Abstract
Major histocompatibility complex (MHC) class I-specific inhibitory natural killer receptors (iNKRs) are expressed by subsets of T cells but the mechanisms inducing their expression are poorly understood, particularly for killer-cell immunoglobulin-like receptors (KIRs). The iNKRs are virtually absent from the surface of cord blood T cells but we found that KIR expression could be induced upon interleukin-2 stimulation in vitro. In addition, KIR expression was enhanced after treatment with 5-aza-2′-deoxycytidine, suggesting a role for DNA methylation. In vivo induction of KIR expression on cord blood T cells was also observed during a human congenital infection with Trypanosoma cruzi which triggers activation of fetal CD8+ T cells. These KIR+ T cells had an effector and effector/memory phenotype suggesting that KIR expression was consecutive to the antigenic stimulation; however, KIR was not preferentially found on parasite-specific CD8+ T cells secreting interferon-γ upon in vitro restimulation with live T. cruzi. These findings show that KIR expression is likely regulated by epigenetic mechanisms that occur during the maturation process of cord blood T cells. Our data provide a molecular basis for the appearance of KIRs on T cells with age and they have implications for T-cell homeostasis and the regulation of T-cell-mediated immune responses.
Keywords: DNA methylation, interleukin-2, killer-cell immunoglobulin-like receptors, T-cell receptors, Trypanosoma cruzi
Introduction
Natural killer cells express inhibitory natural killer cell receptors (iNKRs) recognizing major histocompatibility complex (MHC) class I molecules that participate in the control of NK-cell tolerance to self. In human adults, the main iNKRs include killer-cell immunoglobulin-like receptors (KIRs) interacting with different allelic groups of MHC class I human leucocyte antigen-A, -B and -C,1 LIR1/ILT2 (CD85j), which recognizes most MHC class I molecules,2 and CD94/NKG2A (CD94/CD159a), which binds to the non-classical human leucocyte antigen-E molecules.3 In addition to NK cells, iNKRs can also be found on subpopulations of peripheral blood T-cell receptor-αβ-positive (TCR-αβ+) and TCR-γδ+ T cells.4,5 They regulate T-cell function by interfering with TCR signalling.5 Most TCR-αβ+ iNKR+ T lymphocytes are memory CD8+ T cells lacking CCR7 expression. Yet, some CD4+ T cells expressing KIRs are detected both in healthy individuals and in diseases.6
Studies so far suggest that the regulation of iNKR expression on T cells differs between receptor families. In humans and mice, induction of CD94/NKG2A is observed subsequent to TCR engagement and is clonally determined.4,7 Cytokines such as interleukin-2 (IL-2), IL-10, IL-12, IL-15 or transforming growth factor-β can also participate in the induction of CD94/NKG2A surface expression on adult T cells.8–10 Conversely, factors driving KIR expression still remain unknown. Expression of KIR has been found mostly on T cells harbouring a memory phenotype and a skewed TCR repertoire, suggesting that its expression is associated with antigenic stimulation. However, in vitro TCR ligation or cytokine stimulations failed to induce KIR expression on adult T cells.5 T cells expressing identical TCR can be KIR-negative or KIR-positive and can express a large variety of combinations of KIRs, which indicates that TCR rearrangement precedes KIR acquisition.11,12 Consistent with this, cord blood and thymic T cells rarely express iNKRs.13,14 The expression of KIRs on NK cells is controlled by epigenetic processes. Expression of KIR has been correlated with DNA methylation of CpG near the transcription start site which maintains allele-specific KIR gene expression in NK cells.15,16 Recent results suggest that KIR expression on T cells may also be regulated by DNA methylation. Indeed, it has been reported that dense DNA methylation patterns in the promoter of specific KIR genes of cord blood KIR-negative T cells and KIR2DL4 and KIR2DL2 transcription were induced in T cells following DNA methyltransferase inhibition.17–19
To attempt to identify factors that could drive KIR expression on T cells, we used cord blood T cells stimulated both in vitro and in vivo. Neonatal T cells are qualitatively different from adult T cells. They display a naive phenotype, rarely express iNKRs and are characterized by a reduced ability to produce T helper type 1 cytokines and a decreased cytotoxicity potential.20 They are considered more immature than adult T cells; however, under some circumstances, neonates can mount an adult-like T-cell response. We reported that a strong cord blood CD8+ T-cell response can develop in newborns congenitally infected with Trypanosoma cruzi, the protozoal agent of Chagas’ disease.21 Expansion and differentiation of these mature CD8+ T cells in utero provided us with a unique setting to look for the induction of KIR in vivo following antigen stimulation. In the present study, we found that both in vitro IL-2 stimulation and congenital infection could induce significant expression of KIRs on cord blood CD8+ T cells.
Materials and methods
Patients and samples
Cord blood was obtained from newborn infants delivered at the maternity hospital ‘German Urquidi’ [Universidad Mayor de San Simon (UMSS), Cochabamba, Bolivia] or from the Department of Obstetrics of the Erasme Hospital (Brussels, Belgium). Adult blood samples were collected from healthy Belgian volunteers. Information about newborn infants and congenital infection with T. cruzi has previously been reported.22 This study had ethical approval from the scientific/ethics committees of UMSS and Université Libre de Bruxelles. We obtained the informed written consent of the mothers before blood collection. Cord blood (CBMC) and peripheral blood (PBMC) mononuclear cells were isolated by Nycoprep density gradient centrifugation (Nycomed Pharma AS, Oslo, Norway) and cryopreserved. No contamination of CBMC with PBMC from the mothers was detected, as previously described.21
Antibodies and flow cytometry
Flow cytometric analysis was carried out using standard protocols on a Becton Dickinson FACScalibur and using Cellquest software. The following monoclonal antibodies (mAbs) and their matched isotype controls were used: anti-CD3 (clone SK7) anti-CD8 (SK1), anti-CD62-L (Dreg 56), anti-CD45RA (L48), anti-interferon-γ (IFN-γ; 25723.11), anti-CCR7 (2H4), anti-CD158a (EB6, anti-KIR2DL1/KIR2DS1), anti-CD158b (GL183, anti-KIR2DL2/3/KIR2DS2) from BD Biosciences (Erembodegem, Belgium) and anti-CD159a (Z199) and anti-TCR-γδ (IMMU510) from Beckman Coulter (Fullerton, CA). Anti-CD158e (DX9, anti-KIR3DL1), anti-CD158k (DX31, anti-KIR3DL2), anti-CD159b (DX22, anti-CD94) mAbs were provided by DNAX (Palo Alto, CA) and anti-CD85j (GHI/75, anti-LIR1/ILT2) was generously donated by D. Mason (Oxford, UK). Unlabelled antibodies were visualized using goat F(ab′)2 fragment anti-mouse immunoglobulin G (IgG; H+L) (BD Biosciences). Intracellular staining was performed as recommended by BD Biosciences. The nuclear antigen KI-67 was used to measure cell cycle activity as recommended by the manufacturer and cell death was determined by staining CBMC with fluorescein isothiocyanate-conjugated annexin-V (BD Biosciences).
Stimulation of T cells with live T. cruzi
The CBMC (0·5 × 106/ml) were resuspended in RPMI-1640 supplemented with 10% fetal calf serum, 100 U/ml penicillin G and 100 μg/ml streptomycin (Cambrex Bio Science, Verviers, Belgium). Then, CBMC were stimulated for 24 hr with live T. cruzi trypomastigotes (TcIIe genotype) in the presence or absence of recombinant human IL-15 (1 ng/ml) (R&D Systems Europe, Abingdon, UK), in a 2 : 1 parasite : cell ratio. Brefeldin A (10 μg/ml; Sigma, St Louis, MO) was added for the last 4 hr of the culture.
In vitro induction of iNKR expression on T cells
The CBMC (1 × 106/ml) were cultured for 4 days with coated anti-CD3 mAb (OKT3; 10 μg/ml) or in medium supplemented with IL-2 at 1 or 10 ng/ml (Peprotech, Rocky Hill, NJ), in the presence or absence of 5-aza-2′-deoxycytidine (5-aza; 1 μm; Sigma). No significant toxicity of 5-aza was observed after 4 days of treatment.
Statistical analysis
The graphpad prism software was used to calculate statistical significance (P < 0·05). The comparison of the medians between the three cohorts (uninfected newborns, congenitally infected newborns and healthy adults) was done by using non-parametric analysis of variance (Kruskal–Wallis test) followed by Dunn’s analysis. Otherwise, statistical differences were determined using the Wilcoxon matched pair tests.
Results
In vitro IL-2 stimulation induces KIR expression on cord blood T cells and KIR expression is increased following treatment with DNA methylase inhibitor
We used cord blood T cells which are mostly iNKR negative to identify factors inducing KIR expression on T cells. The CBMCs were stimulated for 4 days with low and high doses of IL-2 or plate-bound anti-CD3 mAb and KIR expression was monitored by flow cytometry on CD8+ T cells. As CD158b was the most frequently induced KIR (data not shown), we focused the study on that particular KIR. Interestingly, CD158b expression was induced on cord blood T cells following IL-2 stimulation but not after TCR triggering (Fig. 1a). The expression of CD158b was found to be a proliferation-independent event as positive cells remained CFSEhigh after 4 days of stimulation with IL-2 (data not shown). By contrast and in agreement with the literature,5 no significant induction of CD158b was observed upon IL-2 and anti-CD3 stimulation on adult CD8+ T cells (Fig. 1a). A significant reduction of the proportion of CD158b+ CD8+ T cells was even observed, correlated with higher proliferation on CD3 cross-linking, further emphasizing that TCR engagement does not induce KIR expression on T cells. Inhibition of DNA methylation using 5-aza induced a further significant increase in the frequency of CD158b-expressing CD8+ T cells both in cord blood and adult T cells (Fig. 1b). The effect of 5-aza was specific to KIR as expression of CD94/NKG2A, which was also induced upon IL-2 stimulation or TCR ligation, was not modulated by 5-aza treatment (data not shown).
Figure 1.
Induction of killer-cell immunoglobulin-like receptors (KIR) expression on cord blood CD8+ T cells in vitro. Frequency of cord blood and adult CD8+ T cells expressing CD158b after incubation for 4 days of cord blood mononuclear cells (healthy newborns) and peripheral blood mononuclear cells (healthy adults) in medium alone, medium supplemented with interleukin-2 (IL-2; 1 or 10 ng/ml) and in the presence of plate-bound anti-CD3 monoclonal antibody (αCD3). (a) without additional treatment. (b) without (white histograms) and with (black histograms) treatment with 5-aza-2′-deoxycytidine (5-aza) for 4 days. Mean ± SEM are shown. Data are representative of (a) 7–13 experiments and (b) 3–13 experiments. Statistical analysis was performed using Wilcoxon matched paired test. P-values are indicated.
Altogether, these results indicate that KIR expression can be induced upon cytokine stimulation on cord blood CD8+ T cells and not on adult T cells. The frequency of KIR-expressing T cells is enhanced following treatment with 5-aza, an inhibitor of DNA methylation.
KIR expression on cord blood T cells is induced upon congenital infection with T. cruzi
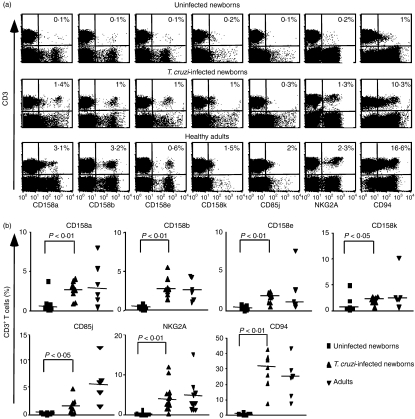
We also investigated whether KIR would also be induced in vivo on cord blood CD8+ T cells, in the context of an acute infection in utero. Human congenital infection with T. cruzi triggers a clonal expansion of activated CD8+ T cells.21 In this study, we confirmed the activation of CD8+ T cells from congenitally infected newborns with a significant increase of CD45R0 expression (median 21·6%, range 14·4–39·3, n = 10) and significant decrease of CD62 ligand expression (median 74·9%, range 18·9–86·6, n = 9) compared with uninfected newborns (median 7·3%, range 0·2–16·1, n = 13 and median 91%, range 54·7–96·3, n = 12, respectively) (P < 0·05). We also confirmed that cord blood T cells barely expressed iNKRs (CD94/NKG2A, CD158a, CD158b, CD158e, CD158k and CD85j) in healthy newborns (Fig. 2). By contrast, we measured a significant expression of KIRs on cord blood CD3+ T cells from congenitally infected newborns: CD158a, P < 0·01; CD158b, P < 0·01; CD158e, P < 0·01; CD158k, P < 0·05, with a frequency that was not too different from that for adults. CD94/NKG2A receptors were also strongly and significantly up-regulated on T cells from congenitally infected newborns (P < 0·01). The acquisition of CD85j surface expression on CD3+ T cells was more variable but the proportion of positive T cells was significantly increased compared with uninfected newborns (P < 0·05). It is of interest to note that such up-regulation was not detected on NK cells,23 providing evidence that the induction of iNKRs on T cells was not a consequence of T. cruzi infection that would up-regulate iNKRs on all lymphocyte populations. Importantly, the expression of not only KIRs but also of CD94/NKG2A was strictly associated with the infection of the baby because uninfected newborn infants of T. cruzi-infected mothers lacked iNKR expression on their T cells (data not shown).
Figure 2.
Inhibitory natural killer cell receptor (iNKR) expression on CD3+ T cells from congenitally Trypanosoma cruzi-infected newborns. (a) iNKR expression on CD3+ T cells from representative cord blood mononuclear cells of uninfected or congenitally T. cruzi-infected newborns and peripheral blood mononuclear cells of a healthy adult. The proportion of iNKR-positive cells among CD3+ T cells is indicated in the quadrants. (b) Percentages of iNKR+ cells among CD3+ T cells in uninfected or congenitally T. cruzi-infected newborns and in adults. Horizontal bars show the medians (n = 7–19) and P-values between groups are indicated. Statistical analysis was performed using Kruskal–Wallis test followed by Dunn’s analysis.
KIR+ cord blood T cells from newborns congenitally infected with T. cruzi are mainly αβ TCR+ CD8+ T cells displaying an effector and effector/memory phenotype
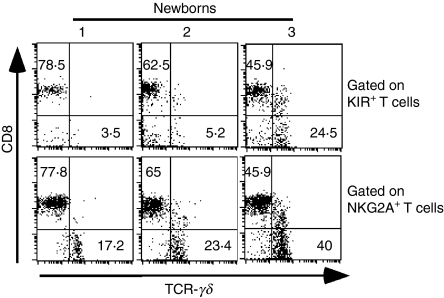
We further characterized the KIR+ T cells in newborns congenitally infected with T. cruzi. Expression of KIR was measured as a whole using a pool of mAbs including anti-CD158a, anti-CD158b, anti-CD158e and anti-CD158k. As indicated in Fig. 3, their expression was detected mostly on TCR-αβ+ TCR-γδ− CD8high T cells, while NKG2A was also frequently expressed on TCR-γδ+ T cells.
Figure 3.
Phenotype of killer-cell immunoglobulin-like receptor (KIR)-expressing and NKG2A-expressing T cells from congenitally Trypanosoma cruzi-infected newborns. Cord blood mononuclear cells from three representative congenitally T. cruzi-infected newborns were stained with anti-CD3, anti-CD8, anti-TCR-γδ and either anti-NKG2A or a pool of anti-KIR monoclonal antibodies (CD158a, CD158b, CD158e and CD158k). The proportions of CD8+ or TCR-γδ+ T cells among gated KIR+ or NKG2A+ CD3+ T cells are indicated.
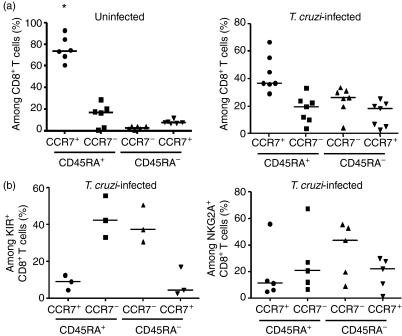
T cells can be divided into subsets whether they are naive, effector or memory cells using CD45RA and CCR7 cell surface markers.24 In agreement with the literature, uninfected newborns exhibited a predominant population of naive CCR7+ CD45RA+ CD8+ T cells (Fig. 4a). By contrast, the percentage of naive CD8+ T cells in congenitally infected newborns decreased significantly (P < 0·01) while the percentage of effector and effector or central memory cells increased. We next analysed the phenotype of KIR+ CD8+ T cells as well as CD94/NKG2A+ CD8+ T cells from congenitally infected newborns (Fig. 4b). As expected, we found a drastic decrease of the proportion of naive CD8+ T cells among CD8+ T cells expressing KIR or CD94/NKG2A, the size of these subsets being smaller than the percentage of naive cells among total CD8+ T cells in these newborns (Fig. 4a,b). The vast majority of KIR+ CD8+ T cells had a phenotype of effector (CCR7− CD45RA+) and effector memory (CCR7− CD45RA−) cells. CD94/NKG2A+ CD8+ T cells had a more distributed pattern but with a slightly higher proportion of effector/memory CD8+ T cells. These results associate the expression of KIR with the differentiation of CD8+ T cells into effector cells upon T. cruzi infection.
Figure 4.
Expression of CCR7 and CD45RA by cord blood T cells. Cord blood mononuclear cells from healthy and congenitally Trypanosoma cruzi-infected newborns were stained with (a) anti-CD8, anti-CD45RA, anti-CCR7 or with (b) anti-CD8, anti-CD45RA, anti-CCR7 and either anti-NKG2A or a pool of anti-killer-cell immunoglobulin-like receptors (KIR) monoclonal antibodies (CD158a, CD158b, CD158e and CD158k). Scatter plots represent the proportion of CCR7+/− CD45RA+/− cells among (a) CD8+ T cells from uninfected or T. cruzi-infected newborns or (b) KIR+ CD8+ and NKG2A+ CD8+ T cells from T. cruzi-infected newborns. Horizontal bars show the medians (n = 3–7). A statistically significant difference was observed in the proportion of naive CCR7+ CD45RA+ CD8+ T cells between total CD8+ T cells of uninfected and T. cruzi-infected newborns (*P < 0·01). P-values were calculated using Wilcoxon matched pair test.
KIR expression on T. cruzi-specific CD8+ T cells
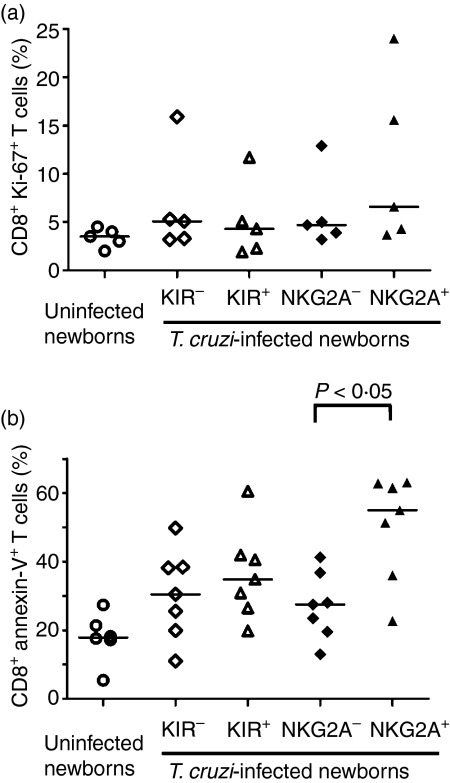
To further characterize the phenotype of KIR+ CD8+ T cells in congenitally infected newborns, we analysed the frequency of cycling cells among KIR+ and CD94/NKG2A+ CD8+ T cells by measuring the intracellular expression of the nuclear protein KI-67 (Fig. 5a). The number of cycling KIR+ CD8+ T cells was not different from KIR− CD8+ T cells and the proportion of KI-67+ CD94/NKG2A+ CD8+ T cells tended to be consistently higher than KI-67+ CD94/NKG2A− CD8+ T cells in infected newborns. We also monitored apoptosis in the circulating pool of CD8+ T cells from newborns congenitally infected with T. cruzi. As shown in Fig. 5(b), we did not detect any differences in the frequency of KIR+ and KIR− CD8+ T cells undergoing apoptosis while a significant increase in the proportion of CD94/NKG2A+ CD8+ T cells undergoing apoptosis was found compared with CD94/NKG2A− CD8+ T cells. Altogether, these data suggest that KIR+ cord blood CD8+ T cells from T. cruzi-infected newborns have a lower dynamic turnover than CD94/NKG2A+ CD8+ T cells.
Figure 5.
Cell cycling and apoptosis in cord blood killer-cell immunoglobulin-like receptors-positive (KIR+) and NKG2A+ CD8+T cells from Trypanosoma cruzi-infected newborns. Cell cycling (a) and apoptosis (b) were measured in CD8+ CD3+ T cells from uninfected newborns and in KIR+/− or NKG2A+/− CD8+ CD3+ T cells from T. cruzi-infected newborns; (a) frequency of KI-67+ CD8+ T cells and (b) frequency of Annexin-V+ CD8+ T cells. Horizontal bars represent the medians (n = 5–7). P-values were calculated using Wilcoxon matched pair test.
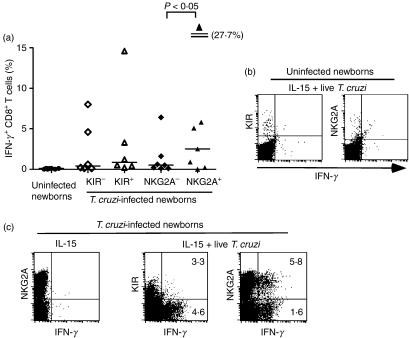
To further assess the link between antigen-specific activation and induction of KIR, we analysed the ability of KIR+ CD8+ T cells to produce IFN-γ upon parasite-specific stimulation and compared it with CD94/NKG2A+ CD8+ T cells. The CBMCs were stimulated with live T. cruzi in the presence of IL-15 for 24 hr, and IFN-γ secretion by CD8+ T cells was measured by intracellular flow cytometry staining. T cells from uninfected newborns were unable to produce IFN-γ upon exposure to both T. cruzi and IL-15 (Fig. 6a,b) whereas most of the congenitally infected newborns tested did (Fig. 6a,c). The parasite was involved in this activation because stimulation with IL-15 alone did not result in IFN-γ production (Fig. 6c). No difference in the proportion of cells secreting IFN-γ was detected between KIR+ and KIR− CD8+ T cells and the percentage of IFN-γ+ cells among CD94/NKG2A+ CD8+ T cells was higher than among CD94/NKG2A− CD8+T cells (P < 0·05) (Fig. 6a,c). These results suggest that KIR expression is not preferentially associated with activation of parasite-specific CD8+ T cells.
Figure 6.
Interferon-γ (IFN-γ) production by cord blood killer-cell immunoglobulin-like receptors-positive (KIR+) and NKG2A+ CD8+T cells upon in vitro stimulation with Trypanosoma cruzi + interleukin-15 (IL-15). (a) Frequency of IFN-γ+ CD8+ T cells from uninfected and from KIR+/− or NKG2A+/− CD8+ T cells of T. cruzi-infected newborns upon in vitro stimulation with live T. cruzi and IL-15. Horizontal bars represent the medians (n = 6–8). P-values were calculated using Wilcoxon matched pair test. (b) Representative uninfected newborns. (c) Representative T. cruzi-infected newborns. (b,c) Intracellular flow cytometry staining for IFN-γ in gated CD8+ T cells following stimulation with live T. cruzi and IL-15 or IL-15 alone. (c) Frequencies of IFN-γ+ cells among KIR+/− or NKG2A+/− CD8+ T cells are indicated.
Discussion
To date, KIR expression on T cells has been associated with memory T-cell differentiation and senescence.5 Expression of KIR by a small proportion of T cells has been demonstrated in inflammatory disorders, tumours and some viral infections, like human cytomegalovirus infection.5,25–28 The proportions of KIR+ CD8+ T cells was also found enriched in human immunodeficiency virus 1-infected subjects and a direct link between KIR expression and viral loads has been suggested.29 The biological functions of KIRs on T cells are still poorly understood. It has been proposed that they are involved in the maintenance of memory T-cell survival and resistance to activation-induced cell death.30,31 They can also modulate the T-cell activation threshold32 and uncouple T-cell effector functions,33 so playing a role in the regulation of T-cell-mediated immune responses.
Factors driving KIR expression on subsets of T cells are also still ill-defined. Many groups failed to induce KIRs on human adult T cells. Similarly, attempts to induce Ly49 receptors on mouse T cells were unsuccessful.5 These findings led to the conclusion that induction of KIR or Ly49 was a rare stochastic event with a randomly distributed expression among T cells,34 consistent with different KIRs being expressed on a small proportion of the progeny of a single T-cell clone. The modelling of KIR gene expression by computer stimulation confirmed this conclusion of a stochastic, cumulative expression of KIR genes during clonal replication.35 It is clear that although all T cells express the transcriptional machinery to express KIR, not many do.36 The expression of KIR on T cells is associated with a memory phenotype suggesting that KIR is induced after antigenic stimulation. However, it has so far been difficult to show that a natural infection could induce KIR expression on T cells in humans. We report here the in vivo induction of KIRs on human CD8+ T cells. Their expression was observed during a congenital infection with T. cruzi and was a direct consequence of the infection because KIR was not induced on T cells of uninfected newborns from T. cruzi-infected mothers. Expression of KIR was found on cord blood T cells with an effector and effector/memory phenotype suggesting that its acquisition occurred during the differentiation of naive CD8+ T cells into effector and memory cells. However, KIR expression did not seem to be tightly associated with activation of parasite-specific T cells. We detected similar proportions of KIR+ and KIR− CD8+ T cells secreting IFN-γ in response to in vitro stimulation with T. cruzi + IL-15 while a higher proportion of CD94/NKG2A+ CD8+ T cells secreted the cytokine compared with CD94/NKG2A− CD8+ T cells. This finding correlates with our in vitro data showing that only IL-2 stimulation and not TCR cross-linking could induce KIR expression on cord blood CD8+ T cells while CD94/NKG2A was induced upon TCR ligation. It is also consistent with KIR being absent on most antigen-specific T cells ex vivo in several viral infections.5,37 Interestingly, van Stijn et al.28 recently reported KIR up-regulation on CD8+ T cells during the acute phase of human cytomegalovirus infection and not during the latency phase. This up-regulation was found on CD45RA+ CD27− CD8+ effector-type T cells but not on pp65-specific T cells recognizing an immunodominant epitope. Whether induction of KIR is restricted to specific subsets of antigen-specific T cells or whether the inflammatory environment is responsible for KIR expression still remains to be investigated. It is interesting to note that we could also induce KIR expression on cord blood T cells upon IL-15 stimulation for 2 weeks. Interleukin-2 and IL-15 might share properties that allow them to contribute to KIR induction. One candidate could be the transcription factor c-myc, which has been implicated in the enhancement of KIR transcription in NK cells.38
Our results both in vitro and in vivo show that induction of KIRs occurs at a higher frequency in cord blood T cells compared with adult T cells. Interestingly, a single study reported Ly49 expression upon IL-2 stimulation on mouse T cells and the experiments were performed with T cells from young mice.39 The differences between adult and neonatal cells are likely to be linked to their level of differentiation. Cord blood T cells are phenotypically and functionally more immature than adult naive T cells.20 One of the main differences resides in the pool of recent thymic emigrants, which is important in neonatal blood.40 Neonates have a higher frequency of naive T cells that show a good response to common γ-chain cytokines such as IL-2, IL-7 and IL-1520 whereas IL-2 is not sufficient to drive extensive proliferation of adult T cells.40,41 Further work is needed to investigate whether the difference of response to IL-2 between cord blood and adult T cells controls KIR induction. It is likely, however, that the quality of the response to activating signals is not the only process involved because it does not explain why some T cells from the same progeny do express KIRs and some do not.
Earlier reports showed that DNA methylation regulates allele-specific KIR gene expression in human NK cells15,16 and KIR2DL4 expression in CD8+ T cells from elderly individuals.18 Molecular mechanisms responsible for active DNA demethylation are poorly characterized; however, components of the DNA repair machinery have recently been implicated.42,43 It therefore remains to establish whether these events could also control KIR expression in neonatal T cells. Liu et al.19 have recently shown that DNA methylation inhibition by 5-aza promoted the expression of KIR2DL2 and KIR2DL4 on mitogen-activated adult T cells through change in both chromatine structure and transcriptions factors. We extend this work by showing that 5-aza specifically induced KIR expression on cord blood and adult CD8+ T cells activated through TCR-dependent or independent pathways. These data suggest that KIR genes are partly silenced by DNA methylation in neonatal T cells. DNA methylation constitutes a biologically and chemically stable epigenetic modification, resulting in long-term gene expression changes. This explains why once KIR expression is acquired on T cells, this expression is maintained in the progeny. DNA methylation is also one of the age-associated changes in mammals44 that could be correlated with changes in DNA repair efficiency. The finding that DNA methylation regulates KIR expression on T cells provides a molecular basis for the increased proportion of KIR+ CD8+ T cells in the elderly. It would also fit with the association of KIR with chronic stimulation and the model of stochastic and cumulative expression of KIR genes during clonal replication. Inflammatory, innate and adaptive immune responses developed not only early in life but also later on, most likely shape the KIR repertoire expressed by T cells that will subsequently participate in immune homeostasis.
Acknowledgments
We thank Eduardo Suarez and the staff of the maternity hospital ‘German Urquidi’ (Cochabamba, Bolivia) for the management of patients, Mary-Cruz Torrico, Marisol Cordova and Marco Antonio Solano (CUMETROP/LABIMED, UMSS, Cochabamba, Bolivia) for performing the serological and parasitological diagnosis of patients, Pascale Deblandre for parasite culture, Anne Lazzari for technical help and Bana Jabri and Markus Uhrberg for stimulating discussions. This study was supported by the ‘Centre de Recherche Interuniversitaire en Vaccinologie’ sponsored by the ‘Région Wallonne’ and Glaxo-Smithkline Biologicals (Rixensart, Belgium), by the Conseil Interuniversitaire de la Communauté française de Belgique, and by the Fonds National de la Recherche Scientifique (Belgium, convention 3.4.615.05.F). C.A-V. is a research fellow of the Association pour la promotion de l’éducation et la formation à l’étranger (Communauté Française de Belgique). V.B. is supported by the Centre National de la Recherche Scientifique.
Glossary
Abbreviations:
- 5-aza
5-aza-2′-deoxycytidine
- iNKRs
inhibitory natural killer cell receptors
- KIR
killer-cell immunoglobulin-like receptors.
Disclosures
The authors declare no financial or commercial conflict of interest.
References
- 1.Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–14. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- 2.Shiroishi M, Tsumoto K, Amano K, et al. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci USA. 2003;100:8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braud VM, Allan DS, O’Callaghan CA, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–9. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 4.Braud VM, Aldemir H, Breart B, Ferlin WG. Expression of CD94-NKG2A inhibitory receptor is restricted to a subset of CD8+ T cells. Trends Immunol. 2003;24:162–4. doi: 10.1016/s1471-4906(03)00064-4. [DOI] [PubMed] [Google Scholar]
- 5.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol. 2004;4:190–8. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 6.Poszepczynska-Guigne E, Schiavon V, D’Incan M, et al. CD158k/KIR3DL2 is a new phenotypic marker of Sezary cells: relevance for the diagnosis and follow-up of Sezary syndrome. J Invest Dermatol. 2004;122:820–3. doi: 10.1111/j.0022-202X.2004.22326.x. [DOI] [PubMed] [Google Scholar]
- 7.Jabri B, Selby JM, Negulescu H, et al. TCR specificity dictates CD94/NKG2A expression by human CTL. Immunity. 2002;17:487–99. doi: 10.1016/s1074-7613(02)00427-2. [DOI] [PubMed] [Google Scholar]
- 8.Mingari MC, Ponte M, Bertone S, et al. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci USA. 1998;95:1172–7. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derre L, Corvaisier M, Pandolfino MC, Diez E, Jotereau F, Gervois N. Expression of CD94/NKG2-A on human T lymphocytes is induced by IL-12: implications for adoptive immunotherapy. J Immunol. 2002;168:4864–70. doi: 10.4049/jimmunol.168.10.4864. [DOI] [PubMed] [Google Scholar]
- 10.Bertone S, Schiavetti F, Bellomo R, et al. Transforming growth factor-beta-induced expression of CD94/NKG2A inhibitory receptors in human T lymphocytes. Eur J Immunol. 1999;29:23–9. doi: 10.1002/(SICI)1521-4141(199901)29:01<23::AID-IMMU23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Vely F, Peyrat M, Couedel C, et al. Regulation of inhibitory and activating killer-cell Ig-like receptor expression occurs in T cells after termination of TCR rearrangements. J Immunol. 2001;166:2487–94. doi: 10.4049/jimmunol.166.4.2487. [DOI] [PubMed] [Google Scholar]
- 12.Uhrberg M, Valiante NM, Young NT, Lanier LL, Phillips JH, Parham P. The repertoire of killer cell Ig-like receptor and CD94 : NKG2A receptors in T cells: clones sharing identical alpha beta TCR rearrangement express highly diverse killer cell Ig-like receptor patterns. J Immunol. 2001;166:3923–32. doi: 10.4049/jimmunol.166.6.3923. [DOI] [PubMed] [Google Scholar]
- 13.Mingari MC, Ponte M, Cantoni C, et al. HLA-class I-specific inhibitory receptors in human cytolytic T lymphocytes: molecular characterization, distribution in lymphoid tissues and co-expression by individual T cells. Int Immunol. 1997;9:485–91. doi: 10.1093/intimm/9.4.485. [DOI] [PubMed] [Google Scholar]
- 14.Warren HS, Rana PM, Rieger DT, Hewitt KA, Dahlstrom JE, Kent AL. CD8 T cells expressing killer Ig-like receptors and NKG2A are present in cord blood and express a more naive phenotype than their counterparts in adult blood. J Leukoc Biol. 2006;79:1252–9. doi: 10.1189/jlb.0905536. [DOI] [PubMed] [Google Scholar]
- 15.Santourlidis S, Trompeter HI, Weinhold S, et al. Crucial role of DNA methylation in determination of clonally distributed killer cell Ig-like receptor expression patterns in NK cells. J Immunol. 2002;169:4253–61. doi: 10.4049/jimmunol.169.8.4253. [DOI] [PubMed] [Google Scholar]
- 16.Chan HW, Kurago ZB, Stewart CA, et al. DNA methylation maintains allele-specific KIR gene expression in human natural killer cells. J Exp Med. 2003;197:245–55. doi: 10.1084/jem.20021127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santourlidis S, Graffmann N, Christ J, Uhrberg M. Lineage-specific transition of histone signatures in the killer cell Ig-like receptor locus from hematopoietic progenitor to NK cells. J Immunol. 2008;180:418–25. doi: 10.4049/jimmunol.180.1.418. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Weyand CM, Goronzy JJ. Epigenetic mechanisms of age-dependent KIR2DL4 expression in T cells. J Leukoc Biol. 2008;84:824–34. doi: 10.1189/jlb.0807583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Kuick R, Hanash S, Richardson B. DNA methylation inhibition increases T cell KIR expression through effects on both promoter methylation and transcription factors. Clin Immunol. 2009;130:213–24. doi: 10.1016/j.clim.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–64. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 21.Hermann E, Truyens C, Alonso-Vega C, et al. Human fetuses are able to mount an adultlike CD8 T-cell response. Blood. 2002;100:2153–8. [PubMed] [Google Scholar]
- 22.Torrico F, Alonso-Vega C, Suarez E, et al. Maternal Trypanosoma cruzi infection, pregnancy outcome, morbidity, and mortality of congenitally infected and non-infected newborns in Bolivia. Am J Trop Med Hyg. 2004;70:201–9. [PubMed] [Google Scholar]
- 23.Hermann E, Alonso-Vega C, Berthe A, et al. Human congenital infection with Trypanosoma cruzi induces phenotypic and functional modifications of cord blood NK cells. Pediatr Res. 2006;60:38–43. doi: 10.1203/01.pdr.0000220335.05588.ea. [DOI] [PubMed] [Google Scholar]
- 24.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 25.Liao YH, Jee SH, Sheu BC, et al. Increased expression of the natural killer cell inhibitory receptor CD94/NKG2A and CD158b on circulating and lesional T cells in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:318–24. doi: 10.1111/j.1365-2133.2006.07301.x. [DOI] [PubMed] [Google Scholar]
- 26.Campillo JA, Martinez-Escribano JA, Moya-Quiles MR, et al. Natural killer receptors on CD8 T cells and natural killer cells from different HLA-C phenotypes in melanoma patients. Clin Cancer Res. 2006;12:4822–31. doi: 10.1158/1078-0432.CCR-06-0019. [DOI] [PubMed] [Google Scholar]
- 27.Bonorino P, Leroy V, Dufeu-Duchesne T, et al. Features and distribution of CD8 T cells with human leukocyte antigen class I-specific receptor expression in chronic hepatitis C. Hepatology. 2007;46:1375–86. doi: 10.1002/hep.21850. [DOI] [PubMed] [Google Scholar]
- 28.van Stijn A, Rowshani AT, Yong SL, et al. Human cytomegalovirus infection induces a rapid and sustained change in the expression of NK cell receptors on CD8+ T cells. J Immunol. 2008;180:4550–60. doi: 10.4049/jimmunol.180.7.4550. [DOI] [PubMed] [Google Scholar]
- 29.Alter G, Rihn S, Streeck H, et al. Ligand independent exhaustion of killer immunoglobulin-like receptor+ CD8+ T cells in HIV-1 infection. J Virol. 2008;82:9668–77. doi: 10.1128/JVI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex-encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933–41. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 31.Ugolini S, Arpin C, Anfossi N, et al. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat Immunol. 2001;2:430–5. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 32.Ferrini S, Cambiaggi A, Meazza R, et al. T cell clones expressing the natural killer cell-related p58 receptor molecule display heterogeneity in phenotypic properties and p58 function. Eur J Immunol. 1994;24:2294–8. doi: 10.1002/eji.1830241005. [DOI] [PubMed] [Google Scholar]
- 33.Henel G, Singh K, Cui D, et al. Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood. 2006;107:4449–57. doi: 10.1182/blood-2005-06-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Veken LT, Campelo MD, van der Hoorn MA, et al. Functional analysis of killer Ig-like receptor-expressing cytomegalovirus-specific CD8+ T cells. J Immunol. 2009;182:92–101. doi: 10.4049/jimmunol.182.1.92. [DOI] [PubMed] [Google Scholar]
- 35.Snyder MR, Muegge LO, Offord C, et al. Formation of the killer Ig-like receptor repertoire on CD4+CD28null T cells. J Immunol. 2002;168:3839–46. doi: 10.4049/jimmunol.168.8.3839. [DOI] [PubMed] [Google Scholar]
- 36.Xu J, Vallejo AN, Jiang Y, Weyand CM, Goronzy JJ. Distinct transcriptional control mechanisms of killer immunoglobulin-like receptors in natural killer (NK) and in T cells. J Biol Chem. 2005;280:24277–85. doi: 10.1074/jbc.M500727200. [DOI] [PubMed] [Google Scholar]
- 37.Anfossi N, Doisne JM, Peyrat MA, et al. Coordinated expression of Ig-like inhibitory MHC class I receptors and acquisition of cytotoxic function in human CD8+ T cells. J Immunol. 2004;173:7223–9. doi: 10.4049/jimmunol.173.12.7223. [DOI] [PubMed] [Google Scholar]
- 38.Cichocki F, Hanson RJ, Lenvik T, et al. The transcription factor c-Myc enhances KIR gene transcription through direct binding to an upstream distal promoter element. Blood. 2009;113:3245–53. doi: 10.1182/blood-2008-07-166389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assarsson E, Kambayashi T, Sandberg JK, et al. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol. 2000;165:3673–9. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 40.Schonland SO, Zimmer JK, Lopez-Benitez CM, et al. Homeostatic control of T-cell generation in neonates. Blood. 2003;102:1428–34. doi: 10.1182/blood-2002-11-3591. [DOI] [PubMed] [Google Scholar]
- 41.Cookson S, Reen D. IL-15 drives neonatal T cells to acquire CD56 and become activated effector cells. Blood. 2003;102:2195–7. doi: 10.1182/blood-2003-01-0232. [DOI] [PubMed] [Google Scholar]
- 42.Barreto G, Schafer A, Marhold J, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445:671–5. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 43.Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–32. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- 44.Issa JP. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109:103–8. doi: 10.1016/s1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]