Abstract
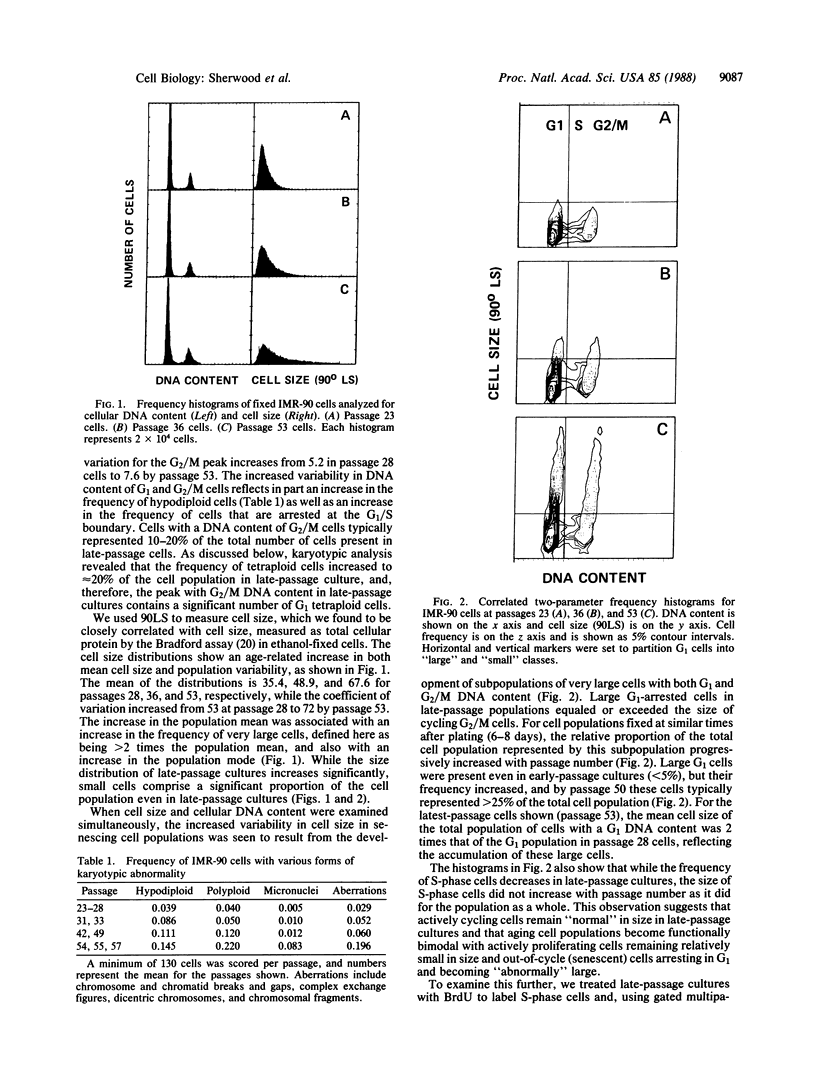
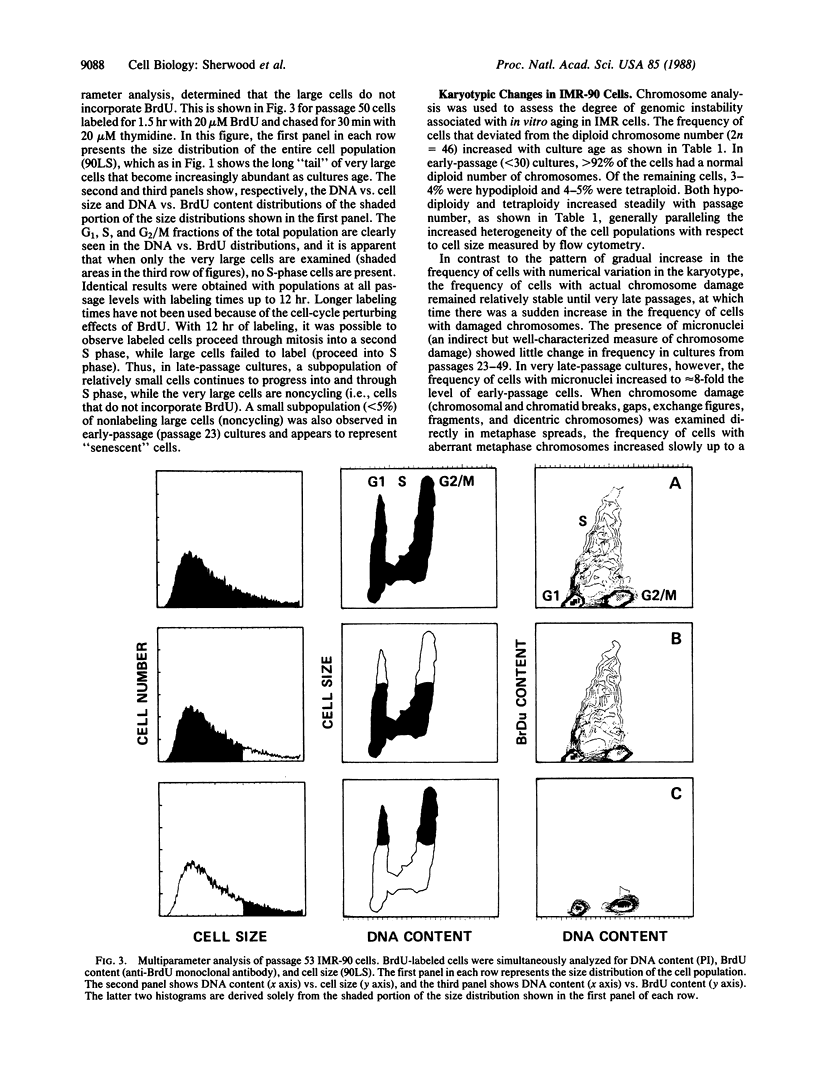
Using multiparameter flow cytometric analysis, we find that senescent cells accumulate in a unique cell-cycle compartment characterized in cell-cycle arrest in G1 and a significantly reduced nucleocytoplasmic ratio (genome size/cell mass) relative to cycling cells. With respect to gross cellular phenotype, the quiescent state of senescent cells differs from quiescence induced by density inhibition; the former is associated with a reduction in the nucleocytoplasmic ratio, while the latter is associated with an increase in the nucleocytoplasmic ratio. Senescent cells were present at all passages examined. The frequency of senescent cells was low in early-passage cultures and increased with passage number. Senescence of populations of IMR-90 cells reflects change in the relative frequency of these cells. The frequency of cells with karyotypic changes increased with the progressive accumulation of out-of-cycle cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benn P. A. Specific chromosome aberrations in senescent fibroblast cell lines derived from human embryos. Am J Hum Genet. 1976 Sep;28(5):465–473. [PMC free article] [PubMed] [Google Scholar]
- Bowman P. D., Meek R. L., Daniel C. W. Aging of human fibroblasts in vitro. Correlations between DNA synthetic ability and cell size. Exp Cell Res. 1975 Jun;93(1):184–190. doi: 10.1016/0014-4827(75)90438-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dolbeare F., Gratzner H., Pallavicini M. G., Gray J. W. Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5573–5577. doi: 10.1073/pnas.80.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa N., Hanaoka F., Hori T., Yamada M. Reevaluation of DNA chain elongation rate in human diploid fibroblasts. Exp Cell Res. 1982 Aug;140(2):443–447. doi: 10.1016/0014-4827(82)90137-9. [DOI] [PubMed] [Google Scholar]
- Hasegawa N., Hanaoka F., Yamada M. Does capacity of DNA replication change during in vitro ageing? Exp Cell Res. 1985 Feb;156(2):478–486. doi: 10.1016/0014-4827(85)90555-5. [DOI] [PubMed] [Google Scholar]
- Heddle J. A., Hite M., Kirkhart B., Mavournin K., MacGregor J. T., Newell G. W., Salamone M. F. The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983 Sep;123(1):61–118. doi: 10.1016/0165-1110(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Houghton B. A., Stidworthy G. H. A growth history comparison of the human diploid cells WI-38 and IMR-90: proliferative capacity and cell sizing analysis. In Vitro. 1979 Sep;15(9):697–702. doi: 10.1007/BF02618249. [DOI] [PubMed] [Google Scholar]
- Loo D. T., Fuquay J. I., Rawson C. L., Barnes D. W. Extended culture of mouse embryo cells without senescence: inhibition by serum. Science. 1987 Apr 10;236(4798):200–202. doi: 10.1126/science.3494308. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Schneider E. L. Increased nuclear sizes in senescent human diploid fibroblast cultures. Exp Cell Res. 1976 Jun;100(1):147–152. doi: 10.1016/0014-4827(76)90336-0. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Murphy D. G., Cristofalo V. J., Toji L. H., Greene A. E., Dwight S. A. Characterization of a new human diploid cell strain, IMR-90. Science. 1977 Apr 1;196(4285):60–63. doi: 10.1126/science.841339. [DOI] [PubMed] [Google Scholar]
- PRITCHARD R. H., LARK K. G. INDUCTION OF REPLICATION BY THYMINE STARVATION AT THE CHROMOSOME ORIGIN IN ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:288–307. doi: 10.1016/s0022-2836(64)80208-4. [DOI] [PubMed] [Google Scholar]
- Pereira-Smith O. M., Fisher S. F., Smith J. R. Senescent and quiescent cell inhibitors of DNA synthesis. Membrane-associated proteins. Exp Cell Res. 1985 Oct;160(2):297–306. doi: 10.1016/0014-4827(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Rabinovitch P. S. Regulation of human fibroblast growth rate by both noncycling cell fraction transition probability is shown by growth in 5-bromodeoxyuridine followed by Hoechst 33258 flow cytometry. Proc Natl Acad Sci U S A. 1983 May;80(10):2951–2955. doi: 10.1073/pnas.80.10.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKSELA E., MOORHEAD P. S. ANEUPLOIDY IN THE DEGENERATIVE PHASE OF SERIAL CULTIVATION OF HUMAN CELL STRAINS. Proc Natl Acad Sci U S A. 1963 Aug;50:390–395. doi: 10.1073/pnas.50.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Fowlkes B. J. Measurement of DNA content and cell volume in senescent human fibroblasts utilizing flow multiparameter single cell analysis. Exp Cell Res. 1976 Mar 15;98(2):298–302. doi: 10.1016/0014-4827(76)90441-9. [DOI] [PubMed] [Google Scholar]
- Sherwood S. W., Schumacher R. I., Schimke R. T. Effect of cycloheximide on development of methotrexate resistance of Chinese hamster ovary cells treated with inhibitors of DNA synthesis. Mol Cell Biol. 1988 Jul;8(7):2822–2827. doi: 10.1128/mcb.8.7.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H., Atkins L. Membrane-associated inhibitor of DNA synthesis in senescent human diploid fibroblasts: characterization and comparison to quiescent cell inhibitor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9030–9034. doi: 10.1073/pnas.83.23.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G. H., Namba M., Corsaro C. M. Relationship of finite proliferative lifespan, senescence, and quiescence in human cells. J Cell Physiol. 1985 Mar;122(3):343–349. doi: 10.1002/jcp.1041220303. [DOI] [PubMed] [Google Scholar]
- Thompson K. V., Holliday R. Chromosome changes during the in vitro ageing of MRC-5 human fibroblasts. Exp Cell Res. 1975 Nov;96(1):1–6. doi: 10.1016/s0014-4827(75)80029-2. [DOI] [PubMed] [Google Scholar]
- Yanishevsky R., Carrano A. V. Prematurely condensed chromosomes of dividing and non-dividing cells in aging human cell cultures. Exp Cell Res. 1975 Jan;90(1):169–174. doi: 10.1016/0014-4827(75)90370-5. [DOI] [PubMed] [Google Scholar]
- Yanishevsky R., Mendelsohn M. L., Mayall B. H., Cristofalo V. J. Proliferative capacity and DNA content of aging human diploid cells in culture: a cytophotometric and autoradiographic analysis. J Cell Physiol. 1974 Oct;84(2):165–170. doi: 10.1002/jcp.1040840202. [DOI] [PubMed] [Google Scholar]



