicts of interest
None declared.
References
- 1.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–722. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwatsuki S, Dvorchik I, Madariaga JR, Marsh JW, Dodson F, Bonham AC, et al. Hepatic resection for metastatic colorectal adenocarcinoma: a proposal of a prognostic scoring system. J Am Coll Surg. 1999;189:291–299. doi: 10.1016/s1072-7515(99)00089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaffe BM, Donegan WL, Watson F, Spratt JS., Jr Factors influencing survival in patients with untreated hepatic metastases. Surg Gynecol Obstet. 1968;127:1–11. [PubMed] [Google Scholar]
- 7.Chang AE, Schneider PD, Sugarbaker PH, Simpson C, Culnane M, Steinberg SM. A prospective randomized trial of regional versus systemic continuous 5-fluorodeoxyuridine chemotherapy in the treatment of colorectal liver metastases. Ann Surg. 1987;206:685–693. doi: 10.1097/00000658-198712000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 9.Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 10.De Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 11.Ekberg H, Tranberg KG, Andersson R, Lundstedt C, Hagerstrand I, Ranstam J, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg. 1986;73:727–731. doi: 10.1002/bjs.1800730917. [DOI] [PubMed] [Google Scholar]
- 12.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 13.Heriot AG, Karanjia ND. A review of techniques for liver resection. Ann R Coll Surg Engl. 2002;84:371–380. doi: 10.1308/003588402760978148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaeck D, Bachellier P, Nakano H, Oussoultzoglou E, Weber JC, Wolf P, et al. One or two-stage hepatectomy combined with portal vein embolization for initially nonresectable colorectal liver metastases. Am J Surg. 2003;185:221–229. doi: 10.1016/s0002-9610(02)01373-9. [DOI] [PubMed] [Google Scholar]
- 15.Muratore A, Polastri R, Bouzari H, Vergara V, Ferrero A, Capussotti L. Repeat hepatectomy for colorectal liver metastases: A worthwhile operation? J Surg Oncol. 2001;76:127–132. doi: 10.1002/1096-9098(200102)76:2<127::aid-jso1023>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki S, Sakaguchi T, Yokoi Y, Kurachi K, Okamoto K, Okumura T, et al. Impact of repeat hepatectomy on recurrent colorectal liver metastases. Surgery. 2001;129:421–428. doi: 10.1067/msy.2001.112486. [DOI] [PubMed] [Google Scholar]
- 17.Yamada H, Katoh H, Kondo S, Okushiba S, Morikawa T. Repeat hepatectomy for recurrent hepatic metastases from colorectal cancer. Hepatogastroenterology. 2001;48:828–830. [PubMed] [Google Scholar]
- 18.Nishio H, Hamady ZZ, Malik HZ, Fenwick S, Rajendra Prasad K, Toogood GJ, et al. Outcome following repeat liver resection for colorectal liver metastases. Eur J Surg Oncol. 2007;33:729–734. doi: 10.1016/j.ejso.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:112–120. doi: 10.1002/bjs.4370. [DOI] [PubMed] [Google Scholar]
- 20.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Prauer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91:1066–1071. doi: 10.1002/bjs.4602. [DOI] [PubMed] [Google Scholar]
- 21.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol. 2006;13:668–676. doi: 10.1245/ASO.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 23.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 24.Menon KV, Al-Mukhtar A, Aldouri A, Prasad RK, Lodge PA, Toogood GJ. Outcomes after major hepatectomy in elderly patients. J Am Coll Surg. 2006;203:677–683. doi: 10.1016/j.jamcollsurg.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Wong VK, Malik HZ, Hamady ZZ, Al-Mukhtar A, Gomez D, Prasad KR, et al. C-reactive protein as a predictor of prognosis following curative resection for colorectal liver metastases. Br J Cancer. 2007;96:222–225. doi: 10.1038/sj.bjc.6603558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55–60. doi: 10.1016/j.ejso.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 28.Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg. 2005;12:351–355. doi: 10.1007/s00534-005-0999-7. [DOI] [PubMed] [Google Scholar]
- 29.Jaeck D, Bachellier P, Guiguet M, Boudjema K, Vaillant JC, Balladur P, et al. Long-term survival following resection of colorectal hepatic metastases. Association Francaise de Chirurgie. Br J Surg. 1997;84:977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 30.Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575–4580. doi: 10.1200/JCO.2007.11.0833. [DOI] [PubMed] [Google Scholar]
- 31.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 32.Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, et al. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55(Suppl 3):iii1–iii8. doi: 10.1136/gut.2006.098053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka K, Shimada H, Miura M, Fujii Y, Yamaguchi S, Endo I, et al. Metastatic tumor doubling time: most important prehepatectomy predictor of survival and nonrecurrence of hepatic colorectal cancer metastasis. World J Surg. 2004;28:263–270. doi: 10.1007/s00268-003-7088-3. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi S, Konishi M, Nakagohri T, Gotohda N, Saito N, Kinoshita T. Short time to recurrence after hepatic resection correlates with poor prognosis in colorectal hepatic metastasis. Jpn J Clin Oncol. 2006;36:368–375. doi: 10.1093/jjco/hyl027. [DOI] [PubMed] [Google Scholar]
- 35.Canna K, McArdle PA, McMillan DC, McNicol AM, Smith GW, McKee RF, et al. The relationship between tumour T-lymphocyte infiltration, the systemic inflammatory response and survival in patients undergoing curative resection for colorectal cancer. Br J Cancer. 2005;92:651–654. doi: 10.1038/sj.bjc.6602419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crumley AB, McMillan DC, McKernan M, Going JJ, Shearer CJ, Stuart RC. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer. 2006;94:1568–1571. doi: 10.1038/sj.bjc.6603150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zakaria S, Donohue JH, Que FG, Farnell MB, Schleck CD, Ilstrup DM, et al. Hepatic resection for colorectal metastases: value for risk scoring systems? Ann Surg. 2007;246:183–191. doi: 10.1097/SLA.0b013e3180603039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen CB, Nagorney DM, Taswell HF, Helgeson SL, Ilstrup DM, Van Heerden JA, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg. 1992;216:493–504. doi: 10.1097/00000658-199210000-00012. discussion 504–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. discussion 869–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghio M, Contini P, Mazzei C, Brenci S, Barberis G, Filaci G, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93:1770–1777. [PubMed] [Google Scholar]
- 41.Opelz G, Terasaki PI. Prolongation effect of blood transfusions on kidney graft survival. Transplantation. 1976;22:380–383. doi: 10.1097/00007890-197610000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Gunter MJ, Stolzenberg-Solomon R, Cross AJ, Leitzmann MF, Weinstein S, Wood RJ, et al. A prospective study of serum C-reactive protein and colorectal cancer risk in men. Cancer Res. 2006;66:2483–2487. doi: 10.1158/0008-5472.CAN-05-3631. [DOI] [PubMed] [Google Scholar]
- 43.Xavier P, Belo L, Beires J, Rebelo I, Martinez-De-Oliveira J, Lunet N, et al. Serum levels of VEGF and TNF-alpha and their association with C-reactive protein in patients with endometriosis. Arch Gynecol Obstet. 2006;273:227–231. doi: 10.1007/s00404-005-0080-4. [DOI] [PubMed] [Google Scholar]
- 44.Bodingbauer M, Tamandl D, Schmid K, Plank C, Schima W, Gruenberger T. Size of surgical margin does not influence recurrence rates after curative liver resection for colorectal cancer liver metastases. Br J Surg. 2007;94:1133–1138. doi: 10.1002/bjs.5762. [DOI] [PubMed] [Google Scholar]
- 45.Are C, Gonen M, Zazzali K, Dematteo RP, Jarnagin WR, Fong Y, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg. 2007;246:295–300. doi: 10.1097/SLA.0b013e31811ea962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Figueras J, Burdio F, Ramos E, Torras J, Llado L, Lopez-Ben S, et al. Effect of subcentimeter nonpositive resection margin on hepatic recurrence in patients undergoing hepatectomy for colorectal liver metastases. Evidences from 663 liver resections. Ann Oncol. 2007;18:1190–1195. doi: 10.1093/annonc/mdm106. [DOI] [PubMed] [Google Scholar]
- 47.Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 48.Rubbia-Brandt L, Audard V, Sartoretti P, Roth AD, Brezault C, Le Charpentier M, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]


