Abstract
Background:
This experimental study was designed to determine if Helicobacter spp. contribute to benign gallbladder disease using polymerase chain reaction (PCR) methods.
Methods:
Patients with benign gallbladder disease scheduled for elective cholecystectomy at New York University Langone Medical Center were recruited from February to May 2008. Bile, gallbladder tissue and gallstones were collected. DNA was isolated from these specimens and amplified via PCR using C97F and C98R primers specific for Helicobacter spp. Appropriate positive and negative controls were used. Products were analysed with agarose gel electrophoresis, sequenced and results aligned using sequencher. Plasma was collected for detection of anti-Helicobacter pylori antibodies via enzyme-linked immunosorbent assay.
Results:
Of 36 patients, 12 patients' bile and/or tissue were positive for Helicobacter spp. by PCR. Species were most homologous with H. pylori, although other Helicobacter spp. were suggested. Six of 12 patients demonstrated anti-Helicobacter antibodies in plasma, suggesting that the remaining six might have demonstrated other species besides H. pylori. Four of six plasma samples with anti-Helicobacter antibodies were anti-CagA (cytotoxin associated gene) negative.
Discussion:
Helicobacter spp. can be detected in bile and gallbladder tissue of patients with benign gallbladder disease. The contribution of these bacteria to the pathophysiology of gallbladder disease and gallstone formation requires further study.
Keywords: Helicobacter, gallstones, gallbladder, cholecystitis
Introduction
Bacterial colonization of tissue plays a critical role in human health and disease.1 This interaction is well demonstrated by the role of Helicobacter pylori in peptic ulcer disease.2 The recognition of this interaction dramatically changed the management of peptic ulcer disease and has led to a broader understanding of the aetiology of benign and malignant disease of the stomach, duodenum and oesophagus.2–6
Gallbladder disease has a significant impact on health care in the USA. It is estimated that 750 000 cholecystectomies are performed annually in the USA (http://www.ssat.com/cgi-bin/chole7.cgi). Although the aetiology of gallbladder disease is multifactorial, bacteria are not traditionally thought to be a priming factor for the development of gallstones or gallbladder inflammation. In our own retrospective study of patients with gallbladder dysfunction, defined as a gallbladder ejection fraction of ≤35% on hepatobiliary iminodiacetic acid (HIDA) scan, 71% had pathological evidence of chronic cholecystitis and 40% of those patients had no evidence of gallstones. Furthermore, we found that 73.2% of 101 such patients also had gastro-oesophageal reflux disease (GORD), whereas 58.4% had gastritis.7 This observation raised the question of whether bacterial colonization of the gallbladder may result in chronic inflammation similar to the association of H. pylori in chronic gastric inflammation. It is generally accepted that biliary obstruction and subsequent bile stasis can lead to bacterial overgrowth and to the development of pigmented gallstones. Stewart and colleagues have demonstrated this and have also suggested that 11–20% of cholesterol gallstones, which had been thought to be sterile, are colonized with bacteria.8,9 These data indicate that bacteria may be important to the formation of all types of gallstones. Furthermore, recent evidence suggests that Helicobacter spp., which are fastidious spiral or rod-shaped Gram negative bacteria, can be found not only in gallstones10–12 but also in bile13 and gallbladder tissue of specimens demonstrating chronic cholecystitis.13–15 This is particularly interesting in view of our finding that 58% of patients with gallbladder dysfunction had been diagnosed with gastritis, a disease associated with H. pylori infection. Stathopoulos et al. reported an association between gallstones and chronic gastritis.16 In their series, 14 of 19 patients with symptomatic gallstones and moderate to marked gastritis had evidence of H. pylori in the stomach, although the authors did not investigate whether H. pylori could be detected in the gallbladder.
The purpose of this study was to determine if bacteria, particularly Helicobacter spp., play a role in benign gallbladder disease. To our knowledge, no study has evaluated all three elements of the gallbladder system (bile, gallbladder tissue and gallstones) in a single cohort of patients for the presence of Helicobacter spp., as we do here.
Materials and methods
Patients and specimen collection
During February–July 2008, 45 patients with benign gallbladder disease undergoing elective cholecystectomy at New York University Langone Medical Center were recruited. Immediately following gallbladder excision, the specimens were collected in a sterile specimen cup. Tissue, bile and gallstones (when available) were collected in a sterile manner and stored whole and unprocessed at −800 °C until the time of experimentation. Both bile and tissue samples were available for 36 of 45 (80%) patients; gallstones were not available for analysis in five (13.9%) of these 36 patients. Clinical history of antibiotic use within the past year, diagnosis of upper gastrointestinal (UGI) disease and specimen pathology (Table 1) were collected and stored in a Health Insurance Portability and Accountability Act (HIPAA)-compliant database.
Table 1.
Clinical presentation of patients enrolled, including all patients and patients positive for Helicobacter by polymerase chain reaction technique
| Overall group | Helicobacter spp.+ | ||
|---|---|---|---|
| n = 36 | n = 12 | ||
| Bile + | 5 | ||
| Tissue + | 4 | ||
| Bile and tissue + | 3 | ||
| Median age, years | 48.5 | 52.0 | |
| % female (median age) | 52.8 (48.0) | 50 (54.0) | |
| % male (median age) | 48.2 (50.5) | 50 (50.5) | |
| Took antibiotics within 1 year, n (%) | 23 (63.9) | 7 (58.3) | |
| Unknown antibiotic use, n (%) | 5 (13.9) | 2 (16.7) | |
| Pathology results | |||
| Chronic cholecystitis, n (%) | 34 (94.4) | 11 (91.7) | |
| Cholesterolosis, n (%) | 10 (27.7) | 4 (33.3) | |
| Clinical history, prior diagnoses | |||
| GORD, n (%) | 16 (44.4) | 5 (41.7) | |
| Gastritis, n (%) | 4 (11.1) | 1 (8.3) | |
| Unknown, n (%) | 3 (8.3) | 1 (8.3) | |
| Helicobacter pylori serology | |||
| Anti-H. pylori, n (%) | 10 (27.8) | 6 (50) | |
| Anti-CagA, n (%) | 5 (13.9) | 2 (16.7) | |
GORD, gastro-oesophageal reflux disease
DNA extraction, polymerase chain reaction and DNA sequencing
DNA was isolated from bile using the Qiamp DNA Stool Minikit (Qiagen, Inc., Valencia, CA, USA) according to the company's protocol. For DNA isolation from gallstones and tissue, the DNeasy Blood and Tissue Kit (Qiagen, Inc.) was used.
Forward and reverse primers were selected to amplify a conserved segment of the Helicobacter spp. 16s rRNA gene (C97F [5′-GCTATGACGGGTATCC] and C98R [5′-GATTTTACC CCTACACCA]). An annealing temperature of 530 °C was used for 35 cycles. Polymerase chain reaction (PCR) was performed in duplicate on separate occasions for each DNA purified from the samples. Products were analysed with agarose gel electrophoresis. A patient sample was considered positive for Helicobacter spp. by PCR method only when there was a positive result on two separate occasions. If a sample demonstrated conflicting results within the two runs (i.e. one positive and one negative result), the sample was run a third time for a final result.
To confirm the presence of Helicobacter spp. DNA in bile and tissue, PCR samples were sequenced (Macrogen Corp., Rockland, MD, USA). The sequencher program was used for sequence alignment. Species identification was performed using the blast (Basic Local Alignment Search Tool) program from the National Center for Biotechnology Information.
Results
Patients
Of 45 patients recruited to this study, 36 (median age 48.5 years, range 24–79 years) had both bile and tissue specimens available for analysis. Nineteen were women (median age 48.0 years, range 25–70 years) and 17 were men (median age 49.0 years, range 24–79 years) (Table 1). Thirty-four of 36 demonstrated biliary colic or symptomatic gallstones, and two were diagnosed with gallbladder-related pancreatitis. One of these two patients had a common bile duct (CBD) and pancreatic duct (PD) stent placed before his scheduled cholecystectomy. Sixteen of 36 (44.4%) had a preoperative diagnosis of GORD, four (11.1%) were diagnosed with gastritis and in three (8.3%) the diagnosis was unknown.
Identification by PCR of Helicobacter spp. in bile and tissue specimens
Helicobacter spp. DNA was identified by PCR in eight of 36 (22.2%) bile samples and seven (19.4%) tissue samples for a total of 12 patients, or 33.3% of the total group (Table 1, Fig. 1). Only three patients demonstrated Helicobacter DNA in both bile and tissue. None of the 31 gallstones available for analysis demonstrated Helicobacter spp. DNA.
Figure 1.
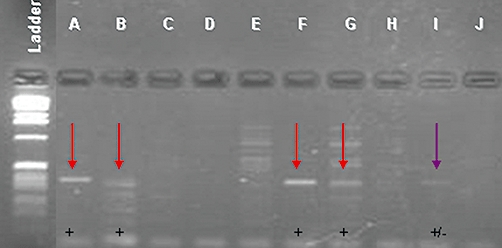
Representative gel of bile samples after polymerase chain reaction (PCR) amplification using Helicobacter-specific primers C97F and C98R. Four water samples were used as a negative control; HP26, a known Helicobacter pylori sample, was used in duplicate as a positive control. In this gel, A, B, F and G were all considered positive and I was considered indeterminate. G and I were considered negative upon repeated PCR experiments. All other samples were considered negative for Helicobacter spp.
H. pylori identification by DNA sequencing in bile and tissue specimens
Specimens positive for Helicobacter spp. DNA by PCR were confirmed by sequencing analysis in four bile and four tissue specimens (from seven patients [19.4%]). Of the four bile and four tissue specimens sequenced, blast results suggested that the bacteria isolated for each sample was most consistent with H. pylori. However, when evaluating results, other candidate bacteria include Helicobacter acinonychis, Helicobacter pullorum, Helicobacter winghamensis, Helicobacter canadensis, Helicobacter bilis, Helicobacter cinaedi and Helicobacter hepaticus because of the high homology in DNA sequence. Only one patient was found to have sequencing results demonstrating H. pylori in both bile and tissue samples.
Overall assessment of Helicobacter in bile and tissue of patients with benign gallbladder disease
The median age of the 12 patients with Helicobacter spp. in bile and/or tissue was 52.0 years, which is similar to that of the total group (Table 1). Six were women (50%, median age 54.0 years) and six were men (50%, median age 50.5 years). Preoperative diagnosis of UGI disease included GORD in five (41.7%), gastritis in one (8.3%) and was unknown in one (8.3%). Only one patient was tested by esophagogastroduodenoscopy (EGD) to evaluate the presence of UGI disease.
Antibiotic use before surgery
Antibiotic use by patients within 1 year prior to cholecystectomy was assessed as this could potentially alter the results. Data regarding antibiotic use were available for 33 of 36 patients. Twelve of 36 (33.3%) had taken antibiotics within 1 month of surgery, 17 (47.2%) had not and four (11.1%) could not recall (Table 1). A total of 23 of 36 (63.9%) had used antibiotics within 1 year of surgery, five (13.9%) had not and five (13.9%) could not recall. Antibiotics reported to have been used included clarithromycin, azithromycin, metronidazole, amoxicillin, levofloxacin, ciprofloxacin and xifaxin. Of the 12 patients with PCR evidence for Helicobacter, seven (58.3%) had taken antibiotics within 1 year of surgery. Two of the 12 (16.7%) patients with PCR evidence did not know whether they had used antibiotics in the year preceding surgery.
Pathology
Pathological evaluation of specimens from the 36 patients demonstrated chronic cholecystitis in 34 patients (94.4%), cholesterolosis in 10 (27.7%), acute on chronic cholecystitis in one (2.7%), eosinophilic cholecystitis in one (2.7%), hyperplasia in one (2.7%), lymphoid hyperplasia in one (2.7%), adenomyomatosis in one (2.7%), and intestinal and pyloric gland metaplasia in association with biliary intraepithelial neoplasia in one (2.7%). Of 12 Helicobacter-positive patients, 11 (91.7%) had pathological changes consistent with chronic cholecystitis and four (33.3%) demonstrated cholesterolosis. One patient had acute on chronic cholecystitis (8.3%), and one demonstrated tissue hyperplasia (8.3%). Gallstone characterization was not recorded.
Anti-H. pylori and anti-CagA antibodies in plasma
Of the 36 patients studied, 34 had plasma available for enzyme-linked immunosorbent assay (ELISA) analysis to evaluate for the presence of H. pylori antibodies and anti-cytotoxin associated gene (CagA) antibodies. Ten (27.8%) had detectable anti-H. pylori antibodies and five of the 10 had detectable anti-CagA antibodies. Of the 12 patients with Helicobacter spp., all had plasma samples available for ELISA analysis. Six demonstrated anti-H. pylori antibodies. This represents 60% of the 10 patients with these antibodies in the whole group. Interestingly, four of these six (66.7%) were anti-CagA− (Table 1).
Discussion
Evidence of Helicobacter spp. was found in 12 of 36 patients with benign gallbladder disease, suggesting that Helicobacter spp. were involved in 33.3% of patients studied. Only one patient whose tissue was positive for Helicobacter had a history of biliary tract manipulation, which may have resulted in direct inoculation of bacteria in the biliary system. Preoperative diagnosis of GORD (41.7%) or gastritis (8.3%) in the 12 positive patients was made by clinical judgement, not by endoscopy or imaging studies, in all but one patient. Therefore, no reliable conclusion can be drawn. The female : male distribution did not differ from that seen overall in the 12 patients and the age distribution was also similar. Of these 12 patients, six were seronegative for H. pylori, suggesting that a different Helicobacter species was probably present (such as H. acinonychis, H. pullorum, H. winghamensis, H. canadensis, H. bilis, H. cinaedi or H. hepaticus as mentioned earlier).
Sequencing analysis suggests that the bacteria identified in this group of seven patients were most probably H. pylori. Serology results further support this in five of these seven patients as they demonstrated anti-H. pylori antibodies in their serum. It is possible that the remaining two seronegative patients had different Helicobacter spp. present in bile or tissue.13 Of the 12 patients who were positive by PCR only, six had antibodies against H. pylori, again suggesting that the other six had different Helicobacter spp. present in bile or tissue.
We also determined whether seropositive patients had evidence of exposure to CagA+ strains of H. pylori. CagA is a virulence factor found in H. pylori that translocates into gastric epithelial cells17 and is associated with increased inflammatory response by gastric mucosal cells18,19 as well as an increased risk of gastric adenocarcinoma.20 In this study, 66.7% of patients whose bile or tissue were positive for Helicobacter spp. by PCR technique only and who demonstrated anti-H. pylori antibodies were anti-CagA−, whereas the remainder demonstrated evidence of infection with CagA+ strains. Although this did not reach statistical significance, perhaps secondary to small sample size, this questions whether CagA− strains of H. pylori are more likely to colonize or infect the gallbladder–biliary tract. This is particularly interesting as the cagA genotype is thought to protect against more severe forms of GORD, including the complication of Barrett's oesophagus.21,22 This observation supports our hypothesis that benign gallbladder disease may be associated with GORD and gastritis, and it may involve infection with CagA− strains of H. pylori. Analysis of this hypothesis with a larger cohort of patients should be performed.
None of the gallstone specimens were found to have Helicobacter DNA, although bacterial DNA was detected in the bile of some patients. As Helicobacter spp. are fastidious organisms, it may be that the gallstone environment is not appropriate to support their growth. It is also possible that we did not detect Helicobacter spp. because we did not analyse gallstone samples from within the centre of the stones; instead, we took samples from the outer surface of each gallstone. Stewart and colleagues' founding work demonstrated bacteria within the whole gallstone as each gallstone studied was crushed before analysis.8,9 Other groups who demonstrated Helicobacter spp. in gallstones took samples from the centre of the stones or used the whole stone for analysis.10–12,23,24
Overall, 58.3% of 12 patients with Helicobacter spp. in bile and/or tissue took antibiotics within 1 year prior to cholecystectomy. Overall, 69.6% of patients analysed reported antibiotic use within the previous year. In this preliminary study, we conclude that antibiotic use probably did not affect the results as we were able to detect Helicobacter spp. in patients who had used these medications.
Pathologically, 91.7% of patients with Helicobacter DNA by PCR had evidence of chronic cholecystitis. This does not differ from findings for the whole group, in which 94.4% of patients demonstrated evidence of chronic cholecystitis.
These results demonstrate that Helicobacter spp., particularly H. pylori, can be detected in the bile and gallbladder tissue of patients with benign gallbladder disease. The mechanism through which these bacteria contribute to the pathophysiology of gallbladder disease, particularly gallstone formation, is unclear at this time. This has been studied in more detail in a mouse model by Maurer et al.,25,26 who suggest that Helicobacter spp. but not necessarily H. pylori may contribute to benign gallbladder disease. Based on our data, the paradigm of gallstone formation and the contribution of Helicobacter spp. should be evaluated critically.
Conflicts of interest
None declared.
References
- 1.Blaser MJ. Who are we? Indigenous microbes and the ecology of human diseases. EMBO Rep. 2006;7:956–960. doi: 10.1038/sj.embor.7400812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA. 1994;272:65–69. [PubMed] [Google Scholar]
- 3.Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 4.Peek RM, Jr, Vaezi MF, Falk GW, Goldblum JR, Perez-Perez GI, Richter JE, et al. Role of Helicobacter pylori cagA(+) strains and specific host immune responses on the development of premalignant and malignant lesions in the gastric cardia. Int J Cancer. 1999;82:520–524. doi: 10.1002/(sici)1097-0215(19990812)82:4<520::aid-ijc9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Chow WH, Blaser MJ, Blot WJ, Gammon MD, Vaughan TL, Risch HA, et al. An inverse relation between cagA+ strains of Helicobacter pylori infection and risk of oesophageal and gastric cardia adenocarcinoma. Cancer Res. 1998;58:588–590. [PubMed] [Google Scholar]
- 6.Ferreri AJ, Ernberg I, Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. J Intern Med. 2009;265:421–438. doi: 10.1111/j.1365-2796.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 7.Sabbaghian MS, Rich BS, Rothberger GD, Cohen J, Batash S, Kramer E, et al. Evaluation of surgical outcomes and gallbladder characteristics in patients with biliary dyskinesia. J Gastrointest Surg. 2008;12:1324–1330. doi: 10.1007/s11605-008-0546-3. [DOI] [PubMed] [Google Scholar]
- 8.Stewart L, Grifiss JM, Jarvis GA, Way LW. Biliary bacterial factors determine the path of gallstone formation. Am J Surg. 2006;192:598–603. doi: 10.1016/j.amjsurg.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Stewart L, Oesterle AL, Erdan I, Griffiss JM, Way LW. Pathogenesis of pigment gallstones in Western societies: the central role of bacteria. J Gastrointest Surg. 2002;6:891–903. doi: 10.1016/s1091-255x(02)00035-5. discussion 904. [DOI] [PubMed] [Google Scholar]
- 10.Abayli B, Colakoglu S, Serin M, Erdogan S, Isiksal YF, Tuncer I, et al. Helicobacter pylori in the aetiology of cholesterol gallstones. J Clin Gastroenterol. 2005;39:134–137. [PubMed] [Google Scholar]
- 11.Monstein HJ, Jonsson Y, Zdolsek J, Svanvik J. Identification of Helicobacter pylori DNA in human cholesterol gallstones. Scand J Gastroenterol. 2002;37:112–119. doi: 10.1080/003655202753387455. [DOI] [PubMed] [Google Scholar]
- 12.Nilsson I, Shabo I, Svanvik J, Monstein HJ. Multiple displacement amplification of isolated DNA from human gallstones: molecular identification of Helicobacter DNA by means of 16S rDNA-based pyrosequencing analysis. Helicobacter. 2005;10:592–600. doi: 10.1111/j.1523-5378.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 13.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, et al. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology. 1998;114:755–763. doi: 10.1016/s0016-5085(98)70589-x. [DOI] [PubMed] [Google Scholar]
- 14.Apostolov E, Al-Soud WA, Nilsson I, Kornilovska I, Usenko V, Lyzogubov V, et al. Helicobacter pylori and other Helicobacter species in gallbladder and liver of patients with chronic cholecystitis detected by immunological and molecular methods. Scand J Gastroenterol. 2005;40:96–102. doi: 10.1080/00365520410009546. [DOI] [PubMed] [Google Scholar]
- 15.Chen DF, Hu L, Yi P, Liu WW, Fang DC, Cao H. H. pylori exist in the gallbladder mucosa of patients with chronic cholecystitis. World J Gastroenterol. 2007;13:1608–1611. doi: 10.3748/wjg.v13.i10.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stathopoulos P, Zundt B, Spelsberg FW, Kolligs L, Diebold J, Goke B, et al. Relation of gallbladder function and Helicobacter pylori infection to gastric mucosa inflammation in patients with symptomatic cholecystolithiasis. Digestion. 2006;73:69–74. doi: 10.1159/000092746. [DOI] [PubMed] [Google Scholar]
- 17.Odenbreit S, Puls J, Sedlmaier B, Gerland E, Fischer W, Haas R. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 18.Peek RM, Jr, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+Helicobacter pylori strains. Lab Invest. 1995;73:760–770. [PubMed] [Google Scholar]
- 19.Bhat N, Gaensbauer J, Peek RM, Bloch K, Tham KT, Blaser MJ, et al. Local and systemic immune and inflammatory responses to Helicobacter pylori strains. Clin Diagn Lab Immunol. 2005;12:1393–1400. doi: 10.1128/CDLI.12.12.1393-1400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corley DA, Kubo A, Levin TR, Block G, Habel L, Zhao W, et al. Helicobacter pylori infection and the risk of Barrett's oesophagus: a community-based study. Gut. 2008;57:727–733. doi: 10.1136/gut.2007.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pereira-Lima JC, Marques DL, Pereira-Lima LF, Hornos AP, Rota C. The role of cagA Helicobacter pylori strains in gastro-oesophageal reflux disease. Eur J Gastroenterol Hepatol. 2004;16:643–647. doi: 10.1097/01.meg.0000108340.41221.9e. [DOI] [PubMed] [Google Scholar]
- 23.Misra V, Misra SP, Dwivedi M, Shouche Y, Dharne M, Singh PA. Helicobacter pylori in areas of gastric metaplasia in the gallbladder and isolation of H. pylori DNA from gallstones. Pathology. 2007;39:419–424. doi: 10.1080/00313020701444473. [DOI] [PubMed] [Google Scholar]
- 24.Farshad S, Alborzi A, Malek Hosseini SA, Oboodi B, Rasouli M, Japoni A, et al. Identification of Helicobacter pylori DNA in Iranian patients with gallstones. Epidemiol Infect. 2004;132:1185–1189. doi: 10.1017/s0950268804002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer KJ, Ihrig MM, Rogers AB, Ng V, Bouchard G, Leonard MR, et al. Identification of cholelithogenic enterohepatic Helicobacter species and their role in murine cholesterol gallstone formation. Gastroenterology. 2005;128:1023–1033. doi: 10.1053/j.gastro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Maurer KJ, Rogers AB, Ge Z, Wiese AJ, Carey MC, Fox JG. Helicobacter pylori and cholesterol gallstone formation in C57L/J mice: a prospective study. Am J Physiol Gastrointest Liver Physiol. 2006;290:175–182. doi: 10.1152/ajpgi.00272.2005. [DOI] [PubMed] [Google Scholar]


