Abstract
The synthetic phospho-ceramide analogue-1 (PCERA-1) down-regulates production of the pro-inflammatory cytokine tumour necrosis factor-α (TNF-α) and up-regulates production of the anti-inflammatory cytokine interleukin-10 (IL-10) in lipopolysaccharide (LPS) -stimulated macrophages. We have previously reported that PCERA-1 increases cyclic adenosine monophosphate (cAMP) levels. The objective of this study was to delineate the signalling pathway leading from PCERA-1 via cAMP to modulation of TNF-α and IL-10 production. We show here that PCERA-1 elevates intra-cellular cAMP level in a guanosine triphosphate-dependent manner in RAW264.7 macrophages. The cell-permeable dibutyryl cAMP was able to mimic the effects of PCERA-1 on cytokine production, whereas 8-chloro-phenylthio-methyladenosine-cAMP, which specifically activates the exchange protein directly activated by cAMP (EPAC) but not protein kinase A (PKA), failed to mimic PCERA-1 activities. Consistently, the PKA inhibitor H89 efficiently blocked PCERA-1-driven cytokine modulation as well as PCERA-1-stimulated phosphorylation of cAMP response element binding protein (CREB) on Ser-133. Finally, PCERA-1 activated cAMP-responsive transcription of a luciferase reporter, in synergism with the phosphodiesterase (PDE)-4 inhibitor rolipram. Our results suggest that PCERA-1 activates a Gs protein-coupled receptor, leading to elevation of cAMP, which acts via the PKA–CREB pathway to promote TNF-α suppression and IL-10 induction in LPS-stimulated macrophages. Identification of the PCERA-1 receptor is expected to set up a new target for development of novel anti-inflammatory drugs.
Keywords: cyclic adenosine monophosphate response element binding protein, interleukin-10, inflammation, lipopolysaccharide, macrophages, tumour necrosis factor-α
Introduction
Pathogen detection by a Toll-like receptor (TLR) initiates a pro-inflammatory process, in which the cytokine tumour necrosis factor-α (TNF-α) plays a key role. This process is followed by an anti-inflammatory response, in which the cytokine interleukin-10 (IL-10) is a key player. Tight regulation of TNF-α production, together with timely up-regulation of IL-10 production, are essential for the prevention of excessive damage to the host.1
Activation of the cyclic adenosine monophosphate (cAMP) pathway is employed by a multitude of ligands, such as β-adrenergic receptor (β-AR) agonists2 and prostaglandin E2 (PGE2)3, to down-regulate production of TNF-α,4,5 as well as to up-regulate production of IL-10.5 The canonical cAMP pathway propagates via protein kinase A (PKA) -mediated phosphorylation of cAMP response element binding protein (CREB),6 a transcription factor that binds to the CRE sites, present at both TNF-α7 and IL-108 promoters.
A novel anti-inflammatory phospholipid-like drug was initially reported by Matsui et al. as a potent inhibitor of TNF-α secretion in lipopolysaccharide (LPS) -challenged rodents.9,10 In addition to TNF-α suppression, the compound, named by us phospho-ceramide analogue-1 (PCERA-1), up-regulates IL-10 production in LPS-challenged mice, and in LPS-stimulated primary macrophages11 or RAW264.7 macrophages.12 Although the target of PCERA-1 remains unidentified, experimental evidence indicates that it is a cell-surface protein, distinct from the known receptors for the endogenous phospholipid mediators sphingosine-1-phosphate (S1P), lysophosphatidic acid (LPA) and low-density lipoprotein-derived oxidized phospholipids.11,12 Importantly, PCERA-1 was found to up-regulate cAMP production in RAW264.7 macrophages.12 Formation of cAMP is generally attributed to G protein signalling,13 but may also result from a Ca2+ influx which activates certain adenylyl cyclase (AC) isoforms,14 from direct activation of AC by compounds like forskolin,14 or from inhibition of the phosphodiesterase (PDE).15 Therefore, the first goal of the research described here was to determine the mechanism of cAMP formation by PCERA-1. Our second objective was to delineate the signalling pathway from cAMP to transcriptional regulation of TNF-α and IL-10.
We show here that PCERA-1 activates AC in a guanosine triphosphate (GTP) -dependent manner, strongly suggesting that the PCERA-1 receptor is a G protein coupled receptor (GPCR). PCERA-1 induced PKA-mediated phosphorylation of CREB as well as transcriptional activation of a cAMP-dependent luciferase reporter activity. PKA activation was found to be required for transcriptional regulation of TNF-α and IL-10 by PCERA-1.
Materials and methods
Reagents and cell culture
The LPS (Escherichia coli serotype 055:B5), dibutyryl cAMP (db-cAMP), H89, rolipram, forskolin, propranolol, 8-(4-chloro-phenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate (8CPT-2Me-cAMP), W7, ethyleneglycoltetraacetic acid (EGTA), BAPTA-AM, isobutylmethylxanthine (IBMX), dexamethasone, cAMP, adenosine triphosphate (ATP), GTP, creatine phosphate, creatine phosphate kinase, Alumina resin, phenylmethylsulphonyl fluoride and dimethylsulphoxide were all purchased from Sigma-Aldrich (St Louis, MO). Ro318220 was purchased from Calbiochem (Darmstadt, Germany). SB203580 was purchased from A.G. Scientific (San Diego, CA). Dowex AG50W-X4 resin was purchased from Bio-Rad Laboratories (Hercules, CA). [α-32P]ATP and the LANCE-cAMP kit were purchased from Perkin-Elmer (Waltham, MA). The PGE2 was purchased from Biomol International (Plymouth Meeting, PA). Trypsin, l-glutamine, penicillin and streptomycin were purchased from Biological Industries (Beit Haemek, Israel). Dulbecco’s modified Eagle’s minimum essential medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco (Carlsbad, CA). Bovine serum albumin was purchased from Amresco (Solon, OH). The enzyme-linked immunosorbent assay (ELISA) reagent sets for TNF-α and IL-10 were purchased from R&D Systems (Minneapolis, MN). The cAMP enzyme immunoassay kit was purchased from Cayman Chemicals (Ann Arbor, MI). The antibody against α-tubulin was from Santa Cruz Biotechnology (Santa Cruz, CA). A CRE-containing EVX-1 promoter luciferase reporter gene construct (hereafter CRE-luciferase)16 and the antibody against phospho Ser-133 CREB were a kind gift from Dr Marc Montminy (Salk Institute, La-Jolla, CA). Infrared dye-labelled secondary antibodies and blocking buffer were obtained from Li-Cor Biosciences (Lincoln, NE). Immobilon-FL polyvinylidene fluoride (PVDF) membranes were from Millipore (Billerica, MA). Complete protease inhibitors mixture and HD-fugene transfection reagent were purchased from Roche (Mannheim, Germany). Endofree Plasmid Maxi Kit was from Qiagen (Hilden, Germany). Dual-luciferase reporter assay kit was from Promega (Madison, WI). DH10B bacteria were from Invitrogen (Carlsbad, CA). The PCERA-1 was synthesized according to published procedures,17,18 dissolved in phosphate-buffered saline (PBS) and freshly diluted in culture media. Mouse RAW264.7 macrophage cells, obtained from the American Type Culture Collection (ATCC, Rockville, MD), were grown to 80–90% confluence in DMEM supplemented with 2 mm l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (hereafter culture medium) with 10% FBS. The cells were grown and maintained at 37° in a humidified incubator with 5% CO2.
Macrophages activation assay and cytokine measurement
RAW264.7 macrophages were maintained for 48 hr before the experiment in 96-well plates, at 2 × 105 cells per well, in culture medium supplemented with 5% FBS. The culture medium was replaced 2 hr before treatment to avoid the artefact of medium replacement on signalling.19 The cells were stimulated with LPS (100 ng/ml) and/or PCERA-1 (1 μm, unless indicated otherwise) at 37° for 2 hr. Secretion of TNF-α and IL-10 into the medium was measured with a commercially available ELISA reagents set, according to the manufacturer’s instructions, using a microplate reader (Bio-Tek, Winooski, Vermont). The samples were stored at − 80° until used.
Whole cell cAMP measurements
The RAW264.7 macrophages were maintained for 48 hr before the experiment in 24-well plates, at 5 × 105 cells per well, in culture medium supplemented with 5% FBS. The cells were pre-incubated with the PDE inhibitor IBMX (0·5 mm) for 10 min at 37°, and then for an additional period of 10 min with either PCERA-1 at the indicated concentrations, or PGE2 (100 ng/ml) and/or LPS (100 ng/ml). The intra-cellular cAMP level was measured with a commercially available enzyme immunoassay kit (Cayman Chemicals), according to the manufacturer’s instructions.
Preparation of RAW264.7 membranes
RAW264.7 macrophages were washed with cold PBS and centrifuged (200 g for 10 min at 4°). The supernatant was discarded and the pellet was re-suspended in an ice-cold medium containing Tris–HCl buffer pH 7·4 (50 mm), ethylenediaminetetraacetic acid (EDTA; 1 mm), MgCl2 (5 mm) and dithiothreitol (6 mm), homogenized in a glass homogenizer (20 strokes) and centrifuged (40 000 g for 20 min at 4°). The final pellet was re-suspended in the above buffer with the addition of 0·3 m sucrose and stored at − 80°.
Adenylyl cyclase (AC) assay in membranes
Production of cAMP in membranes was measured by the LANCE-cAMP kit, according to the manufacturer’s (Perkin-Elmer) instructions. In short, RAW264.7 membranes (6 μg protein) were re-suspended in PBS and incubated for 30 min at 25° with creatine phosphate (5 mm), creatine phosphate kinase (7·7 U), MgCl2 (5 mm), IBMX (0·5 mm), propranolol (1 μm) and ATP (0·1 mm). GTP (0–10 μm), and PCERA-1 (1 μm) or forskolin (10 μm), were added as indicated (Fig. 1c). The final volume was 12 μl in a 384-well plate. Termination and cAMP measurement were performed according to the manufacturer’s instructions using the Synergy 2 time-resolved fluorescence plate reader (BioTek, Winooski, VT). In addition, we repeated the experiment using a different protocol with [α-32P]ATP as a trace substrate, as previously described.20 Radioactive cAMP was measured according to the method of Salomon et al.21 The results (data not shown) were similar to those obtained using the LANCE-cAMP kit (Perkin-Elmer) (Fig. 1c).
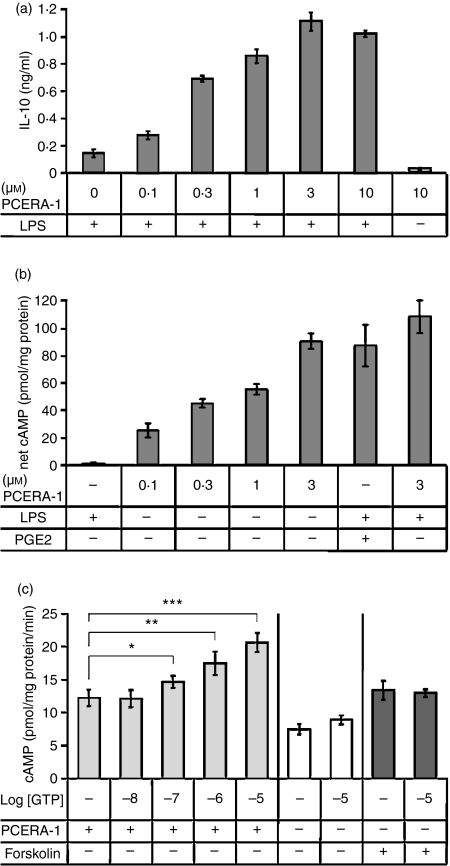
Figure 1.
Synergistic interleukin-10 (IL-10) production by lipopolysaccharide (LPS) and phospho-ceramide analogue-1 (PCERA-1) is associated with a PCERA-1-induced GTP-dependent cAMP increase. (a) Mouse macrophage RAW264.7 cells were incubated at 37° for 2 hr with LPS (100 ng/ml) and/or the indicated concentrations of PCERA-1. IL-10 release to the medium was measured by enzyme-linked immunosorbent assay. Each data point represents mean ± SD (n = 6). Background IL-10 level was 25 ± 6 pg/ml. P<0·003 for cells treated with PCERA-1 and LPS, compared with LPS only. (b) The cells were pre-incubated with the phosphodiesterase (PDE) inhibitor isobutylmethylxanthine (IBMX; 0·5 mm) for 10 min at 37° before the addition of either PCERA-1 at the indicated concentrations, prostaglandin E2 (PGE2; 0·1 μm), or LPS (100 ng/ml) for an additional period of 10 min. Intra-cellular cAMP was then measured by enzyme immunoassay. Each data point represents mean ± SD (n = 6) following reduction of control value (64 pmol cAMP/mg protein). P<0·005 for cells treated with PCERA-1 or PGE2, compared with vehicle. (c) Membranes of RAW264.7 cells were prepared and adenylyl cyclase (AC) activation by PCERA-1 (1 μm) was assayed for 30 min at 25° in the presence of increasing concentrations of GTP, as indicated. A detailed protocol of the LANCE-cAMP method is described in the Materials and methods. As controls, AC activation by forskolin (10 μm) or vehicle was similarly measured in the presence or absence of GTP (10 μm). The β-AR antagonist, propranolol (1 μm), was added to all treatments including control to reduce the background of constitutive β-AR activity. Each data point represents mean ± SD (n = 5). *P<0·02, **P<0·003, ***P<0·0002.
CREB phosphorylation assay
RAW264.7 macrophages were maintained for 24 hr before the experiment in 12-well plates, at 5 × 105 cells per well, in culture medium supplemented with 0·1% FBS. The cells were stimulated with PCERA-1 (1 μm) at 37° for 15 min, unless indicated otherwise. The cells were then washed twice with cold PBS and lysed for 1 hr at 4° with buffer containing Triton X-100 (1%), Tris–HCl buffer pH 8·0 (50 mm), NaCl (100 mm), β-glycerophosphate (50 mm), sodium orthovanadate (1 mm), EDTA (1 mm), EGTA (1 mm), glycerol (30%), phenylmethylsulphonyl fluoride (1 mm) and a complete protease inhibitor mixture diluted according to the manufacturer’s instructions. Cell extracts were centrifuged (14 000 g, 15 min at 4°) and the supernatants were stored at − 80°.
Western blotting
Cell extracts (30 μg protein) were boiled for 5 min in sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) buffer, subjected to 10% SDS–PAGE, and proteins were transferred to Immobilon-FL PVDF membrane. An antibody raised against phospho-Ser-133 CREB was used together with an antibody against α-tubulin, or separately from an antibody against CREB (total). Two-colour imaging and quantitative analysis of Western blots were performed using the Odyssey infrared imaging system (Li-Cor Biosciences), according to the manufacturer’s instructions.
Protein determination
Protein was determined by a modification of the Bradford procedure, which yields linear results, increased sensitivity and reduced detergent interference, as we have previously described.22 Bovine serum albumin served as standard.
Transfection and reporter gene assay
The CRE-luciferase plasmid was amplified using DH10B bacteria, and purified using an Endofree Plasmid Maxi Kit (Qiagen). The resulting plasmid preparation showed no ability to induce TNF-α production in RAW264.7 macrophages, indicating that it was indeed free of LPS contamination (< 0·1 ng/ml). RAW264.7 macrophages were grown for 24 hr in six-well plates, at 8 × 105 cells per well, in culture medium supplemented with 10% FBS. The cells were then co-transfected with 1·5 μg reporter plasmid and 0·5 μg pRL-TK vector which contains the herpes simplex virus thymidine kinase (HSV-TK) promoter to provide low to moderate levels of renilla luciferase expression (for normalization). The two plasmids were pre-incubated with 6 μl HD-fugene transfection reagent in culture medium for 15 min at room temperature, before addition to the cells. Following a 24 hr transfection, the cells were washed and stimulated for 3 hr at 37° with db-cAMP (0·1 mm) or with PCERA-1 (1 μm) and/or rolipram (0·2–20 μm). Luciferase activity in cell extracts was determined following the manufacturer’s (Promega) instructions. Data were expressed as a ratio of CRE-driven firefly luciferase activity divided by the renilla luciferase activity. Transfection with the empty reporter vector, performed as a control, yielded no detectable activity.
Statistical analysis
All the data were analysed using Student’s t-test wherever applicable. In all cases, differences of P<0·05 were considered to be significant. The 50% effective concentration (EC50) values were calculated by non-linear regression curve fitting, using the Graphpad prism software (GraphPad Software Inc., La Jolla, CA). Error bars were calculated for six replicates (with the exception of Fig 1c where n = 5) within a single experiment. All experiments were repeated as least three times.
Results
Synergistic IL-10 production by LPS and PCERA-1 is associated with a PCERA-1-induced cAMP increase
We have previously demonstrated that PCERA-1 increased intracellular cAMP level12 and synergized LPS-induced IL-10 production by macrophages.23 We therefore decided to examine the correlation between these activities. PCERA-1 induced IL-10 production in LPS-activated RAW264.7 macrophages with an EC50 value of 0·15 ± 0·05 μm (Fig. 1a), consistent with a previous determination.12 Treatment with PCERA-1 in the absence of LPS did not result in IL-10 production over the background level (Fig. 1a). Yet, as shown in Fig. 1(b), PCERA-1 elicited cAMP accumulation in resting macrophages (i.e. in the absence of LPS) with a dose response similar to that measured for the elevation of LPS-induced IL-10 production. The observed cAMP increase was comparable to that induced by PGE2 (Fig. 1b), consistent with the similar efficacy of PCERA-1 and PGE2 in synergistic IL-10 production. LPS neither elicited cAMP production, nor significantly augmented PCERA-1-elicited cAMP production (Fig. 1b). Taken together, our results point to a strong correlation between the LPS-independent ability of PCERA-1 to elevate intracellular cAMP and its ability to up-regulate IL-10 production in LPS-activated macrophages.
PCERA-1 stimulates GTP-dependent and Ca2+-independent AC activity in macrophages
The ability of PCERA-1 to increase cAMP level in the intact macrophages raised the question of an AC activation mechanism. As several AC isozymes can be stimulated by either GTP-binding proteins or by Ca2+, we decided to examine the possible role of Ca2+ in PCERA-1 signalling. To this end we measured cAMP formation in PCERA-1-stimulated RAW264.7 macrophages pre-incubated for 30 min with various inhibitors of Ca2+ signalling. We found that cAMP formation in response to PCERA-1 was not inhibited by any of the following Ca2+ modulators: the extra-cellular chelator EGTA, the intra-cellular chelator BAPTA-AM, or the calmodulin antagonist W7 (data not shown). These results indicate that activation of AC by PCERA-1 is not mediated by Ca2+ signalling.
To further probe the mechanism of AC activation we used membrane preparations of RAW264.7 macrophages and compared cAMP formation by PCERA-1, in the presence and absence of GTP. The β-AR antagonist propranolol was added to the reaction mixture to reduce the high background resulting from constitutive β-AR activity. In the presence of 10 μm GTP, treatment of the macrophages membranes with PCERA-1 evoked a fold-increase of 2·3 ± 0·2 in AC activity, comparable to treatment with the direct AC activator forskolin (Fig. 1c), or with PGE2 (data not shown). The finding that cAMP is elevated by PCERA-1 also in membranes is therefore consistent with the lack of effect of Ca2+ inhibitors on that activity in intact cells. Our results further show that reduction of GTP concentration gradually decreased PCERA-1-induced AC activity in the membranes. In the absence of GTP, specific (net) PCERA-1-induced AC activity was reduced by over 70%, whereas forskolin-activated AC was unaffected by GTP absence (Fig. 1c). The residual activity of PCERA-1 in the apparent absence of GTP may be partially attributed to constitutive G-protein-independent AC activity, and also may be partially attributed to a GTP trace present in the ATP reagent used as an AC substrate. It should be noted that a similar residual activity in the apparent absence of GTP was observed also for the β-AR agonist isoproterenol (data not shown). These results indicate that PCERA-1 activates AC in a Ca2+-independent but GTP-dependent manner, suggesting the involvement of a Gs protein-coupled receptor.
Modulation of cytokine production by PCERA-1 is PKA-dependent
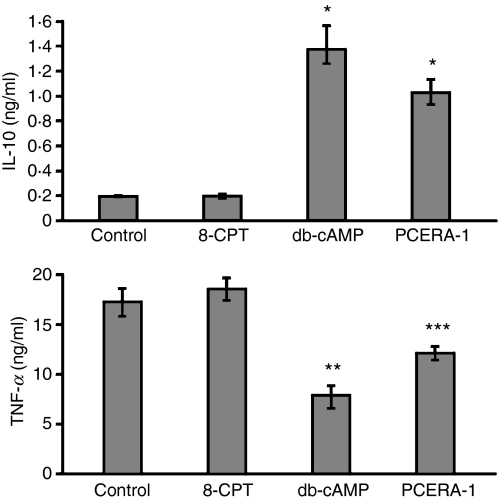
To further correlate between cAMP increase and IL-10 induction by PCERA-1, we tested the ability of the cell-permeable db-cAMP to mimic the effects of PCERA-1 on cytokine modulation. Figure 2 shows that db-cAMP efficiently suppressed LPS-induced TNF-α production, and elevated LPS-induced IL-10 production. These effects were even more pronounced than those of PCERA-1, implying that the exogenous cAMP level exceeds agonist-induced endogenous cAMP formation, in agreement with our previous findings.23 The cAMP pathway is involved in a wide variety of cellular processes that are routinely thought to be mediated by PKA. The discovery of Rap guanine nucleotide exchange proteins directly activated by cAMP (EPAC-1 and EPAC-2) raised the possibility that some cAMP effects, previously attributed to PKA, were in fact mediated by EPAC.24,25 To distinguish a putative role of EPAC in TNF-α and IL-10 modulation, we measured production of these cytokines by LPS-stimulated RAW264.7 cells treated with or without the EPAC-specific activator 8CPT-2Me-cAMP.26 Our results show that the EPAC-specific activator, used at a concentration in which the general activator db-cAMP is maximally active, had no effect on the production of TNF-α or IL-10 in LPS-stimulated macrophages (Fig 2). These results suggest that PKA rather than EPAC modulates LPS-induced TNF-α and IL-10 expression in RAW264.7 macrophages.
Figure 2.
Modulation of lipopolysaccharide (LPS) -induced cytokine production by phospho-ceramide analogue-1 (PCERA-1) is mimicked by dibutyryl (db-) cAMP and not by an exchange protein directly activated by cAMP (EPAC) agonist. RAW264.7 macrophages were incubated at 37° for 2 hr with LPS (100 ng/ml) and with either a specific EPAC activator – 8CPT-2Me-cAMP (0·3 mm), db-cAMP (0·3 mm) or PCERA-1 (1 μm). Tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) release to the medium was measured by enzyme-linked immunosorbent assay. Each data point represents mean ± SD (n = 6). The cytokines were undetectable (< 20 pg/ml) in the absence of LPS. *P<0·0002, **P<0·004, ***P<0·05.
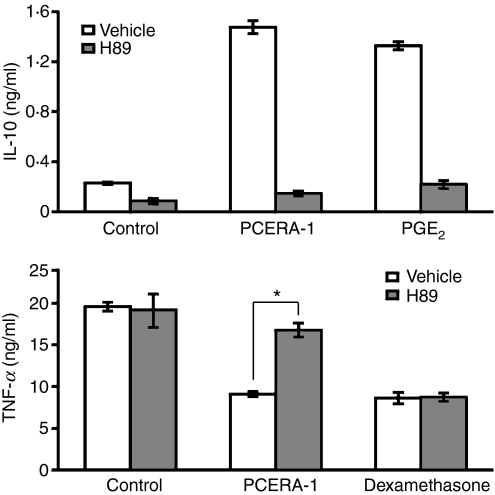
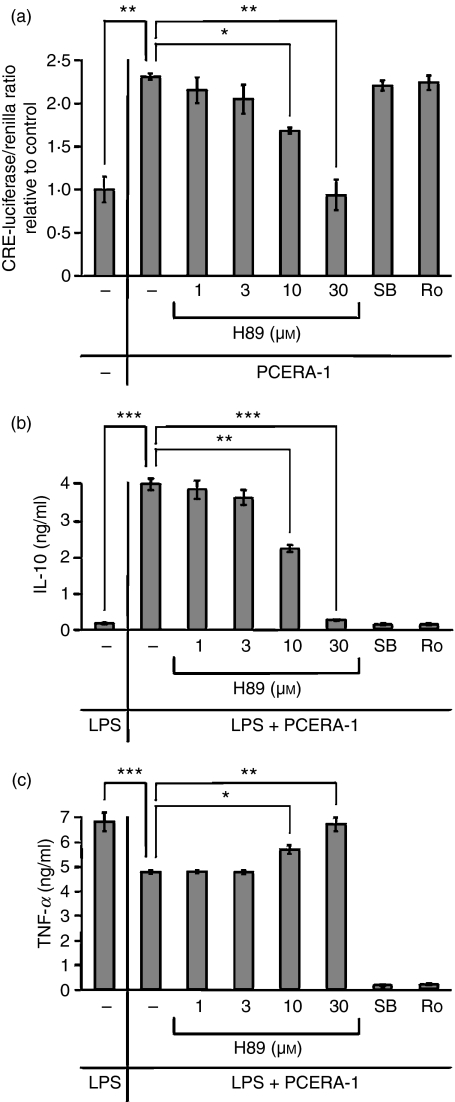
To further study the involvement of the cAMP-PKA pathway in the activity of PCERA-1, we used the PKA inhibitor H89. We found that H89 reversed the inhibitory effect of PCERA-1 on LPS-induced TNF-α production, as well as the stimulatory synergistic effect of PCERA-1 on IL-10 induction in LPS-activated macrophages (Fig. 3). As expected, H89 reversed also the cAMP-dependent activity of PGE2, whereas it failed to reverse the cAMP-independent inhibitory effect of dexamethasone on LPS-induced TNF-α production (Fig. 3). These results imply that the effects of PCERA-1 on expression of both cytokines are mediated, at least in part, by cAMP-activated PKA. Interestingly, the PKA inhibitor significantly inhibited LPS-induced IL-10 production also in the absence of PCERA-1 (Fig. 3), implying that basal cAMP is required for IL-10 induction by LPS alone.
Figure 3.
Phospho-ceramide analogue-1 (PCERA-1) modulates tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) production via protein kinase A (PKA). RAW264.7 macrophages were pre-incubated for 30 min at 37° with the PKA inhibitor H89 (30 μm, solid bars) or with vehicle (open bars). The cells were then stimulated with lipopolysaccharide (LPS; 100 ng/ml) and with either PCERA-1 (1 μm), prostaglandin E2 (PGE2; 0·1 μm) or dexamethasone (1 μm), and further incubated for 2 hr. TNF-α and IL-10 release to the medium was measured by enzyme-linked immunosorbent assay. Each data point represents mean ± SD (n = 6). The cytokines were undetectable (< 20 pg/ml) in the absence of LPS. *P<0·0002.
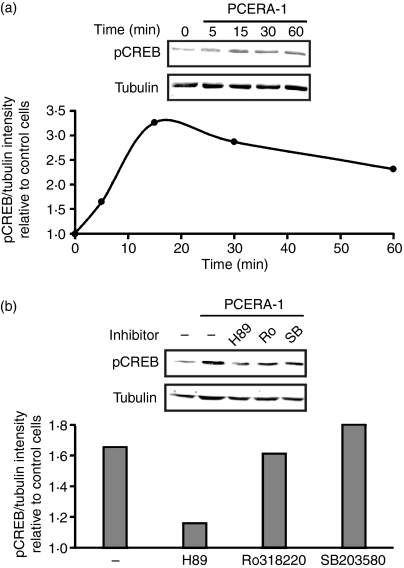
PCERA-1 induces PKA-mediated CREB phosphorylation
Activation of PKA by PCERA-1 was further studied on the level of phosphorylation of the downstream transcription factor CREB. As shown in Fig. 4(a), PCERA-1 rapidly induced phosphorylation of CREB at Ser-133, up to 3·3-fold over basal, within 15 min. Previous reports have shown that CREB phosphorylation can also be mediated by p38-activated MSK1,27,28 and that H89 inhibits MSK1 in addition to PKA 27. Therefore, we examined which of these pathways is involved in the PCERA-1 effect. Figure 4(b) shows that H89 decreased PCERA-1-induced CREB phosphorylation, whereas the specific p38 inhibitor SB203580 and the specific MSK1 inhibitor Ro318220 were ineffective with regards to PCERA-1 activity. These results indicate that phosphorylation of CREB on Ser-133 in response to PCERA-1 is carried out by PKA.
Figure 4.
Phospho-ceramide analogue-1 (PCERA-1) stimulates protein kinase A (PKA) -mediated phosphorylation of cAMP response element binding protein (CREB). (a) RAW264.7 macrophages were stimulated with PCERA-1 (1 μm) for the indicated time at 37°. The proteins in cell lysates (30 μg) were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis and transferred to Immobilon-FL poylvinylidene difluoride. The membrane was probed with antibodies against phospho Ser-133 CREB, and against α-tubulin (for normalization). Quantitative Western blot analysis is shown as the ratio of intensities of phospho-CREB and tubulin, relative to unstimulated control cells. The treatments had no effect on the total level of CREB (data not shown). The results are representative of five independent experiments. (b) Pre-incubation of RAW264.7 macrophages for 30 min at 37° with either H89 (PKA and MSK1 inhibitor, 30 μm), Ro318220 (‘Ro’, MSK1 inhibitor, 5 μm), SB203580 (‘SB’, p38 inhibitor, 30 μm) or vehicle, was followed by the addition of PCERA-1 (1 μm) or vehicle for an additional 15 min. Cell lysates were analysed as in (a). The results are representative of three independent experiments.
PCERA-1 stimulates CRE-regulated transcriptional activity
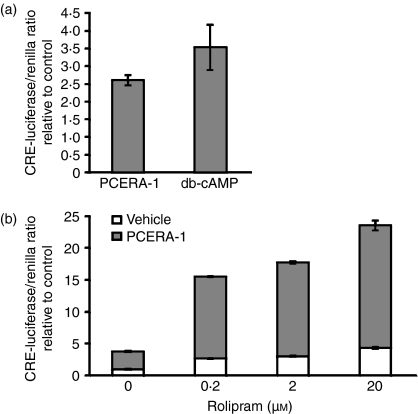
To asses the effect of CREB phosphorylation on its activity as a transcription factor, RAW264.7 macrophages were transiently transfected with a reporter gene construct which codes for luciferase under the regulation of CRE. Figure 5(a) shows that PCERA-1 and the cell-permeable db-cAMP elevated CRE-dependent luciferase activity, 2·6-fold and 3·5-fold, respectively. The concentration of exogenous db-cAMP in this experiment was sub-optimal to better correspond to the endogenous level physiologically reached by receptor-mediated adenylyl cyclase activation, as previously compared by a detailed dose–response of IL-10 activation.23 Strikingly, the PDE-4 inhibitor, rolipram, amplified PCERA-1-induced luciferase expression up to 11-fold (Fig. 5b). The synergistic luciferase activity by PCERA-1 and rolipram was 3·4-fold higher than the sum of their separate effects (Fig. 5b). These results support our findings regarding the involvement of the cAMP pathway in the cytokine modulating activity of PCERA-1, and suggest that PDE-4 activity critically regulates the transcriptional outcome in macrophages exposed to PCERA-1 or other cAMP-elevating agents.
Figure 5.
Phospho-ceramide analogue-1 (PCERA-1) induces CRE-luciferase reporter gene activation. RAW264.7 macrophages were transiently transfected for 24 hr at 37° with a reporter gene construct which codes for firefly luciferase under the regulation of CRE, and with a renilla luciferase construct for normalization. Luciferase activity assay was performed as described in the Materials and methods. Each data point represents mean ± SD (n = 6) of values normalized against renilla luciferase activity, and relative to unstimulated control cells. (a) The cells were washed and incubated with PCERA-1 (1 μm), dibutyryl (db-) cAMP (0·1 mm) or vehicle, for 3 hr at 37°. P<0·004 for cells treated with PCERA-1 or db-cAMP, compared with control. (b) The cells were washed and pre-incubated for 20 min at 37° with the specific PDE-4 inhibitor rolipram at the indicated concentrations, before PCERA-1 (1 μm, upper bars) or vehicle (lower bars) was added for an additional 3 hr. P<0·001 for cells treated with rolipram, compared with control (either with or without PCERA-1).
Finally, the effect of relevant kinase inhibitors on expression of a CRE-luciferase reporter gene and of endogenous TNF-α and IL-10 was studied in the RAW264.7 macrophages. The PKA inhibitor H89 dose-dependently blocked the stimulatory effects of PCERA-1 on CRE-driven luciferase expression (Fig. 6a), and on IL-10 expression (Fig. 6b), as well as the inhibitory effect of PCERA-1 on TNF-α expression (Fig. 6c). In contrast, the p38 inhibitor SB203580 and the specific MSK1 inhibitor Ro318220 had no effect on PCERA-1-dependent CRE-luciferase expression (Fig. 6a), in agreement with their ineffectiveness on CREB phosphorylation (Fig. 4b). These results therefore confirm that PCERA-1 induces CREB phosphorylation and transcriptional activation via PKA. Notably, both SB203580 and Ro318220 completely blocked TNF-α and IL-10 expression in macrophages exposed to LPS and PCERA-1 (Fig. 6b,c), implying that the p38-MSK1 pathway is essential for TNF-α and IL-10 expression in a CREB-independent manner.
Figure 6.
Phospho-ceramide analogue-1 (PCERA-1) regulates transcriptional activation in a protein kinase A (PKA) -dependent manner. RAW264.7 macrophages were pre-incubated for 30 min at 37° with H89 (PKA inhibitor, 1-30 μm), Ro318220 (‘Ro’, MSK1 inhibitor, 5 μm), SB203580 (‘SB’, p38 inhibitor, 30 μm) or vehicle, and then further incubated with PCERA-1 (1 μm) and/or lipopolysaccharide (LPS; 100 ng/ml) for 3 hr (a) or 2 hr (b, c). (a) Before the the above treatment, the cells were transiently transfected for 24 hr at 37° with a reporter gene construct which codes for firefly luciferase under the regulation of CRE, and with a renilla luciferase construct for normalization. Following treatment, luciferase activity assay was performed as described in the Materials and methods. Each data point represents mean ± SD (n = 6) of values normalized against renilla luciferase activity, and relative to unstimulated control cells. (b, c) TNF-α and IL-10 release to the medium were measured by enzyme-linked immunosorbent assay. Each data point represents the mean ± SD (n = 6). The cytokines were undetectable (< 20 pg/ml) in the absence of LPS. *P<0·02, **P<0·005, ***P<0·0007.
Discussion
The ceramide-1-phosphate analogue PCERA-1 exerts a potent anti-inflammatory activity in LPS-challenged mice,11 in part by reciprocally modulating TNF-α and IL-10 production in stimulated macrophages, via an as yet unidentified cell surface receptor.12 Previous studies indicated that this receptor is distinct from the known phospholipid-binding receptors for S1P, LPA and oxidized phospholipids.11,12 The potential therapeutic benefit of PCERA-1 in inflammatory diseases currently treated by TNF-α-neutralizing protein drugs, warrants a detailed study of the mechanism of activity of this putative drug. The results presented here indicate that PCERA-1 signalling involves the Gs protein activating the cAMP pathway and leading to TNF-α suppression and IL-10 induction at the transcription level.
We have previously shown that PCERA-1 increases cAMP formation in macrophages,12 so we decided to determine the mechanism of this activity. The intra-cellular cAMP level can be elevated either by AC activation resulting in induced formation of new cAMP, or by PDE inhibition resulting in preservation of basally formed cAMP. In our experimental settings, the general PDE inhibitor IBMX was used to increase the signal-to-noise ratio of cAMP measurements, implying that PCERA-1 enhances the enzymatic activity of AC, rather than acts as a PDE inhibitor. This conclusion is further supported by the synergistic effect of PCERA-1 and the PDE-4 inhibitor rolipram on CRE reporter activity (Fig. 5b) and on IL-10 production in LPS-stimulated RAW264.7 macrophages.23
Transmembrane AC is conventionally activated by Gs-coupled receptors.13 However, AC isoforms I, III and VIII can alternatively be activated by Ca2+-bound calmodulin.14 Of relevance, AC isoforms III and VIII are expressed in the RAW264.7 cell line studied here.29 Three experiments argue against the involvement of Ca2+ signalling in AC stimulation by PCERA-1. First, PCERA-1-induced cAMP formation in intact cells was not affected by the chelation of extra-cellular Ca2+ by EGTA or intra-cellular Ca2+ by BAPTA-AM, or by the inhibition of calmodulin with W7. Second, cAMP formation in response to PCERA-1 treatment is demonstrated not only in intact cells where Ca2+ may possibly act as a second messenger, but also in membrane preparations where Ca2+ signalling is irrelevant. Finally, the GTP-dependence of PCERA-1-stimulated cAMP formation in membrane preparations provides conclusive evidence in favour of G protein mediation. It should be mentioned that AC can also be directly activated by agents such as forskolin. However, Fig. 1(c) shows that cAMP formation in response to forskolin does not depend on GTP being present in the reaction mixture, in contrast to AC activation by PCERA-1. Taken together, our findings indicate that PCERA-1 activates AC via a G protein, and so suggest that the PCERA-1 receptor is a GPCR. Importantly, this GPCR is distinct from the known GPCRs for the endogenous phospholipid mediators S1P and LPA, and for LDL-derived oxidized phospholipids.11,12 Furthermore, recently, Gómez-Muñoz and co-workers have elegantly demonstrated that the endogenous sphingolipid ceramide-1-phosphate (C1P) binds and activates an unidentified Gi-coupled receptor in RAW264.7 macrophages.30 In spite of the structural similarity between PCERA-1 and C1P, our recent data indicate that the receptors for these two compounds are distinct.31 The data presented here implicate a GPCR in PCERA-1 signalling, suggesting that this unidentified receptor directly binds to and is activated by extra-cellular PCERA-1. Nonetheless, GPCRs can also be transactivated by receptor tyrosine kinases,32,33 and so a mechanism whereby PCERA-1 indirectly activates a GPCR via a receptor tyrosine kinase would also be consistent with our data.
Multiple lines of experiments (such as pertussis toxin sensitivity or direct binding) suggest that while a GPCR mediates C1P-stimulated macrophage migration, there are also GPCR-independent C1P effects, such as calcium entry,34–36 cPLA2 activation,37, 38 cell survival and proliferation.31, 39, 40 It is therefore possible that GPCR-independent effects also exist for PCERA-1, in addition to the GPCR-mediated TNF-α and IL-10 modulation.
For many years PKA was considered to be the sole mediator of cAMP cellular effects. However, it has been found that some activities of cAMP are actually mediated by EPAC rather than by PKA.24, 25 In LPS-stimulated microglial cells, the PKA inhibitor H89 completely prevented the stimulatory effect of db-cAMP on IL-10 production, but only modestly prevented its inhibitory effect on TNF-α production, suggesting the involvement of an alternative cAMP target.41 The mechanism of TNF-α suppression by cAMP is cell type-dependent as PKA (but not EPAC) mediates that activity in alveolar and peritoneal macrophages42 and in monocyte-derived macrophages,43 while both PKA and EPAC are involved in that activity in dendritic cells.42 Two lines of evidence argue in favour of PKA as the mediator of cytokine modulation by the cAMP inducer PCERA-1 in RAW264.7 macrophages. First, we show that PKA inhibition by H89 effectively blocks both TNF-α suppression and IL-10 induction by PCERA-1 in these cells. These H89 effects are specifically attributed to the inhibition of PKA, rather than to the inhibition of p38-activated MSK1,27,28 because PCERA-1-induced CREB phosphorylation and CRE-dependent transcription were blocked only by H89 and not by the p38 inhibitor SB203580 or by the MSK1 inhibitor Ro318220 (Figs 4b and 6a). Second, the general cAMP analogue, db-cAMP, mimicked the effects of PCERA-1, whereas a specific EPAC agonist was ineffective. As EPAC is upstream to the p38-MSK1 pathway,25, 44 these results suggest that PKA, rather than EPAC and MSK1, mediates CREB phosphorylation and subsequent TNF-α suppression and IL-10 induction by PCERA-1 in RAW264.7 macrophages. This cell line expresses mainly EPAC-1 and also EPAC-2,45 but as far as we know was not tested before for EPAC involvement in these processes. While PKA is clearly essential for cytokine modulation by PCERA-1, and activation of EPAC alone is totally ineffective, we cannot rule out the possibility that EPAC participates in TNF-α suppression or IL-10 induction in addition to PKA. As discussed below, we suggest that PKA mediates cytokine modulation via CREB. Magocsi et al. have suggested that PKA suppresses TNF-α production in peritoneal macrophages by inhibiting the extracellular signal-regulated kinase (ERK) pathway.46 This mechanism, however, is unlikely to be relevant for PCERA-1 activity in RAW264.7 cells, as we have previously shown that PCERA-1 does not affect ERK phosphorylation.12
Activation of the cAMP pathway leads to transcriptional activation of the IL-10 promoter,23 suggesting that CREB is a positive regulator of IL-10 transcription. The role of CREB in transcription of TNF-α is less understood, as cAMP inducers negatively regulate TNF-α expression. Substitution or deletion mutations of the TNF-α CRE site result in the reduction of reporter activity in stimulated mouse RAW264.7 macrophages47, 48 or human THP-1 monocytes,49 apparently suggesting that CREB may function as a positive regulator of TNF-α transcription. However, other reports suggest that stimulation of mouse RAW264.7 macrophages50–52 or human THP-1 monocytes53 with LPS leads to the displacement of CREB by activated c-Jun at the TNF-α CRE site, whereas activation of the cAMP pathway in LPS-stimulated RAW264.7 macrophages was suggested to set back the transcription factor composition at the TNF-α CRE site to that observed in unstimulated cells, leading to suppression of TNF-α transcription.50–52 Interestingly, deletion of the CRE results in an increase of basal TNF-α reporter activity, suggesting that CRE has a repressive role in unstimulated cells.49 The evidence shown here for activation of CREB by PCERA-1, together with inhibition of TNF-α expression by PCERA-1, suggest that PCERA-1-activated CREB negatively regulates TNF-α production. Moreover, the finding that the PKA inhibitor H89 reversed TNF-α suppression exerted by PCERA-1, whereas p38 and MSK1 inhibitors blocked TNF-α expression (Fig. 6c), further supports the conclusion regarding the inhibitory role of CREB in TNF-α expression. Our results are therefore consistent with the reports regarding competition between cAMP-activated CREB and LPS-activated c-Jun in binding of CRE at the TNF-α promoter.50–53 Interestingly, similar roles were suggested for CREB and c-Jun in LPS-induced COX-2 expression in RAW264.7 macrophages.54, 55
To conclude, we have demonstrated here that PCERA-1 sequentially activates the signalling components of the cAMP pathway, as it elevates cAMP level, induces PKA-mediated CREB phosphorylation and up-regulates CRE-dependent reporter transcription. Our results suggest that the unidentified PCERA-1 receptor is a novel GPCR that upon ligation activates the classical cAMP-PKA-CREB pathway, and diverts LPS-stimulated macrophages in an anti-inflammatory direction, by reciprocal regulation of TNF-α and IL-10 expression. Future identification of the PCERA-1 receptor has a potential value for the translational research in the area of inflammatory diseases.
Acknowledgments
This paper is dedicated to the memory of the late Dr Zvi Selinger, a pioneer of the G proteins field, a mentor and a colleague. The research was supported by grants from the European Commission (IRG #021862), from Teva Pharmaceutical Industries Ltd., from the public committee for allocation of Estate funds at Israel’s ministry of justice (#3223), and from the Israel Science Foundation (#907/07). T.Z. was financially supported by Israel’s Ministry of Absorption. We are grateful to Mrs Nava Silberstein for superb technical assistance and to Dr Hugh Rosen, Dr Nathanael S. Gray, Dr Iris Ben-Dror and Dr Lily Vardimon for helpful discussions and for the supply of reagents. We thank Mr Roi Mashiach, Mr Peter Ding and Dr Mark Parnell for chemical synthesis of PCERA-1. We are grateful to Dr Marc Montminy for the gift of pCREB antibody and CRE-luciferase construct. Finally, thanks to Dr Zvi Naor, Orna Ernst, Meir Goldsmith and Yifat Glucksam for their critical reading of the manuscript.
Glossary
Abbreviations:
- AC
adenylyl cyclase
- β-AR
β-adrenergic receptor
- C1P
ceramide-1-phosphate
- cAMP
cyclic adenosine monophosphate
- db-cAMP
dibutyryl cAMP
- EPAC
exchange protein directly activated by cAMP
- GPCR
G protein coupled receptor
- IBMX
isobutylmethylxanthine
- PCERA-1
phospho-ceramide analogue-1
- PDE
phosphodiesterase
- PGE2
prostaglandin E2
- PKA
protein kinase A
- PVDF
Immobilon-FL polyvinylidene fluoride
- TLR
Toll-like receptor
Disclosures
The authors declare no financial conflicts of interests.
References
- 1.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–59. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Szelenyi J, Kiss JP, Puskas E, Szelenyi M, Vizi ES. Contribution of differently localized alpha 2- and beta-adrenoceptors in the modulation of TNF-alpha and IL-10 production in endotoxemic mice. Ann N Y Acad Sci. 2000;917:145–53. doi: 10.1111/j.1749-6632.2000.tb05378.x. [DOI] [PubMed] [Google Scholar]
- 3.Takano M, Nishimura H, Kimura Y, Washizu J, Mokuno Y, Nimura Y, Yoshikai Y. Prostaglandin E2 protects against liver injury after Escherichia coli infection but hampers the resolution of the infection in mice. J Immunol. 1998;161:3019–25. [PubMed] [Google Scholar]
- 4.Kast RE. Tumor necrosis factor has positive and negative self regulatory feed back cycles centered around cAMP. Int J Immunopharmacol. 2000;22:1001–6. doi: 10.1016/s0192-0561(00)00046-1. [DOI] [PubMed] [Google Scholar]
- 5.Zidek Z. Adenosine – cyclic AMP pathways and cytokine expression. Eur Cytokine Netw. 1999;10:319–28. [PubMed] [Google Scholar]
- 6.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–80. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 7.Kuprash DV, Udalova IA, Turetskaya RL, Kwiatkowski D, Rice NR, Nedospasov SA. Similarities and differences between human and murine TNF promoters in their response to lipopolysaccharide. J Immunol. 1999;162:4045–52. [PubMed] [Google Scholar]
- 8.Platzer C, Fritsch E, Elsner T, Lehmann MH, Volk HD, Prosch S. Cyclic adenosine monophosphate-responsive elements are involved in the transcriptional activation of the human IL-10 gene in monocytic cells. Eur J Immunol. 1999;29:3098–104. doi: 10.1002/(SICI)1521-4141(199910)29:10<3098::AID-IMMU3098>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 9.Matsui T, Kondo T, Nishita Y, et al. Highly potent inhibitors of TNF-α production. Part 1: discovery of chemical leads. Bioorg Med Chem Lett. 2002;12:903–5. doi: 10.1016/s0960-894x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 10.Matsui T, Kondo T, Nishita Y, et al. Highly potent inhibitors of TNF-α production. Part 2: identification of drug candidates. Bioorg Med Chem Lett. 2002;12:907–10. doi: 10.1016/s0960-894x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 11.Avni D, Goldsmith M, Ernst O, et al. Modulation of TNFα, IL-10 and IL-12p40 levels by a ceramide-1-phosphate analog, PCERA-1, in-vivo and ex-vivo in primary macrophages. Immunol Lett. 2009;123:1–8. doi: 10.1016/j.imlet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith M, Avni D, Levy-Rimler G, et al. A ceramide-1-phosphate analogue, PCERA-1, simultaneously suppresses tumour necrosis factor (TNF)-α and induces interleukin (IL)-10 production in activated macrophages. Immunology. 2008;127:103–15. doi: 10.1111/j.1365-2567.2008.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selinger Z. Discovery of G protein signaling. Annu Rev Biochem. 2008;77:1–13. doi: 10.1146/annurev.biochem.76.082906.094316. [DOI] [PubMed] [Google Scholar]
- 14.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–39. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 16.Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413–23. doi: 10.1016/j.molcel.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Matsui T, Kondo T, Nakatani S, et al. Synthesis, further biological evaluation and pharmacodynamics of newly discovered inhibitors of TNF-α production. Bioorg Med Chem. 2003;11:3937–43. doi: 10.1016/s0968-0896(03)00409-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsui T, Kondo T, Nishita Y, et al. Highly potent inhibitors of TNF-α production. Part II: metabolic stabilization of a newly found chemical lead and conformational analysis of an active diastereoisomer. Bioorg Med Chem. 2002;10:3787–805. doi: 10.1016/s0968-0896(02)00380-2. [DOI] [PubMed] [Google Scholar]
- 19.Smith ER, Jones PL, Boss JM, Merrill AH., Jr Changing J774A.1 cells to new medium perturbs multiple signaling pathways, including the modulation of protein kinase C by endogenous sphingoid bases. J Biol Chem. 1997;272:5640–6. doi: 10.1074/jbc.272.9.5640. [DOI] [PubMed] [Google Scholar]
- 20.Zor T, Bar-Yaacov M, Elgavish S, Shaanan B, Selinger Z. Rescue of a mutant G-protein by substrate-assisted catalysis. Eur J Biochem. 1997;249:330–6. doi: 10.1111/j.1432-1033.1997.00330.x. [DOI] [PubMed] [Google Scholar]
- 21.Salomon Y, Londos C, Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974;58:541–8. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- 22.Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236:302–8. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- 23.Goldsmith M, Avni D, Ernst O, Glucksam Y, Levy-Rimler G, Meijler MM, Zor T. Synergistic IL-10 induction by LPS and the ceramide-1-phosphate analog PCERA-1 is mediated by the cAMP and p38 MAP kinase pathways. Mol Immunol. 2009;46:1979–87. doi: 10.1016/j.molimm.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–8. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 25.Bos JL. Epac proteins: multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–6. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Enserink JM, Christensen AE, de Rooij J, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–6. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 27.Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1 beta in RAW264 macrophages. J Immunol. 2000;164:3018–25. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- 28.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21:4831–40. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osawa Y, Lee HT, Hirshman CA, Xu D, Emala CW. Lipopolysaccharide-induced sensitization of adenylyl cyclase activity in murine macrophages. Am J Physiol Cell Physiol. 2006;290:C143–51. doi: 10.1152/ajpcell.00171.2005. [DOI] [PubMed] [Google Scholar]
- 30.Granado MH, Gangoiti P, Ouro A, Arana L, Gonzalez M, Trueba M, Gomez-Munoz A. Ceramide 1-phosphate (C1P) promotes cell migration. Involvement of a specific C1P receptor. Cell Signal. 2009;21:405–12. doi: 10.1016/j.cellsig.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Levi M, Meijler MM, Gomez-Munoz A, Zor T. Distinct receptor-mediated activities in macrophages for natural ceramide-1-phosphate (C1P) and for phospho-ceramide analogue-1 (PCERA-1) Mol Cell Endocrinol. 2009 doi: 10.1016/j.mce.2009.05.007. in press, doi 10.1016/j.mce.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Pyne NJ, Waters CM, Long JS, Moughal NA, Tigyi G, Pyne S. Receptor tyrosine kinase-G-protein coupled receptor complex signaling in mammalian cells. Adv Enzyme Regul. 2007;47:271–80. doi: 10.1016/j.advenzreg.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waters C, Pyne S, Pyne NJ. The role of G-protein coupled receptors and associated proteins in receptor tyrosine kinase signal transduction. Semin Cell Dev Biol. 2004;15:309–23. doi: 10.1016/j.semcdb.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Hogback S, Leppimaki P, Rudnas B, Bjorklund S, Slotte JP, Tornquist K. Ceramide 1-phosphate increases intracellular free calcium concentrations in thyroid FRTL-5 cells: evidence for an effect mediated by inositol 1,4,5-trisphosphate and intracellular sphingosine 1-phosphate. Biochem J. 2003;370:111–9. doi: 10.1042/BJ20020970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tornquist K, Blom T, Shariatmadari R, Pasternack M. Ceramide 1-phosphate enhances calcium entry through voltage-operated calcium channels by a protein kinase C-dependent mechanism in GH4C1 rat pituitary cells. Biochem J. 2004;380:661–8. doi: 10.1042/BJ20031637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tornquist K, Ramstrom C, Rudnas B, et al. Ceramide 1-(2-cyanoethyl) phosphate enhances store-operated Ca2+ entry in thyroid FRTL-5 cells. Eur J Pharmacol. 2002;453:1–11. doi: 10.1016/s0014-2999(02)02362-2. [DOI] [PubMed] [Google Scholar]
- 37.Pettus BJ, Bielawska A, Subramanian P, et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J Biol Chem. 2004;279:11320–6. doi: 10.1074/jbc.M309262200. [DOI] [PubMed] [Google Scholar]
- 38.Wijesinghe DS, Subramanian P, Lamour NF, et al. The chain length specificity for the activation of group IV cytosolic phospholipase A2 by ceramide-1-phosphate. Use of the dodecane delivery system for determining lipid-specific effects. J Lipid Res. 2009;50:1985–95. doi: 10.1194/jlr.M800367-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gomez-Munoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal. 2008;20:726–36. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 40.Gómez-Muñoz A, Gangoiti P, Granado MH, Arana L, Ouro A. Ceramide 1-phosphate in cell survival and inflammatory signaling. In: Chalfant C, Del Poeta M, editors. Sphingolipids as Signal and Regulatory Molecules. Austin: Landes Bioscience; 2009. in press. [Google Scholar]
- 41.Woo MS, Jang PG, Park JS, Kim WK, Joh TH, Kim HS. Selective modulation of lipopolysaccharide-stimulated cytokine expression and mitogen-activated protein kinase pathways by dibutyryl-cAMP in BV2 microglial cells. Brain Res Mol Brain Res. 2003;113:86–96. doi: 10.1016/s0169-328x(03)00095-0. [DOI] [PubMed] [Google Scholar]
- 42.Aronoff DM, Carstens JK, Chen GH, Toews GB, Peters-Golden M. Short communication: differences between macrophages and dendritic cells in the cyclic AMP-dependent regulation of lipopolysaccharide-induced cytokine and chemokine synthesis. J Interferon Cytokine Res. 2006;26:827–33. doi: 10.1089/jir.2006.26.827. [DOI] [PubMed] [Google Scholar]
- 43.Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol. 2006;176:7361–70. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- 44.Kanda Y, Watanabe Y. Adrenaline increases glucose transport via a Rap1-p38MAPK pathway in rat vascular smooth muscle cells. Br J Pharmacol. 2007;151:476–82. doi: 10.1038/sj.bjp.0707247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aronoff DM, Canetti C, Serezani CH, Luo M, Peters-Golden M. Cutting edge: macrophage inhibition by cyclic AMP (cAMP): differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J Immunol. 2005;174:595–9. doi: 10.4049/jimmunol.174.2.595. [DOI] [PubMed] [Google Scholar]
- 46.Magocsi M, Vizi ES, Selmeczy Z, Brozik A, Szelenyi J. Multiple G-protein-coupling specificity of beta-adrenoceptor in macrophages. Immunology. 2007;122:503–13. doi: 10.1111/j.1365-2567.2007.02658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donnell PM, Taffet SM. The proximal promoter region is essential for lipopolysaccharide induction and cyclic AMP inhibition of mouse tumor necrosis factor-alpha. J Interferon Cytokine Res. 2002;22:539–48. doi: 10.1089/10799900252982016. [DOI] [PubMed] [Google Scholar]
- 48.Roach SK, Lee SB, Schorey JS. Differential activation of the transcription factor cyclic AMP response element binding protein (CREB) in macrophages following infection with pathogenic and nonpathogenic mycobacteria and role for CREB in tumor necrosis factor alpha production. Infect Immun. 2005;73:514–22. doi: 10.1128/IAI.73.1.514-522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz B, Lopez-Berestein G. A distinct element involved in lipopolysaccharide activation of the tumor necrosis factor-alpha promoter in monocytes. J Interferon Cytokine Res. 2000;20:741–8. doi: 10.1089/10799900050116453. [DOI] [PubMed] [Google Scholar]
- 50.Delgado M, Munoz-Elias EJ, Kan Y, Gozes I, Fridkin M, Brenneman DE, Gomariz RP, Ganea D. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide inhibit tumor necrosis factor alpha transcriptional activation by regulating nuclear factor-kB and cAMP response element-binding protein/c-Jun. J Biol Chem. 1998;273:31427–36. doi: 10.1074/jbc.273.47.31427. [DOI] [PubMed] [Google Scholar]
- 51.Leceta J, Gomariz RP, Martinez C, Abad C, Ganea D, Delgado M. Receptors and transcriptional factors involved in the anti-inflammatory activity of VIP and PACAP. Ann N Y Acad Sci. 2000;921:92–102. doi: 10.1111/j.1749-6632.2000.tb06954.x. [DOI] [PubMed] [Google Scholar]
- 52.Pozo D, Delgado M, Martinez M, Guerrero JM, Leceta J, Gomariz RP, Calvo JR. Immunobiology of vasoactive intestinal peptide (VIP) Immunol Today. 2000;21:7–11. doi: 10.1016/s0167-5699(99)01525-x. [DOI] [PubMed] [Google Scholar]
- 53.Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 54.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–66. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 55.Xie W, Herschman HR. v-src induces prostaglandin synthase 2 gene expression by activation of the c-Jun N-terminal kinase the c-Jun transcription factor. J Biol Chem. 1995;270:27622–8. doi: 10.1074/jbc.270.46.27622. [DOI] [PubMed] [Google Scholar]