Abstract
Background:
Biliary tree malignancies including cholangiocarcinoma and gallbladder cancer are aggressive cancers with a high disease-specific mortality despite resection. The aim of the present study was to identify predictors of survival after resection.
Methods:
A retrospective review of all patients that underwent radical resection of biliary malignancies was performed. Demographics, elevated CA19-9 (>35 U/ml), treatment and outcome data were collected and compared according to tumour location. Kaplan–Meier survival curves were created and compared using log-rank analysis. Multivariate analysis was undertaken using Cox proportional hazards regression.
Results:
Ninety-one patients with biliary malignancies underwent surgical resection between 1992 and 2007. There were 46 (50.5%) extrahepatic cholangiocarcinomas (EHC), 23 (25.2%) intrahepatic cholangiocarcinomas (IHC) and 22 (24.2%) gallbladder carcinomas (GBC). The median (range) age was 64 (24–92) years. An elevated CA19-9 was recorded in 45 (55%) patients (52% of IHC, 63% of EHC, and 41% of GBC). The overall median (range) survival was 22.5 (0.3–153.3) months. All three groups were similar in terms of age, gender, pre-operative CA 19-9 level, completeness of resection and tumour histopathological characteristics. GBC were associated with the shortest median survival (14.3 months) followed by EHC (24.8 months) and IHC (30.4 months); however, this did not meet statistical significance (P= 0.971). Only elevated pre-operative CA 19-9 level (>35 U/ml) was predictive of poor median survival by univariate (P= 0.003) and multivariate analysis (15.1 months vs. 67.4, P= 0.047).
Conclusions:
Elevated pre-operative CA 19-9 levels were found to be independent predictors of poor survival after attempted resection for biliary tree malignancies. It is recommended that CA19-9 be routinely measured prior resection.
Keywords: gallbladder cancer, cholangiocarcinoma
Introduction
Biliary tree malignancies are aggressive cancers with a high disease-specific mortality despite resection.1–4 While their incidence seems to be increasing, they are still relatively uncommon, accounting for 9520 newly diagnosed patients and 3340 deaths in the United States in 2008.5,6 Aggressive surgical resection remains the only hope for a cure, however, for the majority of patients palliation remains the only option as a result of advanced state of the disease at presentation. As such, extensive experience with resections of such malignancies are generally lacking even in large volume centres. The majority of existing studies are drawn either from multi-institutional studies or cancer registries, introducing variability of treatment and surgical techniques.2,7–10
The biliary tree contains a heterogeneous group of structures with similar embryologic origin.11 The intrahepatic and extrahepatic cholangiocarcinomas are frequently compared against other tumours of the area such as pancreatic or gallbladder cancer to establish comparative outcomes.8,11,12 However, for the purpose of clinical trials comparing clinical strategies and/or the use and type of adjuvant or neoadjuvant chemotherapy, intrahepatic and extrahepatic cholangiocarcinomas are often coupled with gallbladder malignancies.13,14 The arguments for this combination are the common embryological origin and similar biological behaviour of these malignancies. In addition, most centres have increasing difficulty amassing a sufficient study population. This design may increase the statistical power of the study. Finally, combining cholangiocarcinoma with gallbladder cancer allows for comparison of several common variables.
The aim of the present study was to identify potential predictors of survival for those patients who had undergone attempted curative resection for biliary tree malignancies.
Materials and methods
The study protocol was approved by the Institutional Review Board at The Ohio State University. A retrospective case note review of all patients who underwent resection of biliary tree malignancies including extrahepatic cholangiocarcinoma (EHC), intrahepatic cholangiocarcinoma (IHC), and gallbladder cancer (GBC) between 1992 and 2007 was undertaken.
Patients were selected by retrospectively reviewing the archives of the James Cancer Hospital tumor registry for patients diagnosed with biliary malignancies who subsequently underwent surgical resection with curative intent. EHC was defined, per National Comprehensive Cancer Network (NCCN) guidelines, as any tumour extending from the ampulla of Vater to the hilar plate and included hilar cholangiocarcinomas.5 IHC was defined, per NCCN guidelines, as a tumour arising within the substance of the liver and involving only biliary radicals above the confluence of the right and left bile ducts.5
Patient demographics, comorbidities (defined as any chronic disease that would potentially increase the risk of peri-operative complications or death), presentation, treatment, histopathological information and outcome/survival data were collected. Data were pooled from three sources: the Ohio State University Medical Center information warehouse, the OSU James Cancer Center Tumor Registry and by review of patients' electronic charts. To assess the effect of CA19-9, a binary variable, ‘elevated CA19-9’ was defined as a value >35 U/ml. This value was chosen as this cutoff point as it represented the upper limit of normal range. Survival data were recorded from the time of resection until death, as determined by the medical record and/or the social security death index (http://ssdi.rootsweb.ancestry.com) as of 31 August 2008.
Comparisons of the three primary anatomical types were made by anova and with contingency table analysis (χ2 and Fisher's exact test, where appropriate). Statistical significance was accepted at a P-value <0.05. Survival was analysed using the Kaplan–Meier method and compared by log-rank analysis.
Serial univariate forward regression procedures were undertaken to identify variables associated with survival using Cox proportional hazards analysis. Subsequently, a multivariate forward Cox regression model was created and variables shown to most likely impact survival in the univariate analysis (i.e. P < 0.2) were entered in the established model in a step-wise fashion. This process was continued until no further covariates were found to be significant in the context of the model.
Statistical analyses were performed using STATA 10.1 for Macintosh (StataCorp LP, College Station, TX, USA). Survival analysis and related graphics were performed with SPSS Statistics 17.0 for Macintosh (SPSS Inc., Chicago, IL, USA).
Results
Between 1992 and 2007, 91 patients with biliary tree malignancies underwent attempted curative resection. Demographics, clinical and pathological factors are shown in Table 1. Peri-operative factors and short-term outcomes are shown in Table 2.
Table 1.
Demographics, clinical and pathological characteristics by anatomical site (denominator given when less than total denominator for that variable)
| Variable | Total | IHC | EHC | GBC | P |
|---|---|---|---|---|---|
| Median (range), n (%) | (n= 91) | n= 23 | n= 46 | n= 22 | |
| Age (years) | 64 (24–92) | 61 (48–89) | 64 (24–92) | 64.5 (24–77) | 0.87 |
| Female | 49 (53.8%) | 12 | 22 | 15 | 0.20 |
| Comorbidities | 51 (56.0%) | 17 | 20 | 14 | 0.04 |
| Elevated CA19-9 (>35 U/ml) | 20/48 | 4/11 | 13/29 | 3/8 | 0.90 |
| CA19-9, U/ml | 24.7 (3.5–8989) | 19.9 (9.8–8989) | 33 (3.5–8210) | 17.8 (8.6–375) | 0.60 |
| Size cm | 2.5 (0.7–10.5) | 6.5 (1–13.5) | 2.5 (0.7–8.0) | 2.1 (0.7–4.8) | <0.001 |
| R0 resection | 57 (62.6%) | 16 | 30 | 11 | 0.30 |
| Poor differentiation | 22 (24.2%) | 4 | 12 | 6 | 0.70 |
| Nodal Metastasis | 31 (34.1%) | 7 | 16 | 8 | 0.90 |
| LVI | 26 (28.6%) | 4 | 13 | 9 | 0.21 |
| Perineural Invasion | 44 (48.3%) | 6 | 31 | 7 | <0.001 |
IHC, intrahepatic cholangiocarcinomas; EHC, extrahepatic cholangiocarcinoma; GBC, gallbladder carcinomas.
Table 2.
Peri-operative characteristics of patients undergoing resection for biliary malignancies
| Variable | Total | IHC | EHC | GBC | P |
|---|---|---|---|---|---|
| Median (range), n (%) | (n= 91) | n= 23 | n= 46 | n= 22 | |
| Primary operation | |||||
| Liver resection | 56 (61.5) | 23 | 18 | 15 | NA |
| Extrahepatic biliary resection | 35 (38.5) | 7 | 28 | 0 | NA |
| Pancreaticoduodenectomy | 18 (19.8) | NA | 18 | NA | NA |
| Complications | 16 (18.5) | 4 | 6 | 6 | 0.34 |
| Length of stay, days | 9 (1–70) | 8.5 (1–70) | 9 (1–69) | 10 (4–41) | 0.8 |
| 30-day mortality | 4 (4.4) | 1 | 2 | 1 | 0.6 |
IHC, intrahepatic cholangiocarcinomas; EHC, extrahepatic cholangiocarcinoma; GBC, gallbladder carcinomas; NA, not applicable.
Median overall survival for all groups was 22.5 months. At the completion of the follow-up period 63 (69.2%) patients had died. The median follow-up for those still alive (n= 28) was 39.7 (5.7–153.3) months. Survival data are shown in Table 3. GBC histology correlated with the shortest median survival (14.3 months) followed by EHC (24.8 months) and IHC (30.4 months), however, this finding did not meet statistical significance. Univariate and multivariate analysis confirmed elevated CA19-9 was the only independent variable to predict poor survival (Table 3). Patients with elevated CA19-9 had a median survival of 12.0 months compared with 30.4 months in those with low CA19-9 (P= 0.01, Fig. 1). Neither total nor direct bilirubin reached statistical significance as a predictor of survival in the univariate Cox regression analysis (P= 0.219 and 0.412, respectively). Hence, the two variables were not included in the final model.
Table 3.
Cox proportional hazards analysis of variables (model P-value = 0.026)
| Variable | Median survival | Univariate analysis | Multivariate analysis |
|---|---|---|---|
| (months) | P-value | P-value | |
| Elevated CA 19-9 (>35 U/ml) | |||
| Yes (n= 20) | 12.0 | 0.01 | 0.017 |
| No (n= 28) | 30.4 | ||
| Poorly differentiated | |||
| Yes (n= 22) | 22.5 | 0.449 | 0.706 |
| No (n= 69) | 33.4 | ||
| Nodal metastasis | |||
| N0 (n= 60) | 30.3 | 0.201 | 0.141 |
| N1 (n= 31) | 15.7 | ||
| R0 resection | |||
| R0 (n= 57) | 24.8 | 0.185 | 0.550 |
| R1 (n= 34) | 15.7 | ||
| Lymphovascular invasion | |||
| Yes (n= 26) | 17.2 | 0.158 | 0.445 |
| No (n= 65) | 28.4 | ||
| Perineural invasion | |||
| Yes (n= 44) | 22.3 | 0.416 | 0.919 |
| No (n= 47) | 24.8 | ||
Figure 1.
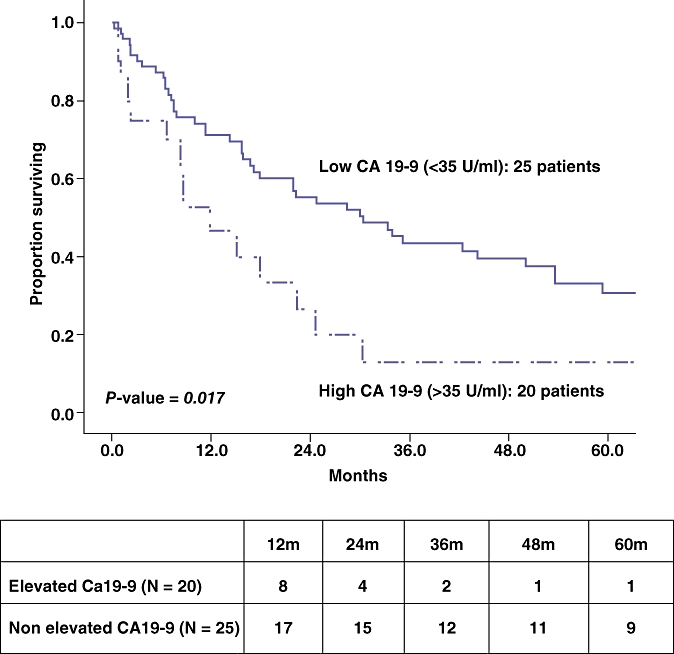
Cumulative survival by high CA 19-9 levels. Table depicts patients at risk at each time interval
The range of CA19-9-values was 3.52–8989. Out of the patients with a recorded value of CA19-9, 20 (44.4%) were higher than 35. The proportion of patients with elevated CA19-9 was not significantly different based upon the extent of resection (19.3% vs. 26.5%, P= 0.29). Pre-operative levels of CA19-9 were available in 45 (55%) patients (52% of IHC, 63% of EHC and 41% of GBC). To assess the effect of missing values in this analysis, we created a binary variable ‘missing CA19-9’ and repeated the Cox regression (data not shown). This variable was not significant in the univariate or multivariate analyses. Inclusion of this variable did not change the variables included in the final model as presented below. While the coefficient of the only significant variable (elevated CA19-9) changed based upon the addition of this new variable, it did not alter its significance.
Discussion
As a result of the rarity of these tumours, there is a paucity of data in the literature regarding the outcomes of patients undergoing curative resection for biliary malignancy. These tumours are challenging to diagnose, and often present at an advanced stage.2,8–10 Hence, accrual of patients that have undergone radical resections of such malignancies, to allow meaningful analysis, is difficult even in large volume centres. In the present study, we analysed our single-institution experience of 91 patients undergoing resection, with the aim of reporting potential predictors of survival in these malignancies.
The current study has shown that, in broad terms, the clinicopathological characteristics of biliary malignancies are similar. Biliary tumours tend to present with advanced disease and IHC typically present with larger size. Attempts at resection reveal similar proportion of margin-negative resection between the three tumor types, however, location, tumour stage, and margin status were not predictive of survival. Elevated CA19-9 appears to be the only predictor of survival in the patients undergoing resection irrespective of the bilirubin.
CA19-9 is an antigen directed at circulating glycoproteins that are coated with sialylated blood group antigens.15,16 CA 19-9 levels can be elevated in other malignancies such as ovarian, stomach, pancreas and colon.16 It can also be elevated in any condition leading to dilation, obstruction or inflammation of the bile ducts such as benign stricture and cholangitis.15 Many authors have described an association between elevated CA19-9 and poor outcomes, in the setting of biliary or other malignancies, using different value cutoffs, or different measurement techniques.4,17–19 In patients with cholangiocarcinoma a cutoff value of 100 U/ml, was previously shown to portend a sensitivity and specificity for detecting cholangiocarcinoma ranging from 53% to 89% and 80% to 91%, respectively.15
Although surgeons are used to seeing extremely high levels, often in the thousands,4,15,17,18 the current study has shown that even slightly abnormally elevated levels (>35 U/ml) appear to be associated with a poorer outcome. To the authors' knowledge, CA19-9 has never been linked to outcome post-surgical treatment of biliary malignancy. In the present study, CA19-9 was the only predictor of survival: elevated levels arbitrarily set at the upper limit of normal (>35 U/ml), were identified to be correlated with poor survival, despite operative resection.
Pre-operative levels of CA19-9 were available in 45 (55%) patients (52% of IHC, 63% of EHC and 41% of GBC). To assess the effect of missing values in this analysis, we created a binary variable ‘missing CA19-9’ and repeated the Cox regression. This variable was not significant in the univariate or multivariate analyses. Inclusion of this variable did not change the variables included in the final model as presented below. While the coefficient of the only significant variable (elevated CA19-9) changed based upon the addition of this new variable, it did not alter its significance. Hence, the final conclusion remains the same. It remains interesting that within the current study typical predictors of survival described by other authors in the literature (R0 resection, nodal involvement, and lymphovascular involvement),2,3,20 did not predict survival and outcome.
This study has several potential weaknesses. It is a retrospective cohort review of patients undergoing radical resection. By design, this study spans 16 years of experience treating these malignancies. With changing approaches to the resection and with evolving chemotherapy, there may be an element of lead time bias – patients treated more recently may be doing better subsequent to lessons learned from previous experiences, better approaches to chemotherapy with newer agents and overall better supportive care. As in the case of most studies evaluating these tumours, this study may also be underpowered to identify subtle differences between the three tumour sites evaluated herein. Despite these potential weaknesses, the effect of elevated CA19-9 was pronounced and we recommend considering it prior to resection.
In summary, while location (i.e. IHC or EHC) is often considered predictive of survival, the current study did not find a difference in biological behaviour based upon location or histology. Pre-operative CA19-9 was more predictive of survival than typical histopathological characteristics when radical resection was possible, suggesting that aggressive surgical resection still be considered even in the presence of advanced disease. In light of these findings it is recommended that CA19-9 should be routinely measured prior to attempted curative resection. This study provides data that could potentially render CA19-9 a useful stratification tool in the setting of future clinical trials assessing the efficacy of chemotherapy or radiation.
Conflicts of interest
Mark Bloomston, MD is supported as a Paul Cabrezi scholar on NIH/NCI 1 K12 CA133250. No other financial disclosures.
References
- 1.Endo I, Shimada H, Sugita M, Fujii Y, Morioka D, Takeda K, et al. Role of three-dimensional imaging in operative planning for hilar cholangiocarcinoma. Surgery. 2007;142:666–675. doi: 10.1016/j.surg.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 3.Endo I, House MG, Klimstra DS, Gönen M, D'Angelica M, Dematteo RP, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol. 2008;15:2104–2112. doi: 10.1245/s10434-008-0003-2. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Bosonnet L, Ghaneh P, Raraty M, Sutton R, Campbell F, et al. Preoperative CA19-9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg. 2008;25:226–232. doi: 10.1159/000140961. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, 3rd, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, et al. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350–391. doi: 10.6004/jnccn.2009.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 7.Wang SJ, Fuller CD, Kim JS, Sittig DF, Thomas CR, Jr, Ravdin PM. Prediction model for estimating the survival benefit of adjuvant radiotherapy for gallbladder cancer. J Clin Oncol. 2008;26:2112–2117. doi: 10.1200/JCO.2007.14.7934. [DOI] [PubMed] [Google Scholar]
- 8.Aljiffry M, Abdulelah A, Walsh M, Peltekian K, Alwayn I, Molinari M. Evidence-based approach to cholangiocarcinoma: a systematic review of the current literature. J Am Coll Surg. 2009;208:134–147. doi: 10.1016/j.jamcollsurg.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Veillette G, Castillo CF. Distal biliary malignancy. Surg Clin North Am. 2008;88:1429–1447. doi: 10.1016/j.suc.2008.07.003. xi. [DOI] [PubMed] [Google Scholar]
- 10.Akoad M, Jenkins R. Proximal biliary malignancy. Surg Clin North Am. 2008;88:1409–1428. doi: 10.1016/j.suc.2008.07.012. x–xi. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett DL, Ramanathan RK, Ben-Josef E. Cancer of the Biliary Tree. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott, Williams, and Wilkens; 2008. pp. 1156–1186. [Google Scholar]
- 12.Reddy SB, Patel T. Current approaches to the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2006;8:30–37. doi: 10.1007/s11894-006-0061-1. [DOI] [PubMed] [Google Scholar]
- 13.Leonard GD, O'Reilly EM. Biliary tree cancers: current concepts and controversies. Expert Opin Pharmacother. 2005;6:211–223. doi: 10.1517/14656566.6.2.211. [DOI] [PubMed] [Google Scholar]
- 14.Okusaka T. Chemotherapy for biliary tree cancer in Japan. Semin Oncol. 2002;29(Suppl 20):51–53. doi: 10.1053/sonc.2002.37377. [DOI] [PubMed] [Google Scholar]
- 15.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–207. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 16.Ragupathi G, Damani P, Srivastava G, Srivastava O, Sucheck SJ, Ichikawa Y, et al. Synthesis of sialyl Lewis(a) (sLe (a), CA19-9) and construction of an immunogenic sLe(a) vaccine. Cancer Immunol Immunother. 2009;58:1397–1405. doi: 10.1007/s00262-008-0654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith RA, Bosonnet L, Ghaneh P, Sutton R, Evans J, Healey P, et al. The platelet-lymphocyte ratio improves the predictive value of serum CA19-9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008;143:658–666. doi: 10.1016/j.surg.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Smith RA, Ghaneh P, Sutton R, Raraty M, Campbell F, Neoptolemos JP. Prognosis of resected ampullary adenocarcinoma by preoperative serum CA19-9 levels and platelet-lymphocyte ratio. J Gastrointest Surg. 2008;12:1422–1428. doi: 10.1007/s11605-008-0554-3. [DOI] [PubMed] [Google Scholar]
- 19.Bloomston M, Bekaii-Saab TS, Kosuri K, Cowgill SM, Melvin WS, Ellison EC, et al. Preoperative carbohydrate antigen 19-9 is most predictive of malignancy in older jaundiced patients undergoing pancreatic resection. Pancreas. 2006;33:246–249. doi: 10.1097/01.mpa.0000236726.34296.df. [DOI] [PubMed] [Google Scholar]
- 20.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, et al. Intrahepatic Cholangiocarcinoma: Prognostic Factors After Surgical Resection. World J Surg. 2009;33:1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]


