Abstract
Advanced glycation endproducts (AGEs) of food proteins resulting from the Maillard reaction after cooking or heating may have particular importance in food allergy. The underlying immunological mechanisms are only poorly understood. The aim of the study was to examine the effects of AGE derived from the model food allergen ovalbumin (AGE-OVA) on dendritic cells (DCs), their immunostimulatory capacity and the T-cell response compared with regular OVA. For this purpose, human immature DCs were exposed to fluorescein isothiocyanate (FITC)-labelled AGE-OVA and FITC-labelled regular OVA and uptake was analysed by flow cytometry and fluorescence microscopy. Furthermore, autologous CD4+ T-cell proliferation and cytokine production induced by mature DCs loaded with AGE-OVA were compared with those induced by mature DCs loaded with OVA. Finally, expression of the receptor for advanced glycation endproducts (RAGE) and activation of the transcription factor nuclear factor (NF)-κB by AGE were investigated. Internalization of FITC-AGE-OVA by immature DCs was significantly increased compared with FITC-OVA. Blocking the mannose receptor, macropinocytosis or the scavenger receptor strongly reduced uptake of both FITC-OVA and FITC-AGE-OVA. In a comparison of CD4+ T cells co-cultured with AGE-OVA-loaded mature DCs versus those co-cultured with OVA-loaded mature DCs, AGE-OVA DCs were found to produce more interleukin (IL)-6 and to induce a stronger T helper type 2 (Th2) and a weaker Th1 cytokine response, while there was no difference in proliferation of CD4+ T cells. The expression of RAGE was higher on immature DCs compared with mature DCs. AGE-OVA-exposed immature DCs showed a stronger expression of RAGE and activation of the transcription factor NF-κB compared with OVA-loaded immature DCs. Our data indicate that AGE-OVA may be more immunogenic/allergenic than regular OVA.
Keywords: advanced glycation endproducts, allergy, dendritic cells, ovalbumin, Th1/Th2
Introduction
In the industrialized nations, the prevalence of food allergy is increasing.1,2 Factors such as food production, processing, conservation, storage, sterilization and final preparation may play an important role in this increase.3 Although heat treatment of food has many advantages, such as improvements in taste, appearance and smell and the destruction of pathogens, it may produce drastic changes in the allergenicity of proteins.4,5
Most food proteins are denaturated by heat treatment, and this denaturation includes the destruction of their three-dimensional structure. Therefore, certain epitopes show a diminished capacity to bind immunoglobulin E (IgE) antibodies and thus reduced allergenic potential. However, there are also examples for the creation of new epitopes by food processing, for example during the Maillard reaction, leading to advanced glycation endproducts (AGEs).6,7 This non-enzymatic reaction of amino acids with non-reducing sugars occurs in the heat treatment during cooking of cakes, biscuits and amylase containing foods or after their long-term storage.8,9 It also takes place in the human body, mainly in aging tissues or in blood vessels of diabetic patients with increased blood sugar levels. Neoantigens induced by the Maillard reaction such as AGEs are more resistant to digestion in comparison to native proteins.10 It is possible that neoantigens bind to completely different receptors, influencing their recognition, uptake and processing by antigen-presenting cells, and possibly activate different signalling pathways.11 This may result in modified immune responses compared with those elicited by the native proteins.12–14
Six receptors that recognize and bind AGEs have been identified.15,16 The best characterized and most extensively studied receptor for AGEs (RAGE), a 46-kD protein, is mainly expressed on the surface of endothelial cells, on smooth muscle cells and on mononuclear phagocytes.17,18 RAGE belongs to the so-called ‘receptors of pattern particles’ of the innate immune system which recognize the 3D structures of proteins rather than specific amino acid sequences. In contrast to the other receptors of the innate immune system that recognize bacterial or foreign structures, the ligands for RAGE can be generated endogenously.18 They persist in the tissues for long periods and thus provoke significant ligand–receptor interactions. This leads to enhanced activation of immune cells instead of tissue clearance.19,20 RAGE-mediated endocytosis followed by lysosomal destruction is a very slow process, in contrast to the much more efficient uptake of antigens via scavenger receptor A on macrophages. The RAGE genes are located within the human and murine major histocompatibility complex (MHC) gene locus and the binding of its ligands leads to enhanced gene transcription, cell activation and inflammation.19 One mechanism that is induced by ligand binding to RAGE is the redox-dependent activation of the transcription factor nuclear factor (NF)-κB,21–23 leading to enhanced expression of the adhesion molecules vascular cell adhesion molecule (VCAM)-1 and intercellular adhesion molecule (ICAM)-1 on leucocytes and macrophages and the production of proinflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1, IL-6 and metalloproteinases. In this study we examined the potentially different effects of the native hen’s egg allergen ovalbumin (OVA) and its glycated form AGE-ovalbumin (AGE-OVA) on antigen uptake and presentation by monocyte-derived human DCs and the induced T-cell response. Additionally, we examined the expression of RAGE and the activation state of NF-κB in DCs.
Methods
Glycation of OVA
AGE-OVA was prepared as described by Gasic-Milenkovic et al.24 Briefly, 1 mm OVA (Sigma-Aldrich, Taufkirchen, Germany) was incubated with 1 m glucose in 100 mm phosphate-buffered saline (PBS), pH 7·4, at 50° for 6 weeks. OVA incubated under the same conditions, but without glucose (thermally processed OVA), was used as a control. At the end of the incubation, the AGE structures Nε-carboxymethyl-lysine (CML), Nε-carboxyethyl-lysine (CEL) and GA-pyridine, but not pyrraline, were detected in AGE-OVA by enzyme-linked immunosorbent assay (ELISA).8 The protein concentration of the samples was measured using a BCA assay kit (Pierce, Rockford, IL). Furthermore, the endotoxin content of all preparations was determined with QCL-1000 chromogenic LAL (Bio-Whittaker, Walkersville, MD) according to the instructions of the manufacturer, and was less than 1 ng/ml.
Blood samples
Leucocyte-enriched buffy coats (transfusion centre, Mainz, Germany) were obtained from non-allergic, non-atopic, tetanus-immunized healthy blood donors. The study was approved by the local ethics committee. Informed consent was obtained from all donors before participation in the study.
Generation of monocytes and monocyte-derived DCs
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by Ficoll-Paque 1·077 g/ml (PAA Laboratories GmbH, Cölbe, Germany) density centrifugation. To enrich CD14+ monocytes, 1 × 107 PBMC per well were incubated for 45 min in a six-well plate (Greiner, Frickenhausen, Germany) in Iscove’s modified Dulbecco’s medium containing l-glutamine and 25 mm Hepes (IMDM; PAA Laboratories GmbH) supplemented with an antibiotic-antimycotic solution containing 100 μg/mL streptomycin, 100 U/mL penicillin, and 250 ng/ml amphotericin B (PAA) and 3% autologous plasma at 37°. After washing of the non-adherent cells with pre-warmed PBS, the remaining monocytes (purity > 90%) were incubated in 3 ml/well IMDM supplemented with 1% heat-inactivated autologous plasma, 1000 U/ml IL-4 (Strathmann Biotech GmbH, Hannover, Germany) and 200 U/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) (Leukine®; Immunex Corp., Seattle, WA). On day 6, the resulting immature DCs were pulsed with different amounts of OVA or AGE-OVA, as indicated in the figures, in the presence or absence of 10 μg/ml polymyxin B sulphate (Sigma-Aldrich) or 1 μg/ml tetanus toxoid (Behring-Werke, Marburg, Germany), and further stimulated with 1000 U/ml TNF-α, 2000 U/ml IL-1β (Strathmann Biotech GmbH) and 1 μg/ml PGE2 (Cayman Chemical, Ann Arbor, MI) to induce their full maturation. Forty-eight hours after stimulation, the supernatant of mature DCs was collected for determination of IL-6 and IL-12p40. The cells were then harvested, washed twice and used in T-cell stimulation assays. Mature DCs expressed high levels (> 90%) of CD80, CD83, CD86 and MHC class II molecules as determined by flow cytometry.
Purification of T cells
Autologous CD4+ T cells were obtained from PBMC using antibody-coated paramagnetic MicroBeads (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the protocol of the manufacturer. Separation was controlled by flow cytometry (purity > 98%).
Co-culture of T cells and autologous allergen-pulsed DCs
For proliferation assays, 1 × 105 CD4+ T cells were co-cultured in 96-well plates (Greiner) in triplicate with 1 × 104 autologous allergen-pulsed DCs in 200 μl of IMDM supplemented with 5% heat-inactivated autologous plasma. After 5 days, the cells were pulsed with 37 kBq/well of [3H]TdR ([methyl-3H]thymidine; ICN, Irvine, CA) for 6 hr, and [3H]TdR incorporation was evaluated in a beta counter (1205 Betaplate; LKB Wallac, Turku, Finland).
For cytokine production assays, 5 × 105 CD4+ T cells were cultured in 48-well plates with 5 × 104 autologous allergen-pulsed DCs in 1 ml of IMDM supplemented with 5% heat-inactivated autologous plasma. After 1 week of culture, T cells were re-stimulated with newly generated allergen-pulsed DCs (5 × 104), and supernatants were collected 24 hr later. For intracellular staining of IL-4 and IFN-γ, co-cultures were further stimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich), 1 μg/ml ionomycin (Sigma-Aldrich) and 0·7 μl GolgiStop™ (BD Biosciences, Heidelberg, Germany) for 5 hr.
Quantification of cytokine production by ELISA
Human IL-6 (R&D Systems, Wiesbaden, Germany), IL-4, IL-5, IL-10, IL-12p40 and IFN-γ (BD Biosciences) were measured by ELISA according to the instructions of the distributors of the pairs of antibodies used. The detection limit was 8 pg/ml for IL-4 and 32 pg/ml for all other cytokines.
Flow cytometry
Surface phenotyping of DCs was performed by staining 5 × 104 cells with specific mouse anti-human mAbs for 20 min at 4°. The following antibodies were used: phycoerythrin (PE)-conjugated CD80 (L307.4), CD83 (HB15e), CD86 [2331 (FUN-1)], FITC-conjugated human leucocyte antigen (HLA)-DR (L243), and mouse IgG isotype controls (all from BD Biosciences, Heidelberg, Germany). For staining of RAGE, DCs were incubated with 0·25 μg of goat anti-human RAGE polyclonal antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) or goat IgG isotype control (R&D Systems) and thereafter with PE-conjugated donkey anti-goat antibody (Dianova, Hamburg, Germany). For determination of intracellular cytokines, co-cultures of 5 × 105 CD4+ T cells and 5 × 104 DCs were fixed with Fix/Perm Buffer (eBioscience, San Diego, CA) for 30 min at 4°. Cells were then permeabilized with Permeabilization Buffer (eBioscience) for 5 min and staining was performed in Permeabilization Buffer for 30 min at 4° using AlexaFluor 647-conjugated CD4 (MT310; Santa Cruz Biotechnology), FITC-conjugated IFN-γ (4S.B3), PE-conjugated IL-4 (MP4-25D2), and mouse or rat isotype controls (all from BD Biosciences). After incubation the cells were washed and analysed in a FACSCalibur (BD Biosciences) equipped with CellQuest software.
Analysis of internalization of OVA or AGE-OVA
OVA and AGE-OVA were labelled with FITC using a FluoroTag™ FITC conjugation kit according to the manufacturer’s protocol (Sigma-Aldrich). The adsorption of the conjugated samples was measured at 280 and 495 nm and the fluorescence/protein molar ratio was calculated. Additionally, the degree of FITC conjugation was verified by ELISA using mAb against FITC (Millipore, Schwalbach, Germany). Labelled allergen (1–10 μg/ml) was added to immature DCs on day 6 of culture and internalization was analysed after 10, 60 and 240 min in a FACSCalibur (BD Biosciences). In some experiments, mannan (200 μg/ml), which blocks the mannose receptor,25 polyinosinic acid (poly I) (20 μg/ml), which blocks the scavenger receptor,26 dimethylamiloride (DMA) (300 μm), which blocks pinocytosis27 (all from Sigma-Aldrich), or goat anti-human RAGE polyclonal antibody (1 μg/ml) (Santa Cruz Biotechnology) was added 30 min before FITC-OVA/FITC-AGE-OVA.
Fluorescence microscopy
FITC-labelled OVA or AGE-OVA was added to immature DCs on day 6 of culture and the internalization was analysed after 4 hr. Cells were spun on slides by cytospinning at 1000 U for 3 min at low acceleration and covered with PBS. Fluorescence microscopy was carried out with a Spot insight camera (model no. 3.1.0; Diagnostic Instruments Inc, Sterling Heights, MI) mounted over an Axiovert S100 microscope (Zeiss, Göttingen, Germany). For image acquisition, Meta Imaging Series 6.1 imaging software (Universal Imaging Corporation, Downington, PA) was used.
Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and western blot
Cell lysates of 1 × 106 immature DCs were mixed with loading buffer (Roth, Karlsruhe, Germany), heated for 5 min at 95°, and subjected to SDS-PAGE on a 10% polyacrylamide gel with 0·1% SDS using standard procedures (constant voltage at 200 V; 100 μg protein/lane). Proteins were blotted onto polyvinylidenfluoride membrane (Millipore, Bedford, MA) using a semidry blotting unit (Trans-Blot SD; Bio-Rad, München, Germany) in a Tris/Glycin buffer for 35 min at 2·5 mA/cm2. After transfer, the membrane was blocked in blocking buffer (PBS containing 0·1% Tween-20 and 5% non-fat dry milk powder) overnight at 4°.
For detection of actin or NF-κB, the membrane was incubated with horseradish peroxidase (HRP)-conjugated mouse anti-human actin mAb (Santa Cruz Biotechnology) at a dilution of 1 : 2000 in blocking buffer for 2 hr or with mouse anti-human phosphorylated NF-κB p65 mAb (BD Biosciences) at a dilution of 1 : 500 for 2 hr and thereafter with HRP-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) at a dilution of 1 : 5000 for 90 min. Blots were developed using chemoluminescence (Roti-Lumin; Roth).
Statistics
Student’s t-test was employed to test the statistical significance of the results; P ≤ 0·05 was considered significant.
Results
AGE-OVA is taken up more efficiently by immature DCs than OVA
First, we analysed the internalization of different concentrations of the FITC-conjugated allergens OVA and AGE-OVA by immature DCs at different time-points. In general, uptake of allergen was increased after application of higher allergen concentrations and time duration. The internalization of FITC-AGE-OVA was significantly enhanced compared with the internalization of FITC-OVA after 1 and 4 hr using the optimal concentration of 10 μg/ml allergen (P ≤ 0·05; Fig. 1a). In order to investigate and characterize the mechanisms of internalization of the allergens OVA and AGE-OVA by immature DCs, inhibitors were used to block the receptor-mediated antigen uptake (mannan and poly I) or to block macropinocytosis (DMA).25–27 All inhibitors were added 30 min before application of the allergen FITC-OVA or FITC-AGE-OVA. Figure 1(a,b) shows that the uptake of allergens was significantly reduced (P ≤ 0·01) by all inhibitors at each examined time-point. The uptake of FITC-OVA and AGE-OVA was completely blocked by mannan, poly I and DMA after 10 min and 1 hr. In the presence of the inhibitor mannan or poly I, FITC-AGE-OVA was taken up at a reduced rate after 4 hr, while the uptake of OVA was still completely blocked (P ≤ 0·05).
Figure 1.
Uptake of ovalbumin (OVA) and advanced glycation endproduct (AGE)-OVA by immature dendritic cells (DCs) with or without inhibitors. (a) Immature DCs were loaded with 10 μg/ml fluorescein isothiocyanate (FITC)-conjugated OVA or AGE-OVA and their uptake was detected by flow cytometry after 10, 60 and 240 min. The inhibitors mannan (200 μg/ml), poly I (20 μg/ml), and dimethylamiloride (DMA) (300 μm) were added to the cells 30 min before the addition of the allergens. The mean ± standard deviation of eight experiments is shown. *P ≤ 0·05 compared with OVA; §P ≤ 0·01 compared with no inhibition. (b) One representative experiment of the uptake of FITC-conjugated OVA and AGE-OVA after 240 min with inhibitors. (c) Immature DCs were treated with goat anti-human receptor for advanced glycation endproducts (RAGE) antibody (1 μg/ml) 30 min prior to application of the allergens and analysed by fluorescence microscopy after 4 hr.
In further experiments, we examined the uptake of OVA and AGE-OVA by immature DCs using fluorescence microscopy and investigated whether this uptake could be reduced by blocking the AGE receptor RAGE. In Fig. 1c it can be seen that a higher amount of fluorescence appeared after incubation with FITC-AGE-OVA compared with FITC-OVA. Blocking of RAGE by a neutralizing antibody did not inhibit internalization of FITC-OVA or FITC-AGE-OVA.
Glycation of OVA has no effect on overall T-cell proliferation
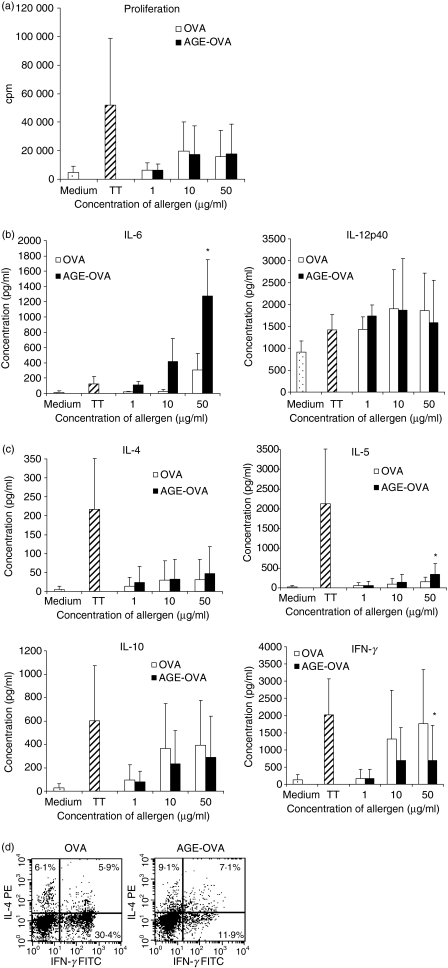
To investigate the proliferation of CD4+ T cells induced by OVA or AGE-OVA, CD4+ T cells were co-cultured together with autologous mature DCs that had been loaded with different concentrations of OVA or AGE-OVA. Figure 2(a) shows that both allergens were able to induce a concentration-dependent proliferation of T cells compared with the background proliferation of unloaded DCs (medium) which did not reach the level of the positive control tetanus toxoid (TT). There was no significant difference between OVA- and AGE-OVA-loaded DC-induced T-cell proliferation. To eliminate a possible influence of lipopolysaccharide (LPS) at the highest concentration of OVA or AGE-OVA, polymyxin B sulphate was added together with the allergen during DC culture, without changing the results (data not shown).
Figure 2.
Proliferation and cytokine production of CD4+ T cells after stimulation with ovalbumin (OVA) or advanced glycation endproduct (AGE)-OVA loaded mature dendritic cells (DCs). (a) Autologous T cells were cultured together with mature DCs pulsed with different concentrations of OVA or AGE-OVA or tetanus toxoid (TT) as a positive control. For the negative control (medium) no allergen was added. After 5 days, the cells were pulsed for 8 hr with [3H]thymidine. (b) DCs were pulsed with different concentrations of OVA or AGE-OVA or TT during their maturation phase from day 6–8. Then, interleukin (IL)-6 and IL-12p40 production was determined by enzyme-linked immunosorbent assay (ELISA). (c) CD4+ T cells were cultured together with allergen-loaded mature DCs. After 8 days the co-cultures were re-stimulated with freshly prepared autologous allergen-loaded DCs. The concentrations of cytokines were determined after 24 hr by ELISA. Shown is the mean ± standard deviation of five (for b) and 10 (for a and c) independent experiments; *P ≤ 0·05 compared with OVA. (d) Co-cultures were further stimulated with phorbol 12-myristate 13-acetate (PMA)/ionomycin/GolgiStop™ for 5 hr and stained for intracellular IL-4 and interferon (IFN)-γ. One representative of four independent experiments is shown.
AGE-OVA mature DCs produce high amounts of IL-6 and preferentially induce Th2 cytokine production compared with OVA-loaded DCs which induce primarily Th1 cytokine production
Next, we wondered whether glycation of OVA induces a change in the cytokine profile of mature DCs and subsequent co-cultures of DCs and CD4+ T cells. Therefore, we measured the secretion of IL-6 and IL-12p40 by DCs as well as the secretion of the Th2 cytokines IL-4 and IL-5 and the Th1 cytokine IFN-γ by CD4+ T cells. Additionally, we measured the production of the regulatory cytokine IL-10. Figure 2(b) shows that AGE-OVA induced a stronger expression of IL-6 in mature DCs than OVA, while IL-12p40 production was not affected by OVA or AGE-OVA. In the co-cultures, stimulation of CD4+ T cells with autologous OVA- or AGE-OVA-loaded mature DCs induced concentration-dependent production of all cytokines (Fig 2c). Compared with tetanus toxoid-pulsed DCs, the Th2 cytokines IL-4 and IL-5 were more weakly expressed after stimulation with OVA- or AGE-OVA-pulsed DCs, while the production of IFN-γ and IL-10 almost reached the levels found in the positive control. Interestingly, AGE-OVA-loaded DCs induced greater Th2 cytokine production (P < 0·05 for IL-5), while OVA-loaded DCs induced a significant Th1 or regulatory cytokine profile. This bias towards Th2 cytokine production after stimulation with AGE-OVA-pulsed DCs was confirmed by intracellular staining of the co-cultures for IFN-γ and IL-4. Again, IFN-γ-producing cells were greatly reduced after stimulation of CD4+ T cells with AGE-OVA-pulsed DCs compared with OVA-pulsed DCs, while IL-4-producing cells were slightly increased (Fig. 2d).
The AGE receptor RAGE is expressed on immature DCs more strongly than on mature DCs and is increased on immature DCs after exposure to AGE-OVA
For analysis of the expression of RAGE on immature and mature DCs, cells were analysed by flow cytometry, and 16·1 ± 5·6% expression of RAGE by immature DCs and 12·8 ± 6·1% expression by mature DCs were found (Fig. 3a,b). As RAGE expression is up-regulated after contact of AGEs with RAGE on monocytes,28,29 we examined whether AGE-OVA also enhances the expression of RAGE on immature and mature DCs. Figure 3(a,b) shows that RAGE expression was significantly increased on immature DCs (23·6 ± 7·0%) after exposure to AGE-OVA, while the addition of OVA had no effect (18·8 ± 6·3%). Increased RAGE expression after exposure to AGE-OVA was not observed in mature DCs (11·8 ± 5·8%).
Figure 3.
Detection and activation of the receptor for advanced glycation endproducts (RAGE) on immature dendritic cells (DCs) and mature DCs. (a, b) Immature DCs were loaded on day 6 of culture with ovalbumin (OVA) and AGE-OVA, matured or not, and RAGE expression was examined by flow cytometry 48 hr later. Shown is one representative experiment (a) and the mean ± standard deviation of 10 experiments (b); *P ≤ 0·05 compared with unpulsed DCs (medium) and §P ≤ 0·02 compared with mature DCs. (c) Protein lysates of immature DCs after treatment with either OVA or AGE-OVA for 48 hr were separated in a 10% polyacrylamid gel and the activated subunit p65 of nuclear factor (NF)-κB was detected by western blot. [Correction added after online publication 22 January 2010: Label in Fig 3a changed from Isotyp-PE to RAGE-PE]
AGE-OVA stimulation of RAGE on immature DCs leads to enhanced activation of NF-κB
As it is known that binding of AGEs to RAGE can activate the transcription factor NF-κB in inflamed tissues, we investigated whether NF-κB was also increased in immature DCs after treatment with AGE-OVA. Figure 3(c) shows that the phosphorylated subunit p65 of NF-κB was indeed expressed more strongly by immature DCs after treatment with AGE-OVA compared with OVA.
Discussion
In this study we have investigated whether glycation of the model food allergen OVA occurring during heat treatment or long-term storage influences its allergenicity and its effects on the human immune system. We found that internalization of glycated AGE-OVA by immature DCs was significantly increased compared with internalization of non-glycated OVA. The finding that incorporation of AGE-OVA occurs faster than incorporation of OVA at every concentration and time-point was also obtained using murine plasmacytoid and myeloid DCs.30 One explanation for the faster uptake of AGE-OVA might be that AGE-OVA had a more condensed structure after heat treatment. However, this possibility could be ruled out by denatured SDS-PAGE, demonstrating that AGE-OVA had a higher molecular weight and size compared with native OVA.30 Gruber et al.12 also showed, with the same method but another allergen (Pru av 1 from cherry), that the addition of sugar residues during the Maillard reaction leads to an irreversible change in the tertiary structure. This resulted in a higher molecular weight and a diffuse protein band in comparison to the native protein. The most likely reason for the faster uptake of AGE-OVA compared with OVA may be the increased number of receptors available for the uptake of AGE-OVA on the cell surface and the induction of an enhanced expression of AGE receptors on immature DCs by the modified protein.18,21,31
The manner and speed of the antigen uptake by APCs and the compartment in which the antigen accumulates might direct the course of the induced immune response. Burgdorf et al.32 showed that DCs are able to incorporate OVA via the mannose receptor pathway as well as by macropinocytosis. OVA that was incorporated via the mannose receptor pathway was only presented to CD8+ T cells, while pinocytosed OVA was presented to CD4+ T cells. In addition, pinocytosed OVA was transported exclusively to late endosomes while mannose receptor-endocytosed OVA was localized in early endosomes.32 Thus, the uptake of antigens and shuttling into certain pathways or compartments strongly influence the presentation of antigens.
Our analysis of the mechanisms of internalization of OVA and AGE-OVA confirmed that both receptor-mediated mechanisms (scavenger receptor and mannose receptor) as well as pinocytosis are involved, as the uptake of both OVA and AGE-OVA was inhibited after addition of inhibitors of mannose receptor (mannan), scavenger receptor (poly I) and pinocytosis (DMA). The results that blockage of RAGE did not affect internalization are consistent with those of Schmidt et al.,18 who characterized RAGE as a signal transduction receptor rather than as a clearance receptor. Accordingly, RAGE mediates long-lasting interactions with its ligands and as a result transcription of genes encoding proinflammatory cytokines is activated.19,20 These cytokines and other factors may cause the up-regulation of other receptors able to recognize and incorporate AGEs. Further experiments using confocal microscopy are under way to delineate the uptake of OVA and AGE-OVA.
When loaded on mature DCs, both allergens induced T-cell proliferation, even in non-allergic healthy donors. However, these donors were not naïve to OVA because of natural exposure to hen’s eggs in everyday life. Although there was no difference in proliferation induced by OVA- or AGE-OVA-pulsed DCs, we observed a shift towards Th2 cytokine production after stimulation of CD4+ T cells with AGE-OVA-loaded compared with OVA-loaded mature DCs. This Th2 bias was linked to the high production of IL-6 by AGE-OVA-pulsed DCs compared with OVA-pulsed DCs. The enhanced Th2 response induced by AGE-OVA-pulsed DCs could still be observed after addition of polymyxin B, indicating that the allergens themselves and not LPS contamination were responsible for the cytokine production. Additionally, any LPS effect on the maturation of DCs could be neglected as DCs were brought to maximal maturity by the proinflammatory cytokines IL-1β, TNF-α and prostaglandin E2 (PGE2).
The finding that T cells were activated similarly by the two antigens in terms of proliferation indicates that differences occurring later in cytokine production may depend not only on the activation potential of antigens in general, for example increased IL-6 production by AGE-OVA-pulsed DCs, but also on the quality, route or concentration of antigens inducing a Th1 or Th2 response. The finding that AGE-OVA induces a Th2 response compared with OVA, even in non-allergic and non-atopic donors, might indicate that AGE-OVA has a greater potential to induce an allergic immune response leading to IgE production. In an in vivo mouse model, AGE-OVA also induced higher titres of specific IgE compared with OVA (Toda et al., unpublished data).
In further experiments we analysed the expression of RAGE on immature DCs and found that it is up-regulated by AGE-OVA compared with native OVA and that the stimulation of immature DCs with AGE-OVA induced activation of the proinflammatory transcription factor NF-κB. Binding of AGEs to RAGE on the surface of DCs activates the signalling domain of RAGE in the cytoplasm, and the transcription factor NF-κB translocates into the nucleus and not only induces the transcription of genes that encode inflammatory cytokines such as IL-6,19,29,31 but also up-regulates the expression of the receptor itself33 in the form of a positive feedback loop, supporting the development of an immune response. Furthermore, the enhanced expression of RAGE by AGE-OVA-loaded immature DCs in comparison to OVA-loaded immature DCs might increase the potential of DCs to interact with AGE-peptides. This is also consistent with other reports showing up-regulation of RAGE in diabetic patients with higher blood sugar levels or aged tissues due to reduced degradation of AGEs.28,34–36
Taken together, our findings of increased uptake of AGE-OVA compared with OVA by immature DCs, induction of increased expression of RAGE, and its activation leading to phosphorylation of NF-κB indicate that glycated antigens might have increased immunogenicity. The fact that this is also relevant for allergens such as OVA, the increased induction of IL-6 by mature DCs by AGE-OVA compared with OVA leading to Th2 rather than Th1 cytokine production and the known increased resistance of glycated proteins to digestion may point to an increased potential of glycated allergens to initiate allergic immune responses, in addition to their known increased ability to elicit allergic reactions.
Acknowledgments
This work was supported by a Deutsche Forschungsgemeinschaft (SFB 548 TP A4) grant.
Acknowledgments
The authors have no financial conflicts of interest.
Glossary
Abbreviations:
- AGE
advanced glycation endproduct
- DC
dendritic cell
- IMDM
Iscove’s modified Dulbecco’s medium
- OVA
ovalbumin
- RAGE
receptor for advanced glycation endproducts
References
- 1.Custovic A, Murray CS. The effect of allergen exposure in early childhood on the development of atopy. Curr Allergy Asthma Rep. 2002;2:417–23. doi: 10.1007/s11882-002-0076-0. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WJ, Watson L. Asthma and the hygiene hypothesis. N Engl J Med. 2001;344:1643–4. doi: 10.1056/NEJM200105243442116. [DOI] [PubMed] [Google Scholar]
- 3.Akdis CA, Blaser K. Regulation of specific immune responses by chemical and structural modifications of allergens. Int Arch Allergy Immunol. 2000;121:261–9. doi: 10.1159/000024352. [DOI] [PubMed] [Google Scholar]
- 4.Carnes J, Ferrer A, Huertas AJ, Andreu C, Larramendi CH, Fernandez-Caldas E. The use of raw or boiled crustacean extracts for the diagnosis of seafood allergic individuals. Ann Allergy Asthma Immunol. 2007;98:349–54. doi: 10.1016/S1081-1206(10)60881-2. [DOI] [PubMed] [Google Scholar]
- 5.Davis PJ, Smales CM, James DC. How can thermal processing modify the antigenicity of proteins? Allergy. 2001;56(Suppl 67):56–60. doi: 10.1034/j.1398-9995.2001.00918.x. [DOI] [PubMed] [Google Scholar]
- 6.Berrens L. Neoallergens in heated pecan nut: products of Maillard-type degradation? Allergy. 1996;51:277–8. doi: 10.1111/j.1398-9995.1996.tb04610.x. [DOI] [PubMed] [Google Scholar]
- 7.Chuyen NV. Toxicity of the AGEs generated from the Maillard reaction: on the relationship of food-AGEs and biological-AGEs. Mol Nutr Food Res. 2006;50:1140–9. doi: 10.1002/mnfr.200600144. [DOI] [PubMed] [Google Scholar]
- 8.Henle T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29:313–22. doi: 10.1007/s00726-005-0200-2. [DOI] [PubMed] [Google Scholar]
- 9.Wal JM. Thermal processing and allergenicity of foods. Allergy. 2003;58:727–9. doi: 10.1034/j.1398-9995.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 10.Sathe SK, Teuber SS, Roux KH. Effects of food processing on the stability of food allergens. Biotechnol Adv. 2005;23:423–9. doi: 10.1016/j.biotechadv.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Thornalley PJ. Dietary AGEs and ALEs and risk to human health by their interaction with the receptor for advanced glycation endproducts (RAGE) – an introduction. Mol Nutr Food Res. 2007;51:1107–10. doi: 10.1002/mnfr.200700017. [DOI] [PubMed] [Google Scholar]
- 12.Gruber P, Vieths S, Wangorsch A, Nerkamp J, Hofmann T. Maillard reaction and enzymatic browning affect the allergenicity of Pru av 1, the major allergen from cherry (Prunus avium) J Agric Food Chem. 2004;52:4002–7. doi: 10.1021/jf035458+. [DOI] [PubMed] [Google Scholar]
- 13.Malanin K, Lundberg M, Johansson SG. Anaphylactic reaction caused by neoallergens in heated pecan nut. Allergy. 1995;50:988–91. doi: 10.1111/j.1398-9995.1995.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 14.Maleki SJ, Chung SY, Champagne ET, Raufman JP. The effects of roasting on the allergenic properties of peanut proteins. J Allergy Clin Immunol. 2000;106:763–8. doi: 10.1067/mai.2000.109620. [DOI] [PubMed] [Google Scholar]
- 15.Li YM, Mitsuhashi T, Wojciechowicz D, et al. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996;93:11047–52. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazaki A, Nakayama H, Horiuchi S. Scavenger receptors that recognize advanced glycation end products. Trends Cardiovasc Med. 2002;12:258–62. doi: 10.1016/s1050-1738(02)00171-8. [DOI] [PubMed] [Google Scholar]
- 17.Brett J, Schmidt AM, Yan SD, et al. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am J Pathol. 1993;143:1699–712. [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt AM, Yan SD, Yan SF, Stern DM. The biology of the receptor for advanced glycation end products and its ligands. Biochim Biophys Acta. 2000;1498:99–111. doi: 10.1016/s0167-4889(00)00087-2. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi A, Blood DC, del Toro G, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405:354–60. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- 21.Valencia JV, Mone M, Zhang J, Weetall M, Buxton FP, Hughes TE. Divergent pathways of gene expression are activated by the RAGE ligands S100b and AGE-BSA. Diabetes. 2004;53:743–51. doi: 10.2337/diabetes.53.3.743. [DOI] [PubMed] [Google Scholar]
- 22.Yan SF, Naka Y, Hudson BI, et al. The ligand/RAGE axis: lighting the fuse and igniting vascular stress. Curr Atheroscler Rep. 2006;8:232–9. doi: 10.1007/s11883-006-0078-9. [DOI] [PubMed] [Google Scholar]
- 23.Zill H, Bek S, Hofmann T, et al. RAGE-mediated MAPK activation by food-derived AGE and non-AGE products. Biochem Biophys Res Commun. 2003;300:311–5. doi: 10.1016/s0006-291x(02)02856-5. [DOI] [PubMed] [Google Scholar]
- 24.Gasic-Milenkovic J, Dukic-Stefanovic S, uther-Conrad W, Gartner U, Munch G. Beta-amyloid peptide potentiates inflammatory responses induced by lipopolysaccharide, interferon -gamma and ‘advanced glycation endproducts’ in a murine microglia cell line. Eur J Neurosci. 2003;17:813–21. doi: 10.1046/j.1460-9568.2003.02506.x. [DOI] [PubMed] [Google Scholar]
- 25.Deslee G, Charbonnier AS, Hammad H, et al. Involvement of the mannose receptor in the uptake of Der p 1, a major mite allergen, by human dendritic cells. J Allergy Clin Immunol. 2002;110:763–70. doi: 10.1067/mai.2002.129121. [DOI] [PubMed] [Google Scholar]
- 26.Finger AN, Bisoffi M, Wetterwald A, et al. Scavenger receptor block as strategy for the identification of bone marrow homing phages by panning in vivo random peptide phage displayed libraries. J Immunol Methods. 2002;264:173–86. doi: 10.1016/s0022-1759(02)00089-3. [DOI] [PubMed] [Google Scholar]
- 27.Liu NQ, Lossinsky AS, Popik W, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol. 2002;76:6689–700. doi: 10.1128/JVI.76.13.6689-6700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miura J, Uchigata Y, Yamamoto Y, et al. AGE down-regulation of monocyte RAGE expression and its association with diabetic complications in type 1 diabetes. J Diabetes Complications. 2004;18:53–9. doi: 10.1016/S1056-8727(02)00281-7. [DOI] [PubMed] [Google Scholar]
- 29.Hou FF, Ren H, Owen WF, Jr, et al. Enhanced expression of receptor for advanced glycation end products in chronic kidney disease. J Am Soc Nephrol. 2004;15:1889–96. doi: 10.1097/01.asn.0000131526.99506.f7. [DOI] [PubMed] [Google Scholar]
- 30.Ilchmann A, Burgdorf S, Waibler Z, et al. Glycation of a food allergen by the Maillard reaction enhances its T-cell immunogenicity: the role of macrophage scavenger receptor class A type I and II. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.08.013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Valencia JV, Weldon SC, Quinn D, et al. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem. 2004;324:68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 32.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–6. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt AM, Yan SD, Brett J, Mora R, Nowygrod R, Stern D. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products. J Clin Invest. 1993;91:2155–68. doi: 10.1172/JCI116442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herold K, Moser B, Chen Y, et al. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: inflammatory signals gone awry in the primal response to stress. J Leukoc Biol. 2007;82:204–12. doi: 10.1189/jlb.1206751. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt AM, Stern DM. RAGE: a new target for the prevention and treatment of the vascular and inflammatory complications of diabetes. Trends Endocrinol Metab. 2000;11:368–75. doi: 10.1016/s1043-2760(00)00311-8. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura K, Yamagishi S, Adachi H, et al. Serum levels of sRAGE, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol Med. 2007;13:185–9. doi: 10.2119/2006-00090.Nakamura. [DOI] [PMC free article] [PubMed] [Google Scholar]