Abstract
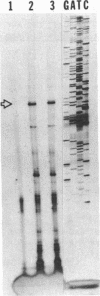
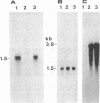
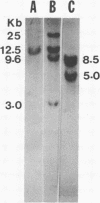
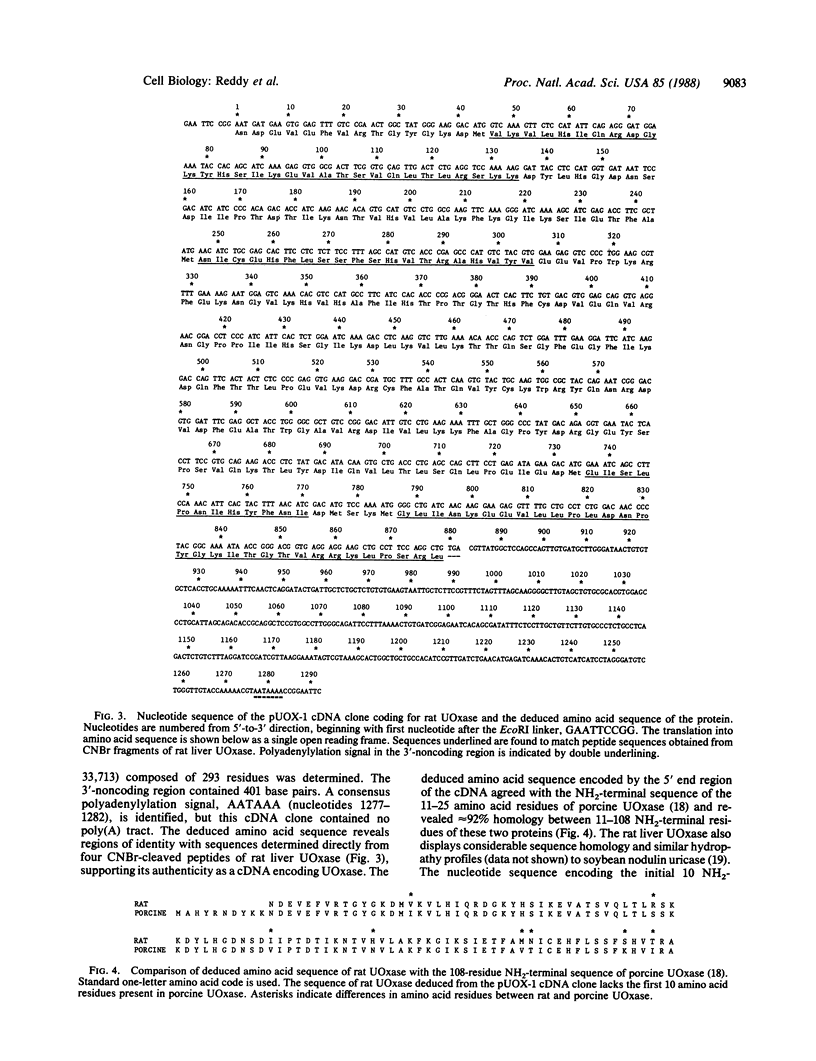
Urate oxidase (UOxase; urate:oxygen oxidoreductase, EC 1.7.3.3), which catalyzes the oxidation of uric acid to allantoin, is present in most mammals but is absent in humans and certain primates. A cDNA clone for UOxase containing an insert of 1.3 kilobases (kb) was isolated from a lambda gt11 cDNA library prepared from rat liver mRNA. This recombinant clone with a 1283-nucleotide insert has sequence for 97% of the coding region together with 401 nucleotides of the 3'-untranslated region of the mRNA. The identity of UOxase cDNA clone was verified by analyzing the fusion protein, immunocytochemical localization with epitope-selected antibody, and hybrid-select translation analysis and by comparing sequences of four CNBr-cleaved peptides of the protein. Blot analysis revealed that the probe hybridizes to a single 1.5-kb mRNA species in the rat liver and a transplantable hepatocellular carcinoma. No UOxase mRNA was detected in 11 nonhepatic tissues of rat, suggesting tissue specificity of expression of this UOxase gene. Blot analysis of RNA from livers of rats treated with a peroxisome proliferator showed 2- to 3-fold increase in UOxase mRNA content, whereas the fatty acyl-CoA oxidase mRNA increased over 30-fold. Southern blot analysis of restriction enzyme digests of rat DNA suggests that there is a single copy of UOxase gene. Analysis of human genomic DNA revealed restriction fragments that are homologous to rat UOxase cDNA, although no UOxase mRNA was detected in human liver.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Reddy J. K. Peroxisomes (microbodies) in cell pathology. Int Rev Exp Pathol. 1984;26:45–84. [PubMed] [Google Scholar]
- Ito M., Suzuki M., Takagi Y. Nucleotide sequence of cDNA and predicted amino acid sequence of rat liver uricase. Eur J Biochem. 1988 Apr 15;173(2):459–463. doi: 10.1111/j.1432-1033.1988.tb14021.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Lee C. C., Wu X. W., Gibbs R. A., Cook R. G., Muzny D. M., Caskey C. T. Generation of cDNA probes directed by amino acid sequence: cloning of urate oxidase. Science. 1988 Mar 11;239(4845):1288–1291. doi: 10.1126/science.3344434. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nguyen T., Zelechowska M., Foster V., Bergmann H., Verma D. P. Primary structure of the soybean nodulin-35 gene encoding uricase II localized in the peroxisomes of uninfected cells of nodules. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5040–5044. doi: 10.1073/pnas.82.15.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Ozasa H., Hashimoto T. Molecular cloning of cDNA for rat acyl-CoA oxidase. J Biol Chem. 1984 Feb 25;259(4):2031–2034. [PubMed] [Google Scholar]
- Osumi T., Ozasa H., Miyazawa S., Hashimoto T. Molecular cloning of cDNA for rat liver catalase. Biochem Biophys Res Commun. 1984 Jul 31;122(2):831–837. doi: 10.1016/s0006-291x(84)80109-6. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Azarnoff D. L., Hignite C. E. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980 Jan 24;283(5745):397–398. doi: 10.1038/283397a0. [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Goel S. K., Nemali M. R., Carrino J. J., Laffler T. G., Reddy M. K., Sperbeck S. J., Osumi T., Hashimoto T., Lalwani N. D. Transcription regulation of peroxisomal fatty acyl-CoA oxidase and enoyl-CoA hydratase/3-hydroxyacyl-CoA dehydrogenase in rat liver by peroxisome proliferators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1747–1751. doi: 10.1073/pnas.83.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda N., Reddy M. K., Hashimoto T., Rao M. S., Reddy J. K. Tissue specificity and species differences in the distribution of urate oxidase in peroxisomes. Lab Invest. 1988 Jan;58(1):100–111. [PubMed] [Google Scholar]
- Weinberger C., Hollenberg S. M., Ong E. S., Harmon J. M., Brower S. T., Cidlowski J., Thompson E. B., Rosenfeld M. G., Evans R. M. Identification of human glucocorticoid receptor complementary DNA clones by epitope selection. Science. 1985 May 10;228(4700):740–742. doi: 10.1126/science.2581314. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]