Abstract
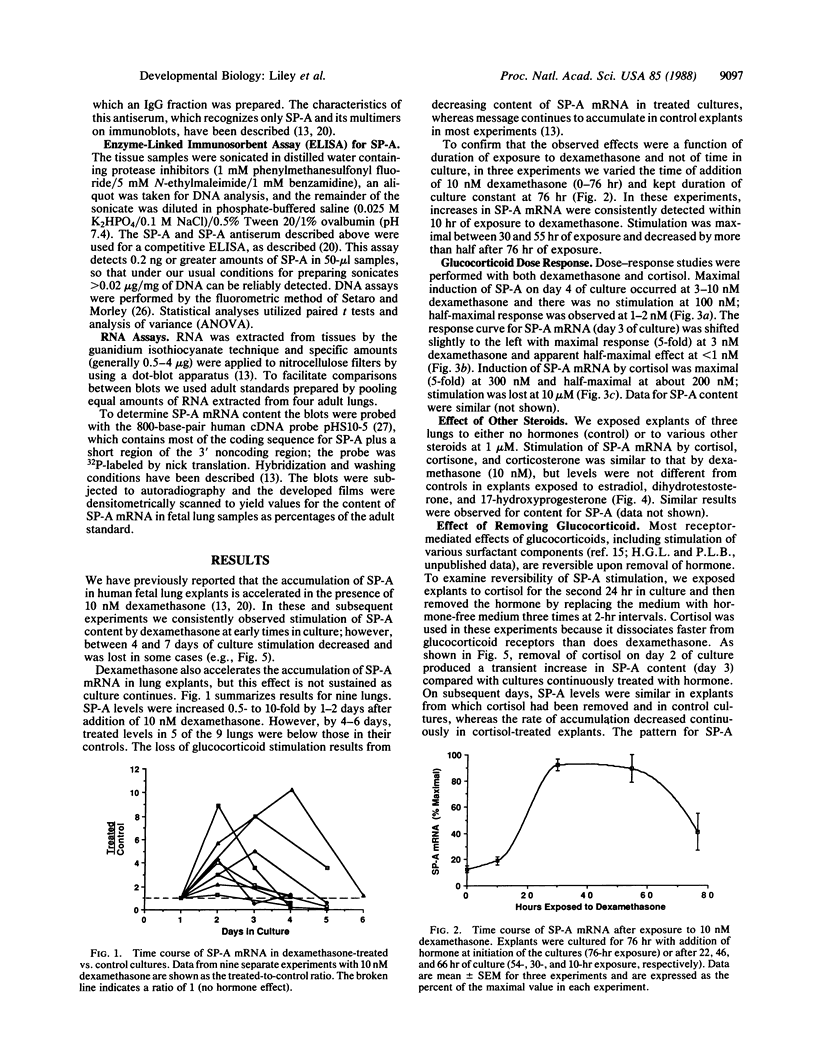
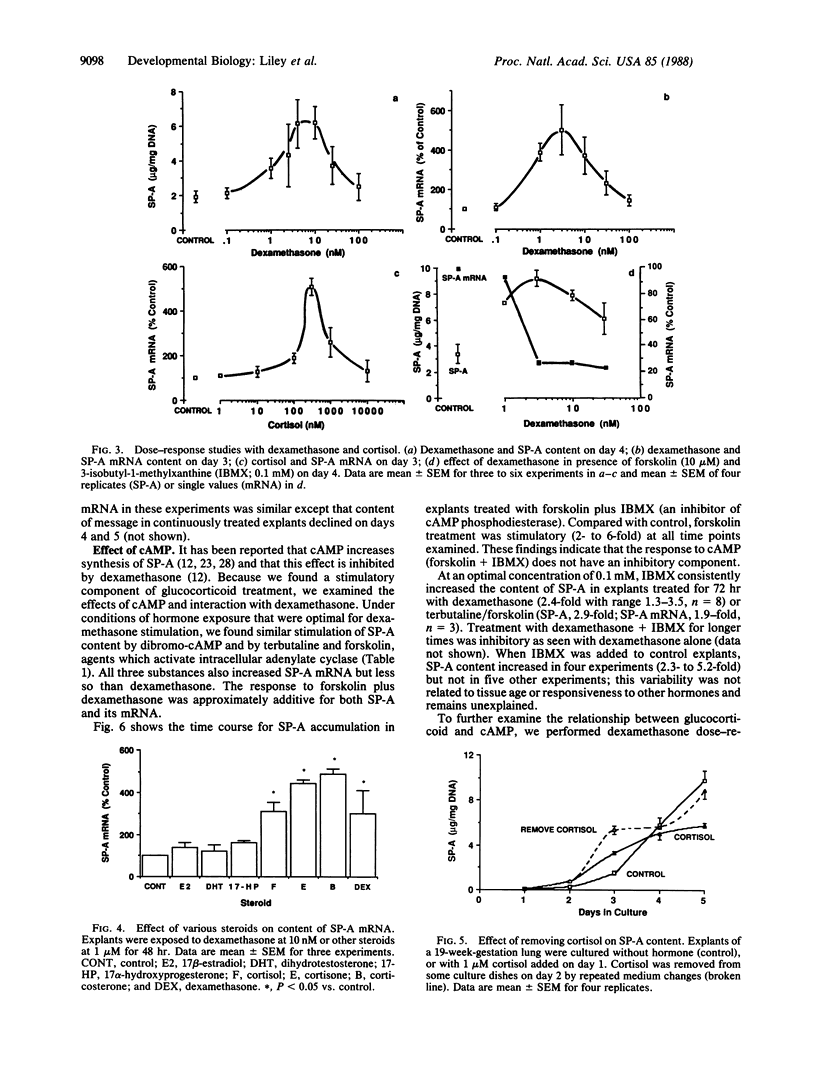
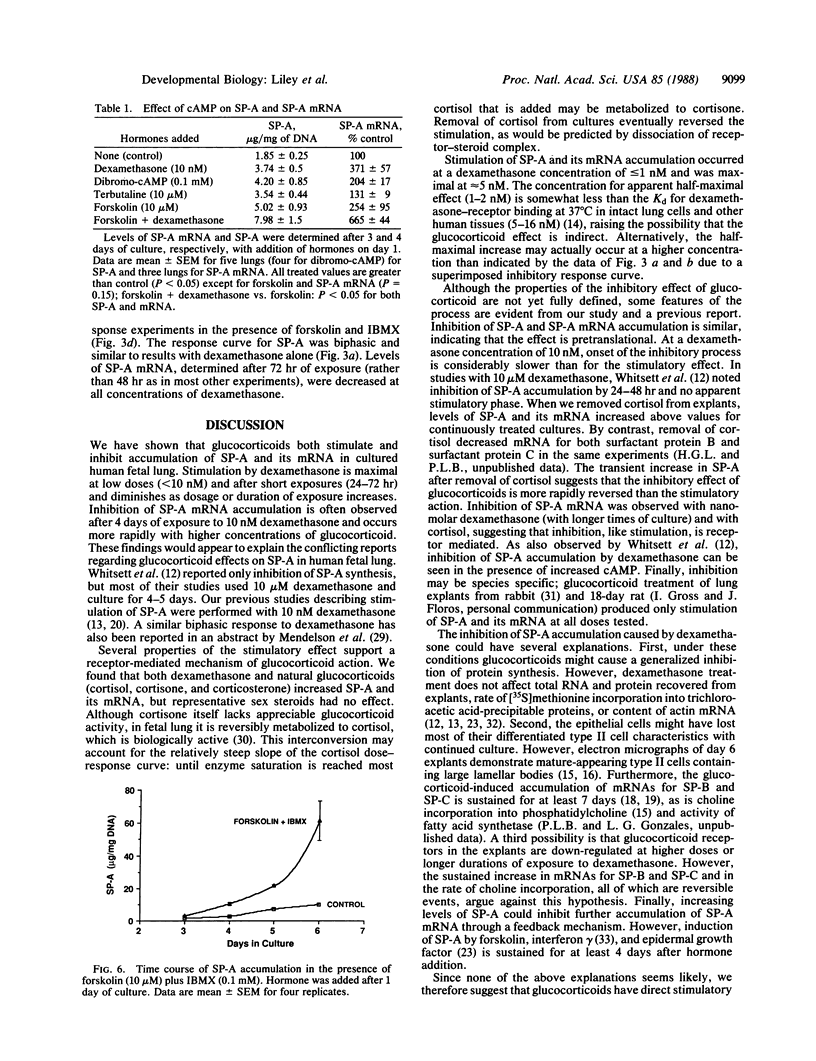
Pulmonary surfactant is a mixture of phospholipids and proteins which stabilizes lung alveoli and prevents respiratory failure. The surfactant-associated protein of Mr = 28,000-36,000 (SP-A) influences the structure, function (film formation), and metabolism of surfactant. We have characterized glucocorticoid regulation of SP-A and SP-A mRNA in explants of fetal human lung. The time course of response to dexamethasone was biphasic, with early stimulation and later inhibition of SP-A accumulation. Maximal induction of SP-A occurred with 3-10 nM dexamethasone and approximately 300 nM cortisol for 72 hr, and stimulation diminished at higher concentrations. SP-A mRNA accumulation was maximally stimulated at 24-48 hr of exposure to dexamethasone (10 nM) and was generally inhibited by 4-6 days. Stimulation was also observed with cortisone and corticosterone but not with sex steroids, suggesting a receptor-mediated process. When explants were exposed to cortisol for only 24 hr, SP-A content was transiently increased above the level in continuously treated tissue and subsequently was similar to control. The content of SP-A and its mRNA was also increased by dibromo-cAMP, terbutaline, and forskolin, and effects were approximately additive with those of dexamethasone. However, elevated in tracellular cAMP did not alter the biphasic time course or dose-response patterns of dexamethasone. We propose that glucocorticoids have both stimulatory and inhibitory effects on SP-A gene expression. This biphasic regulation is not consistent with generalized toxic effects, product-feedback inhibition, or receptor down-regulation, and it appears to be specific for SP-A among the various surfactant components.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramovitz M., Branchaud C. L., Murphy B. E. Cortisol-cortisone interconversion in human fetal lung: contrasting results using explant and monolayer cultures suggest that 11 beta-hydroxysteroid dehydrogenase (EC 1.1.1.146) comprises two enzymes. J Clin Endocrinol Metab. 1982 Mar;54(3):563–568. doi: 10.1210/jcem-54-3-563. [DOI] [PubMed] [Google Scholar]
- Ballard P. L., Hawgood S., Liley H., Wellenstein G., Gonzales L. W., Benson B., Cordell B., White R. T. Regulation of pulmonary surfactant apoprotein SP 28-36 gene in fetal human lung. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9527–9531. doi: 10.1073/pnas.83.24.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. J., Dattel B. J., Ballard P. L., Roberts J. M. Beta-adrenergic receptors and cyclic adenosine monophosphate generation in human fetal lung. Pediatr Res. 1987 Feb;21(2):142–147. doi: 10.1203/00006450-198702000-00007. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Wright J. R., Hawgood S., Gonzalez R., Venstrom K., Nellenbogen J. Pulmonary surfactant and its components inhibit secretion of phosphatidylcholine from cultured rat alveolar type II cells. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1010–1014. doi: 10.1073/pnas.84.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales L. W., Ballard P. L., Ertsey R., Williams M. C. Glucocorticoids and thyroid hormones stimulate biochemical and morphological differentiation of human fetal lung in organ culture. J Clin Endocrinol Metab. 1986 Apr;62(4):678–691. doi: 10.1210/jcem-62-4-678. [DOI] [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. J., MacBeth M. C. Interaction of the lipid and protein components of pulmonary surfactant. Role of phosphatidylglycerol and calcium. Biochim Biophys Acta. 1981 Oct 2;647(2):159–168. doi: 10.1016/0005-2736(81)90242-x. [DOI] [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liley H. G., Hawgood S., Wellenstein G. A., Benson B., White R. T., Ballard P. L. Surfactant protein of molecular weight 28,000-36,000 in cultured human fetal lung: cellular localization and effect of dexamethasone. Mol Endocrinol. 1987 Mar;1(3):205–215. doi: 10.1210/mend-1-3-205. [DOI] [PubMed] [Google Scholar]
- Mendelson C. R., Chen C., Boggaram V., Zacharias C., Snyder J. M. Regulation of the synthesis of the major surfactant apoprotein in fetal rabbit lung tissue. J Biol Chem. 1986 Jul 25;261(21):9938–9943. [PubMed] [Google Scholar]
- Mendelson C. R., Johnston J. M., MacDonald P. C., Snyder J. M. Multihormonal regulation of surfactant synthesis by human fetal lung in vitro. J Clin Endocrinol Metab. 1981 Aug;53(2):307–317. doi: 10.1210/jcem-53-2-307. [DOI] [PubMed] [Google Scholar]
- Odom M. J., Snyder J. M., Mendelson C. R. Adenosine 3',5'-monophosphate analogs and beta-adrenergic agonists induce the synthesis of the major surfactant apoprotein in human fetal lung in vitro. Endocrinology. 1987 Sep;121(3):1155–1163. doi: 10.1210/endo-121-3-1155. [DOI] [PubMed] [Google Scholar]
- Ono M., Oka T. alpha-Lactalbumin-casein induction in virgin mouse mammary explants: dose-dependent differential action of cortisol. Science. 1980 Mar 21;207(4437):1367–1369. doi: 10.1126/science.6986657. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Taeusch H. W., Jr, Benson B., Hawgood S. An electrophoretic and immunochemical characterization of human surfactant-associated proteins. Biochim Biophys Acta. 1984 Dec 7;791(2):226–238. doi: 10.1016/0167-4838(84)90013-x. [DOI] [PubMed] [Google Scholar]
- Revak S. D., Merritt T. A., Degryse E., Stefani L., Courtney M., Hallman M., Cochrane C. G. Use of human surfactant low molecular weight apoproteins in the reconstitution of surfactant biologic activity. J Clin Invest. 1988 Mar;81(3):826–833. doi: 10.1172/JCI113391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setaro F., Morley C. G. A modified fluorometric method for the determination of microgram quantities of DNA from cell or tissue cultures. Anal Biochem. 1976 Mar;71(1):313–317. doi: 10.1016/0003-2697(76)90043-9. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem Biophys Res Commun. 1986 Mar 13;135(2):527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Warr R. G., Hawgood S., Buckley D. I., Crisp T. M., Schilling J., Benson B. J., Ballard P. L., Clements J. A., White R. T. Low molecular weight human pulmonary surfactant protein (SP5): isolation, characterization, and cDNA and amino acid sequences. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7915–7919. doi: 10.1073/pnas.84.22.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Ohning B. L., Ross G., Meuth J., Weaver T., Holm B. A., Shapiro D. L., Notter R. H. Hydrophobic surfactant-associated protein in whole lung surfactant and its importance for biophysical activity in lung surfactant extracts used for replacement therapy. Pediatr Res. 1986 May;20(5):460–467. doi: 10.1203/00006450-198605000-00016. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Pilot T., Clark J. C., Weaver T. E. Induction of surfactant protein in fetal lung. Effects of cAMP and dexamethasone on SAP-35 RNA and synthesis. J Biol Chem. 1987 Apr 15;262(11):5256–5261. [PubMed] [Google Scholar]
- Whitsett J. A., Weaver T. E., Clark J. C., Sawtell N., Glasser S. W., Korfhagen T. R., Hull W. M. Glucocorticoid enhances surfactant proteolipid Phe and pVal synthesis and RNA in fetal lung. J Biol Chem. 1987 Nov 15;262(32):15618–15623. [PubMed] [Google Scholar]
- Whitsett J. A., Weaver T. E., Lieberman M. A., Clark J. C., Daugherty C. Differential effects of epidermal growth factor and transforming growth factor-beta on synthesis of Mr = 35,000 surfactant-associated protein in fetal lung. J Biol Chem. 1987 Jun 5;262(16):7908–7913. [PubMed] [Google Scholar]
- Williams M. C., Benson B. J. Immunocytochemical localization and identification of the major surfactant protein in adult rat lung. J Histochem Cytochem. 1981 Feb;29(2):291–305. doi: 10.1177/29.2.7019304. [DOI] [PubMed] [Google Scholar]
- Wright J. R., Wager R. E., Hawgood S., Dobbs L., Clements J. A. Surfactant apoprotein Mr = 26,000-36,000 enhances uptake of liposomes by type II cells. J Biol Chem. 1987 Feb 25;262(6):2888–2894. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Possmayer F. Reconstitution of surfactant activity by using the 6 kDa apoprotein associated with pulmonary surfactant. Biochem J. 1986 May 15;236(1):85–89. doi: 10.1042/bj2360085. [DOI] [PMC free article] [PubMed] [Google Scholar]


