Abstract
Dietary very long chain omega (ω)-3 polyunsaturated fatty acids (PUFA) have been associated with reduced CVD risk, the mechanisms of which have yet to be fully elucidated. LDL receptor null mice (LDLr-/-) were used to assess the effect of different ratios of dietary ω-6 PUFA to eicosapentaenoic acid plus docosahexaenoic acid (ω-6:EPA+DHA) on atherogenesis and inflammatory response. Mice were fed high saturated fat diets without EPA and DHA (HSF ω-6), or with ω-6:EPA+DHA at ratios of 20:1 (HSF R=20:1), 4:1 (HSF R=4:1), and 1:1 (HSF R=1:1) for 32 weeks. Mice fed the lowest ω-6:EPA+DHA ratio diet had lower circulating concentrations of non-HDL cholesterol (25%, P<0.05) and interleukin-6 (IL-6) (44%, P<0.05) compared to mice fed the HSF ω-6 diet. Aortic and elicited peritoneal macrophage (Mϕ) total cholesterol were 24% (P=0.07) and 25% (P<0.05) lower, respectively, in HSF R=1:1 compared to HSF ω-6 fed mice. MCP-1 mRNA levels and secretion were 37% (P<0.05) and 38% (P<0.05) lower, respectively, in elicited peritoneal Mϕ isolated from HSF R=1:1 compared to HSF ω-6 fed mice. mRNA and protein levels of ATP-binding cassette A1, and mRNA levels of TNFα were significantly lower in elicited peritoneal Mϕ isolated from HSF R=1:1 fed mice, whereas there was no significant effect of diets with different ω-6:EPA+DHA ratios on CD36, Mϕ scavenger receptor 1, scavenger receptor B1 and IL-6 mRNA or protein levels. These data suggest that lower ω-6:EPA+DHA ratio diets lowered some measures of inflammation and Mϕ cholesterol accumulation, which was associated with less aortic lesion formation in LDLr-/- mice.
Keywords: ω-6:EPA+DHA ratio, ω-3 fatty acids, atherosclerosis, inflammation, macrophage cholesterol accumulation, LDLr-/- mouse, diet, elicited peritoneal Mϕ
Introduction
Cardiovascular disease (CVD) is the leading cause of death and disability in developed countries. Atherosclerosis is the major cause of CVD, accounting for about half of the attributable deaths [1]. One component of diet that appears to play a multifactorial role in CVD risk is very long chain omega (ω)-3 fatty acids, eicosapentaenoic acid (EPA, C20:5 ω-3) and docosahexaenoic acid (DHA, C22:6 ω-3). Observational data in humans suggest that consumption of 1 to 2 servings of fish per week is associated with decreased CVD mortality [2]. Concern has been raised that the current low absolute intakes of EPA+DHA and high ratios of ω-6 polyunsaturated fatty acids (ω-6 PUFA) to EPA+DHA (ω-6:EPA+DHA) may predispose some individuals to increased CVD risk [3].
Historically, plasma lipoprotein profiles and lipid accumulation in the blood vessel wall have been the major focus of research related to atherogenesis. More recently, a preponderance of evidence from clinical and experimental data suggests that inflammatory responses play a critical role [4]. Mϕ scavenger receptor 1 (MSR1) and CD36 are two scavenger receptors involved in cholesterol influx [5]. Two Mϕ membrane receptors involved in cholesterol efflux are ATP-transporter cassette A1 (ABCA1) and scavenger receptor B class 1 (SR-B1) [6]. When Mϕ cholesterol influx is greater than efflux, cholesterol homeostasis is disturbed and cholesteryl ester (CE) accumulates in cytoplasmic droplets. The resulting Mϕ-derived foam cells secrete pro-inflammatory factors, which amplify the local inflammatory reaction and produce reactive oxygen species, which in turn modify lipoproteins. Interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα) are major pro-inflammatory factors. IL-6 promotes the synthesis of acute-phase reactants and monocyte chemoattractant protein-1 (MCP-1) [7, 8].
MCP-1 and its receptor, CC chemokine receptor 2 (CCR2), direct the migration of monocytes into the intima. Subsequent exposure of the monocytes to macrophage colony-stimulating factor promotes their differentiation to macrophages (Mϕ). Mϕ avidly take up modified apolipoprotein (apo) B-containing lipoproteins, resulting in the formation of Mϕ-derived foam cells. These cells secrete pro-inflammatory factors that amplify the local inflammatory response and produce reactive oxygen species, which modify lipoproteins resulting in increased uptake by scavenger receptors. Overexpression of MCP-1 is positively associated with accumulation of monocytes in fatty streaks [9]. It has been reported that dietary saturated fatty acids (SFA) increase and ω-3 PUFA decrease blood levels of these inflammatory biomarkers, whereas the effect of ω-6 PUFA has yet to be elucidated [10, 11].
To better understand the impact of dietary ω-6:EPA+DHA ratios in atherosclerotic lesion formation, LDL receptor null (LDLr-/-) mice were fed diets high in saturated fat and cholesterol, differing only in ω-6:EPA+DHA ratios. The effect of these diets on aortic lipid accumulation, serum lipoprotein profile, plasma inflammatory biomarkers, and elicited peritoneal Mϕ fatty acid composition, cholesterol content, and secretion of inflammatory factors was measured. mRNA and protein levels of genes involved in cholesterol accumulation and inflammation in elicited peritoneal Mϕ were simultaneously determined.
Materials and Methods
Animals and diets
Forty-one eight-week old, male LDLr-/- mice (Jackson Laboratory, Bar Harbor, Maine) initially weighing 20.1 ± 2.5 g were placed in individual cages with stainless-steel wire bottoms in a windowless room maintained at 22 to 24° C, 45% relative humidity and a daily 10/14 hour light/dark cycle with the light period from 06:00 to 16:00. After 1-week of acclimatization, mice were weighed and randomly assigned to one of four groups. Mice were fed high saturated fat (HSF; 20% fat, w/w) and cholesterol (0.2%, w/w) diets ad libitum, differing only in the ω-6:EPA+DHA ratios: no EPA+DHA (HSF ω-6), and ω-6:EPA+DHA ratio of 20:1 (HSF R=20:1), 4:1 (HSF R=4:1), and 1:1 (HSF R=1:1) (Tables 1). The HSF ω-6 diet has previously been shown to induce atherosclerotic lesion formation in the LDLr-/- mouse [12]. The ratio of ω-6:EPA+DHA in the diets was manipulated by adding different amounts of fish oil (Omega Protein Inc., Houston, TX) and safflower oil. The endogenous cholesterol in the fish oil was compensated for by adding purified cholesterol so the final amount was equivalent among diets. The experimental diets were designed so that the saturated fatty acid (SFA), monounsaturated fatty acid (MUFA) and PUFA content, with the exception of ω-6 PUFA, EPA and DHA, was equivalent. This was achieved by altering the amounts of butter fat, safflower oil and fish oil used to prepare the diets (Table 2). Body weight and food intake were monitored weekly over the 32-week feeding period. In order to ensure that adequate quantities of Mϕ would be available for all measurements, five days prior to killing mice were given an intraperitoneal injection of 1.0 ml Brewer thioglycollate broth (4.05 g/100 ml) to elicit Mϕ accumulation. Elicited peritoneal Mϕ were collected as previously described [13]. After a 16-18 hour fast the mice were anesthetized with CO2 and killed by exsanguination. Blood was collected by retro-orbital bleeding to harvest serum at week 0, 12 and 32, and by cardiac puncture to harvest plasma at week 32. Serum and plasma were obtained by blood centrifugation at 1,500 × g at 4°C for 25 minutes.
Table 1.
Composition of the experimental diets
| Ingredienta | Diet | |||
|---|---|---|---|---|
| HSFb ω-6 | HSF Rc=20:1 | HSF R=4:1 | HSF R=1:1 | |
| g/kg diet | ||||
| Casein | 240 | 240 | 240 | 240 |
| L-methionine | 3.6 | 3.6 | 3.6 | 3.6 |
| Cornstarch | 150 | 150 | 150 | 150 |
| Sucrose | 299 | 299 | 299 | 299 |
| Cellulose | 50 | 50 | 50 | 50 |
| Mineral mix, AIN-76 | 42 | 42 | 42 | 42 |
| Vitamin mix | 12 | 12 | 12 | 12 |
| Calcium carbonate | 3.6 | 3.6 | 3.6 | 3.6 |
| Lipid | 201.5 | 201.5 | 201.5 | 201.5 |
| Cholesterol | 1.5 | 1.5 | 1.5 | 1.5 |
| Anhydrous milk fat | 191.0 | 190.0 | 185.5 | 179.0 |
| Safflower oil | 9.0 | 8.1 | 6.0 | 0.1 |
| Fish oild | 0.0 | 1.9 | 8.5 | 20.9 |
| SFA | 125.3 | 125.0 | 123.6 | 122.0 |
| MUFA | 59.0 | 59.0 | 58.7 | 58.5 |
| PUFA | 12.5 | 12.5 | 13.2 | 13.3 |
| ω-6 PUFA | 12.5 | 11.8 | 10.6 | 6.9 |
Harlan Teklad (Madison, WI), with the exception of the fish oil. Vitamin mix was Teklad 40060. Anhydrous milk fat provided an additional 0.05% of cholesterol, the total cholesterol was 0.2%.
HSF: high saturated fatty acid and high cholesterol diet
R: ratio of ω-6:EPA+DHA
Omega Protein Inc. (Houston, TX). To compensate for the small amounts of cholesterol in the fish oil (0.24g/100g fish oil), 1.48 g/kg and 1.45 g/kg of cholesterol was added to HSF R=4:1 and HSF R=1:1 diets, respectively.
Table 2.
Fatty acid profile of the experimental diets
| Fatty acid | Diet | |||
|---|---|---|---|---|
| HSF ω-6 | HSF R=20:1 | HSF R=4:1 | HSF R=1:1 | |
| % | ||||
| SFA | 68.30±0.29 | 68.42±0.26 | 67.88±0.07 | 66.85±0.00 |
| C12:0 | 3.25±0.07 | 3.33±0.07 | 3.17±0.02 | 3.18±0.01 |
| C14:0 | 10.83±0.06 | 10.92±0.02 | 10.93±0.03 | 11.10±0.06 |
| C16:0 | 28.87±0.06 | 28.75±0.13 | 28.98±0.03 | 28.65±0.09 |
| C18:0 | 21.84±0.14 | 21.46±0.28 | 21.58±0.11 | 20.93±0.03 |
| C24:0 | 0.06±0.00 | 0.07±0.00 | 0.07±0.00 | 0.07±0.00 |
| MUFA | 25.37±0.18 | 25.32±0.25 | 25.62±0.07 | 26.47±0.04 |
| C16:1 ω-7 | 1.33±0.03 | 1.44±0.00 | 1.74±0.00 | 2.33±0.02 |
| C18:1 ω-9 | 18.83±0.10 | 18.67±0.20 | 18.60±0.04 | 18.36±0.04 |
| C18:1 ω-7 | 4.26±0.05 | 4.24±0.05 | 4.33±0.05 | 4.87±0.05 |
| C24:1 | 0.01±0.00 | 0.01±0.00 | 0.03±0.00 | 0.04±0.00 |
| PUFA | 6.32±0.11 | 6.26±0.01 | 6.50±0.01 | 6.67±0.14 |
| ω-6 | 5.36±0.10 | 5.07±0.00 | 4.42±0.00 | 2.85±0.02 |
| C18:2 | 4.47±0.05 | 4.16±0.04 | 3.42±0.01 | 1.80±0.00 |
| C18:3 | 0.45±0.01 | 0.48±0.00 | 0.47±0.03 | 0.46±0.02 |
| C20:3 | 0.04±0.00 | 0.05±0.00 | 0.05±0.00 | 0.07±0.00 |
| C20:4 | 0.09±0.04 | 0.06±0.00 | 0.09±0.00 | 0.15±0.01 |
| C22:2 | 0.20±0.01 | 0.21±0.03 | 0.26±0.02 | 0.25±0.00 |
| C22:5 | 0.11±0.02 | 0.11±0.01 | 0.13±0.00 | 0.12±0.00 |
| ω-3 | 0.96±0.09 | 1.19±0.01 | 2.07±0.00 | 3.82±0.06 |
| C18:3 ω-3 | 0.74±0.00 | 0.74±0.00 | 0.78±0.00 | 0.86±0.01 |
| C20:5 ω-3 | 0.10±0.00 | 0.18±0.00 | 0.54±0.00 | 1.25±0.02 |
| C22:5 ω-3 | 0.13±0.00 | 0.14±0.00 | 0.23±0.00 | 0.39±0.01 |
| C22:6 ω-3 | 0.01±0.00 | 0.12±0.00 | 0.52±0.00 | 1.32±0.02 |
Atherosclerotic lesion quantitation
Mouse hearts were perfused in situ for 1 minute with diethylpyrocarbonate treated-phosphate buffered saline (PBS) containing 1.5 μmol/l aprotinin and 0.1 mmol/l phenylmethylsulfonyl fluoride through a cannula inserted into the left ventricle. Aortas were dissected from the aortic root to the iliac bifurcation using a stereoscopic zoom microscope. As a measure of atherosclerotic lesion formation, total cholesterol (TC) and free cholesterol (FC) were quantified (8 aortas/group) as previously described [14, 15]. CE was calculated as the difference between the two measures. The residual delipidated aortic tissue was digested in 1N NaOH and total protein was determined using a bicinchoninic acid (BCA) kit (Pierce Ins., Rockford, IL).
Immunohistochemistry
In two randomly chosen mice from each diet group, aortic arches were isolated from the left aortic valve to the right subclavian artery branch and embedded in OCT compound (Tissue-Tek 4583; Sakura Finetek), snap-frozen in liquid nitrogen and stored at −80°C until sectioning. Aortic arches were sectioned at a 5μm thickness and stained in the pathology facility at the New England Medical Center Hospital. Rat anti-mouse monocyte/Mϕ antibody (MCA519G; Serotec, Raleigh, NC) was used to identify monocyte/Mϕ.
Serum lipid and lipoprotein concentrations
Serum TC, HDL-cholesterol (HDL-C) and triglyceride (TG) concentrations were measured using an Olympus AU400 analyzer with enzymatic reagents (Olympus America, Melville, NY). Non HDL-C was calculated as the difference between TC and HDL-C [16].
Plasma concentrations of inflammatory factors
Plasma TNFα, IL-6 and MCP-1 concentrations were measured using Quantikine® ELISA kits (R&D Systems, Minneapolis, MN).
Elicited peritoneal Mϕ culture and stimulation
Elicited peritoneal Mϕ were cultured in RPMI1640 medium (ATCC, Manassas, VA) containing 2% heat-inactivated fetal bovine serum (Invitrogen, Carlsbad, CA) for 2 hours (1×106 cells per ml). One million cells were stimulated with 1 μg/ml lipopolysaccharide (LPS) for 15 hours and inflammatory factors released into the culture medium were measured. The remaining cells were washed with PBS three times and used for the measurement of cholesterol content, fatty acid profile, mRNA and protein levels of genes involved in inflammation and cholesterol accumulation.
Cholesterol content and fatty acid profile
Elicited peritoneal Mϕ TC, FC and protein concentrations were determined as described for the aorta. Mϕ fatty acid profiles were determined as previously described [17].
Secretion of inflammatory factors
TNFα, IL-6 and MCP-1 secretion by LPS-stimulated elicited peritoneal Mϕ were measured using DuoSet® ELISA kits (R&D Systems, Minneapolis, MN). Elicited peritoneal Mϕ attached to the culture plates were digested by 0.5N NaOH and total protein was measured using BCA kits (Pierce Ins., Rockford, IL).
Western blot
Protein was extracted from elicited peritoneal Mϕ using a RIPA kit (Santa Cruz, Santa Cruz, CA). Western blotting was performed as previously described [16] with the following primary antibodies, MSR1 (Serotec, South San Francisco, CA), SR-B1 (Novus Biologicals, Littleton, CO), ABCA1 (Novus Biologicals, Littleton, CO), CD36 (Cascade Bioscience, Winchester, MA) and β-actin (Sigma, St. Louis, MO). Signals were visualized by chemiluminescence (Amersham Biosciences, Piscataway, NJ), quantified using a GS-800 calibrated densitometer (Bio-Rad, Hercules, CA), and normalized by β-actin.
Real-time PCR
RNA was extracted from elicited peritoneal Mϕ using the RNeasy mini kit (Qiagen, Valencia, CA). Single strand cDNA was synthesized from RNA using SuperScript™ ∏ reverse transcriptase according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). Primers for peroxisomal proliferator activated receptor (PPAR)β, PPARγ, MSR1, CD36, SR-B1, ABCA1, MCP-1, TNFα and β-actin (Table 3) were designed using Primer Express version 2.0 (Applied Biosystems, Foster City, CA). β-actin was used as an endogenous control. Primer amplification efficiency and specificity were verified for each set of primers. cDNA levels of the genes of interest were measured using power SYBR green master mix on real-time PCR 7300 (Applied Biosystems, Foster City, CA). cDNA levels of IL-6 and PPARα were measured using Taqman® primers and Taqman® PreAmp master mix kit (Applied Biosystems, Foster City, CA). The real-time PCR reaction condition was 95°C for 10 minutes, 40 cycles of 95°C for 15 seconds and 60°C for 1 minute, 1 cycle of dissociation stage. mRNA fold change was calculated using the 2(-Delta Delta C(T)) method [18].
Table 3.
Mouse oligonucleotide sequences of primers
| Gene name | Accession No. | Forward primer | Reverse primer |
|---|---|---|---|
| ABCA1 | NM_013454 | CCTGCTAAAATACCGGCAAGG | GTAACCCGTTCCCAACTGGTTT |
| SR-B1 | NM_016741 | TGGAACGGACTCAGCAAGATC | AATTCCAGCGAGGA TTCGG |
| CD36 | NM_007643 | ATTAATGGCACAGACGCAGC | CCGAACACAGCGTAGATAGACC |
| MSR1 | AF203781 | TCTACAGCAAAGCAACAGGAGG | TCCACGTGCGCTTGTTCTT |
| TNFα | NM_013693 | TGTAGCCCACGTCGTAGCAAA | GCTGGCACCACTAGTTGGTTGT |
| MCP-1 | NM_011333 | TCTCTCTTCCTCCACCACCATG | GCGTTAACTGCATCTGGCTGA |
| PPARβ/δ | NM_011145 | AGTGCGATCGGATCTGCAAGA | TCCAAAGCGGATAGCGTTGTG |
| PPARγ | NM_011146 | TCTTAACTGCCGGATCCACAAA | CCAAACCTGATGGCATTGTGA |
| β-actin | NM_007393 | CTTTTCCAGCCTTCCTTCTTGG | CAGCACTGTGTTGGCATAGAGG |
Statistical methods
Data were checked for normality and appropriate transformations were performed when necessary prior to statistical analysis (PROC UNIVARIATE; SAS version 9.1, SAS Institute Inc, Cary, NC). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed to compare multiple group means. Differences were considered significant at P<0.05. Untransformed data are presented in text, figures and tables as mean ± standard deviation (SD).
Results
Animal body weight and food intake
There were no significant differences in mouse body weight or food intake among the groups at baseline and throughout the 32-week feeding period (data not shown).
Aortic lesion composition
Aortic TC and FC content was lower in the HSF R=1:1 compared to HSF ω-6 diet fed mice, whereas content was intermediate in the other diet groups (Figures 1A and 1B). The dietary ω-6:EPA+DHA ratios had no significant effect on aortic CE content. There was less staining for Mϕ in the cross sections of aortic arches isolated from lower ratio diet fed mice relative to HSF ω-6 diet fed mice (Figure 1C).
Figure 1.
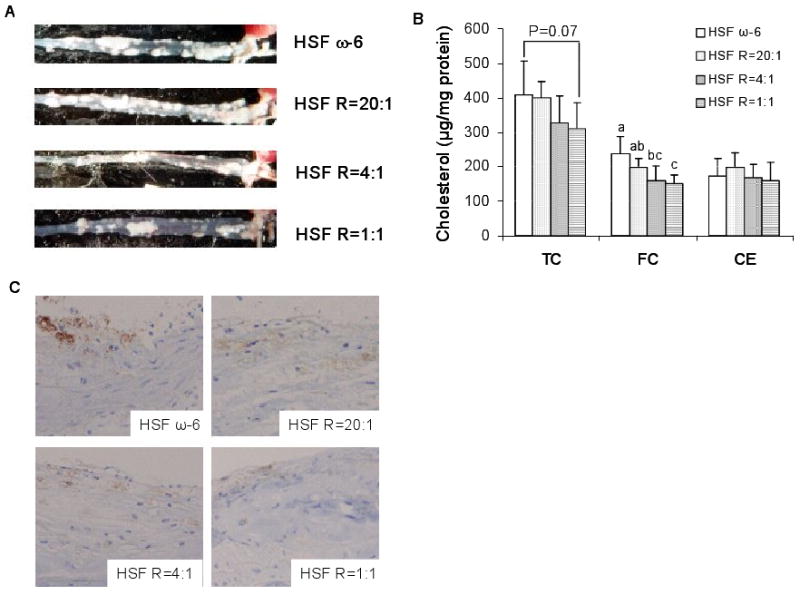
Representative aorta from each diet group, white areas denote atherosclerotic lesion (A), aortic cholesterol content in whole aortas (B), and Mϕ immunostaining by an antibody to mouse Mϕ surface marker in the cross-sections of aortic arches (C) in LDLr-/- mice. Magnified (200×) images of aortic arch cross-sections. The intensity of the brown color corresponds to the presence levels of Mϕ. n =11 in HSF R=4:1 group, n=10 in other groups. Values are mean ± SD of untransformed data. Prior to statistical analysis, the following transformation was made, aortic FC (Log). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed to compare group means. Bars without a common superscript differ, P<0.05.
Serum lipid and lipoprotein concentrations
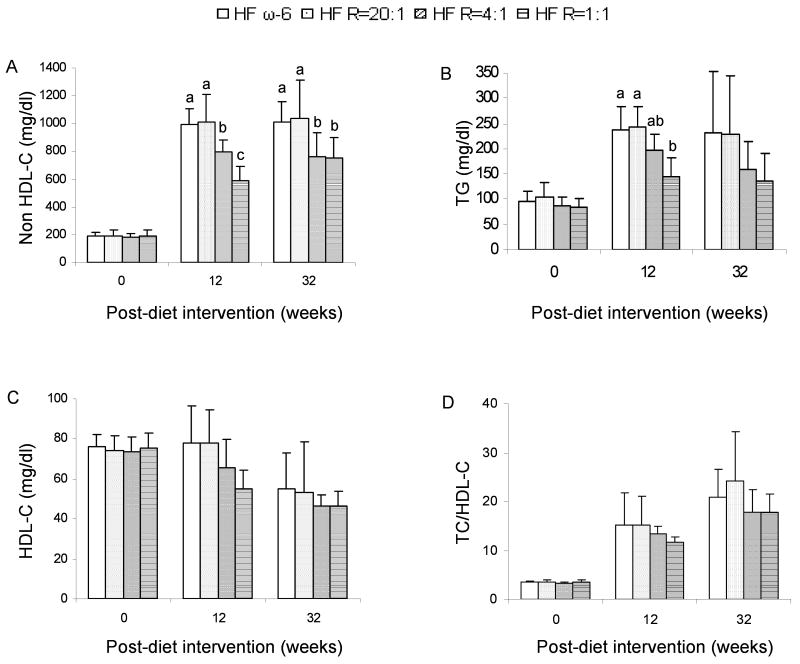
Serum TC (data no shown) and non HDL-C concentrations were both approximately 25% lower in the HSF R=4:1 and HSF R=1:1 compared with the HSF ω-6 diet group (P<0.05) (Figure 2A). There was no significant difference in serum ratios of TC to HDL-C among the different diet groups. Serum TG concentrations were 30% and 40% lower in the mice fed the HSF R=1:1 and HSF R=4:1 diets, respectively, compared to mice fed the HSF ω-6 diet at 12 weeks. These differences did not reach statistical significance at 32 weeks due to high degree of variability in response among mice.
Figure 2.
Serum lipid and lipoprotein concentrations in LDLr-/- mice. Serum was obtained at 0-, 12- and 32-week post-diet interventions. n =11 in HSF R=4:1 group, n=10 in other groups. Values are mean ± SD of untransformed data. Prior to statistical analysis, HDL-C at 32-week point was transformed (inverse). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed for multiple comparisons. Bars without a common superscript differ, P<0.05.
Plasma concentrations of inflammatory factors
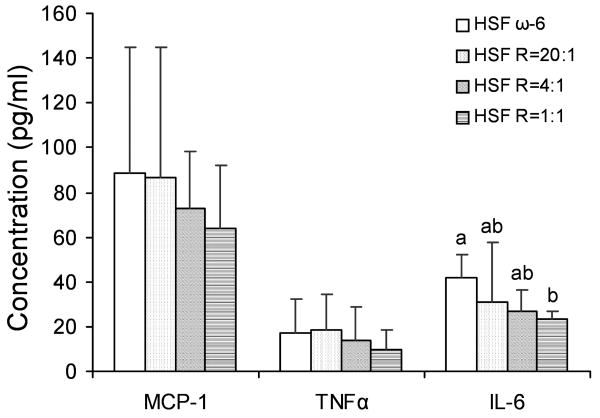
IL-6 concentrations were 44% lower in the HSF R=1:1 compared to HSF ω-6 diet fed mice (P<0.05), whereas concentrations were intermediate in the other diet groups (Figure 3). Due to high degree of variability in response among mice, there was no significant difference in MCP-1 and TNFα concentrations among the diet groups.
Figure 3.
Plasma concentrations of inflammatory factors in LDLr-/- mice. n =11 in HSF R=4:1 group, n=10 in all other groups. Values are mean ± SD of untransformed data. Prior to statistical analysis, the following transformations were made, plasma MCP-1 (log), plasma TNFα (log), plasma IL-6 (inverse). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed for multiple comparisons. Bars without a common superscript differ, P<0.05.
Elicited peritoneal Mϕ fatty acid profiles and cholesterol content
The fatty acid profile of the elicited peritoneal Mϕ reflected that of the diets (Table 4). As the ratio of ω-6:EPA+DHA in the diet was lower, the proportion of EPA, docosapentaenoic acid (DPA, C22:5) and DHA in the elicited peritoneal Mϕ was higher. These changes were concurrent with a decrease in the proportion of ω-6 fatty acids, resulting in a progressive decrease in the ω-6:EPA+DHA ratio to 4.9, 3.2, 1.7 and 0.9 in elicited peritoneal Mϕ isolated from HSF ω-6, HSF R=20:1, HSF R=4:1 and HSF R=1:1 diet fed mice, respectively.
Table 4.
Membrane fatty acid profile in elicited peritoneal Mϕa
| Fatty acid | Diet | |||
|---|---|---|---|---|
| HSF ω-6 | HSF R=20:1 | HSF R=4:1 | HSF R=1:1 | |
| % | ||||
| SFA | 34.97±5.97 | 33.30±2.30 | 34.00±3.16 | 33.49±2.53 |
| C12:0 | 0.38±0.34 | 0.29±0.20 | 0.31±0.13 | 0.27±0.09 |
| C14:0 | 1.88±0.19 | 1.67±0.47 | 1.98±0.28 | 1.74±0.33 |
| C16:0 | 21.67±3.23 | 21.91±4.41 | 21.29±3.21 | 21.62±2.04 |
| C18:0 | 10.66±3.03 | 9.10±3.09 | 10.01±0.92 | 9.47±0.86 |
| C20:0 | 0.14±0.07 | 0.12±0.05 | 0.10±0.03 | 0.10±0.03 |
| C22:0 | 0.11±0.07 | 0.09±0.04 | 0.10±0.02 | 0.11±0.02 |
| C24:0 | 0.14±0.04 | 0.13±0.05 | 0.20±0.23 | 0.18±0.05 |
| MUFA | 42.13±5.55 | 46.00±5.72 | 42.80±2.57 | 43.08±2.37 |
| C16:1 ω-9 | 1.31±0.07 | 1.19±0.27 | 1.55±0.26 | 1.55±0.32 |
| C16:1 ω-7 | 4.07±1.27 | 4.17±1.37 | 4.67±0.93 | 4.77±0.79 |
| C18:1 ω-9 | 28.28±4.76 | 32.03±8.43 | 28.45±3.59 | 29.05±2.93 |
| C18:1 ω-7 | 5.50±1.05 | 5.71±1.52 | 5.20±0.76 | 4.88±0.41 |
| C20:1 | 1.14±0.19 | 1.10±0.35 | 1.03±0.15 | 0.81±0.13 |
| C22:1 | 0.31±0.12 | 0.32±0.14 | 0.32±0.19 | 0.31±0.13 |
| C24:1 | 0.67±0.28 | 0.63±0.28 | 0.63±0.13 | 0.66±0.12 |
| PUFA | 22.90±2.51 | 20.70±6.16 | 23.20±4.86 | 23.44±1.17 |
| ω-6 | 16.75±1.91a | 13.31±3.90b | 11.21±1.74b | 8.46±1.14c |
| C18:2 | 4.72±0.90a | 3.88±0.82a | 3.80±0.68a | 1.67±0.46b |
| C18:3 | 0.02±0.01 | 0.02±0.03 | 0.03±0.03 | 0.02±0.03 |
| C20:2 | 1.04±0.14 | 0.84±0.24 | 0.54±0.05 | 0.35±0.04 |
| C20:3 | 1.32±0.30 | 1.32±0.43 | 1.12±0.19 | 0.90±0.14 |
| C20:4 | 6.48±1.18a | 5.34±2.02ab | 4.47±0.85b | 4.14±0.77b |
| C22:2 | 1.34±1.42 | 0.64±0.42 | 0.50±0.34 | 0.69±0.57 |
| C22:5 | 0.15±0.19 | 0.07±0.05 | 0.13±0.09 | 0.19±0.08 |
| C22:4 | 1.68±0.44 | 1.21±0.50 | 0.64±0.16 | 0.51±0.10 |
| ω-3 | 6.16±0.68c | 7.39±2.27c | 11.98±3.21b | 14.98±0.69a |
| C18:3 ω-3 | 0.19±0.04 | 0.20±0.05 | 0.21±0.05 | 0.19±0.02 |
| C20:5 ω-3 | 1.03±0.73b | 0.93±0.35b | 1.98±0.56a | 3.29±0.72a |
| C22:5 ω-3 | 2.47±0.37b | 2.99±1.02b | 4.89±1.21a | 5.33±2.10a |
| C22:6 ω-3 | 2.46±0.71b | 3.27±0.99ab | 4.89±1.48a | 6.17±1.42a |
| ω-6:EPA+DHA | 4.88±0.78a | 3.18±0.20a | 1.74±0.46b | 0.91±0.11c |
Values are mean ± SD of untransformed data, n = 11 in HSF R=4:1 group, n=10 in all other groups. Prior to the statistical analysis the following transformations were made, C20:5 (square root), C22:5 (square), C22:6 (log), ω-6:EPA+DHA (inverse), MUFA (log), PUFA (log) and ω-6 PUFA (log). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed for multiple comparison. Within a row means without a common superscript differ, P<0.05.
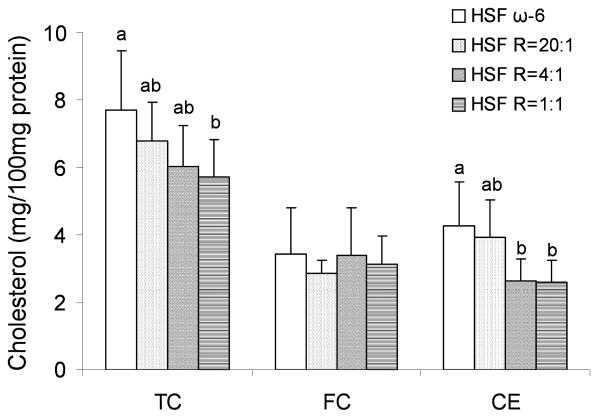
The pattern of differences in the TC content of the elicited peritoneal Mϕ harvested from the mice fed the different diets were similar to that seen in the aortas of the respective mice (Figures 1 and 4). Mϕ TC content was12%, 22% and 26% lower in the HSF R=20:1, HSF R=4:1, HSF R=1:1, respectively, compared to the HSF ω-6 fed mice. In contrast to that observed in the aorta, the difference in TC content was in the CE rather than FC fraction.
Figure 4.
Elicited peritoneal Mϕ cholesterol content in LDLr-/- mice. n =11 in HSF R=4:1 group, n=10 in all other groups. Values are mean ± SD of untransformed data. Prior to statistical analysis, the following transformations were made, Mϕ FC (square root), Mϕ CE (square). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed for multiple comparisons. Bars without a common superscript differ, P<0.05.
mRNA and protein levels of genes involved in cholesterol accumulation in elicited peritoneal Mϕ
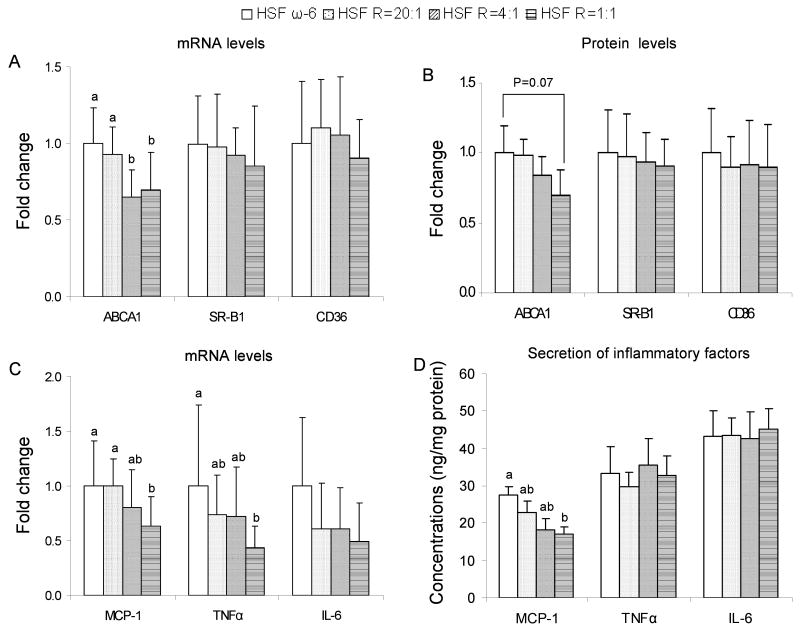
To investigate the mechanism(s) underlying the lower CE content in the elicited peritoneal Mϕ harvested from the mice fed the lower ω-6:EPA+DHA ratio diets, mRNA and protein levels of genes involved in Mϕ cholesterol accumulation were measured. Mϕ ABCA1 mRNA levels were 8%, 35% and 30% lower, and protein levels were 2%, 26% and 30% lower, in the HSF R=20:1, HSF R=4:1, HSF R=1:1 fed mice, respectively, compared to the HSF ω-6 fed mice (Figure 5A and 5B). MSR1, SR-B1 and CD36 mRNA and protein levels were similar among diet groups. There was no significant effect of dietary treatment on mRNA levels of MSR1, PPARα, PPARβ/δ, PPARγ (data not shown).
Figure 5.
mRNA (A) and protein (B) levels of ABCA1, SR-B1, CD36; mRNA levels (C) and secretion (D) of MCP-1, TNFα and IL-6 in/from elicited peritoneal Mϕ harvested from LDLr-/- mice at the end of the 32-week feeding period. n =11 in HSF R=4:1 group, n=10 in all other groups. Values are mean ± SD of untransformed data. Prior to statistical analysis, the following transformations were made, mRNA levels of ABCA1 (log), MCP-1 (log), TNFα (square root), IL-6 (log) and MCP-1 secretion (square root). An analysis of variance (PROC GLM) followed by Tukey's post hoc test was performed for multiple comparisons. Bars without a common superscript differ, P<0.05.
mRNA levels and secretion of inflammatory factors in elicited peritoneal Mϕ
To explore whether plasma concentrations of inflammatory factors depend on Mϕ secretion, we measured MCP-1, TNFα and IL-6 mRNA levels and secretion in elicited peritoneal Mϕ. Mϕ MCP-1 mRNA levels were 1%, 20% and 27% lower, and protein secretion were 17%, 34% and 38% lower, in the HSF R=20:1, HSF R=4:1, HSF R=1:1 fed mice, respectively, compared to the HSF ω-6 fed mice (Figure 5C and 5D). A similar pattern was seen in TNFα mRNA levels but not secretion. There was no significant effect of dietary ω-6:EPA+DHA ratios on IL-6 secretion and mRNA levels.
Discussion
Observational data are somewhat inconsistent with respect to the relationship between dietary very long chain ω-3 fatty acids (EPA and DHA) and risk of developing CVD [19-21]. This inconsistency has spawned a debate as to whether the critical variable is the ratio of ω-6:EPA+DHA or the absolute level of EPA and DHA in the diet [22]. The former issue was addressed in the current study using LDLr-/- mice fed an atherogenic diet containing different ratios of ω-6:EPA+DHA as an experimental model. The results demonstrated that the diet with the lowest ratio of ω-6:EPA+DHA, HSF R=1:1, resulted in a less atherogenic plasma lipid profile, less Mϕ deposition in the aortic wall, and lower plasma IL-6 concentrations, with intermediate outcomes in the HSF R=20:1 and HSF R=4:1 diet groups. In addition, elicited peritoneal Mϕ isolated from HSF R=1:1 diet fed mice had fatty acid profiles that mirrored the dietary fatty acids, lower cholesterol content, and lower MCP-1 mRNA levels and secretion in response to stimulation. These observations were associated with less atherosclerotic lesion development.
The experiment diets were designed to have equivalent compositions with respect to SFA, MUFA, and total PUFA. The only difference among the diets was the ratio of ω-6 PUFA to EPA+DHA. Currently recommendations for individuals with CVD and hypertriglyceridemia are to consume 1 gram to 3 grams of EPA+DHA, respectively [2]. For a person consuming 2200 kcal that is equivalent to approximately 0.4% to 1% energy from. Five grams of EPA+DHA per kilogram diet is equivalent to 1% energy in the HSF R=1:1 diet. The amount of EPA+DHA in the HSF R=4:1 diet and HSF R=20:1 diets is 0.5% and 0.1% energy, respectively.
Mice fed the lowest ratio of ω-6:EPA+DHA diet had lower serum TC and non HDL-C concentrations and lower aortic TC and FC accumulation compared to mice fed the HSF ω-6 diet. Changes in LDLr-/- mice aortic cholesterol content in response to an atherogenic diet have previously been reported in both FC and CE fractions [23]. The smaller difference and higher variability in the CE fraction among mice might have accounted for larger differences in FC than CE accumulation in this study. Two previous studies have reported that LDLr-/- mice fed fish oil had less atherosclerotic lesion area and lower plasma TC concentrations compared to mice fed olive oil or corn oil [24, 25]. Apolipoprotein E and LDL receptor double null mice fed a low ω-6:α-linolenic acid ratio diet compared to a high ω-6:α-linolenic acid ratio diet also resulted in less atherosclerotic lesion area and lower plasma TC concentrations [26]. Mice have been reported to have a higher capacity to convert α-linolenic acid to EPA than humans, albeit under more extreme dietary conditions prior to and during the period of assessment [27, 28]. Under these circumstances the authors speculated that it was the EPA and DHA, rather than the α-linolenic acid that resulted in lowered plasma TC concentrations.
Mϕ play a major role in the uptake of oxidized LDL, and accumulation and deposition of cholesterol in the arterial wall [29, 30]. Based on the results from previous studies [13, 31, 32], the expression and production of inflammatory factors is comparable between resident and elicited peritoneal Mϕ. In addition, there was no significant difference in the response of nutritional interventions between the two types of peritoneal Mϕ. In our study, elicited peritoneal Mϕ were used to ensure an adequate number of cells were harvested. The fatty acid profile of the diet was reflected in the fatty acid profile of the elicited peritoneal Mϕ isolated from the respective groups of mice. The TC content of these Mϕ followed a similar pattern to that of the corresponding aortas. Lower Mϕ TC and CE content in the mice fed the HSF R=1:1 diet suggests lower uptake and accumulation of modified LDL and/or more cholesterol efflux.
In order to understand the mechanism causing the difference in Mϕ cholesterol content, the expression of two proteins involved in cholesterol influx, MSR1 and CD36, and two proteins involved in cholesterol efflux, SR-B1 and ABCA1, was assessed. Mice fed diets with ω-6 and the highest ratio of ω-6:EPA+DHA had the highest ABCA1 mRNA and protein levels compared to mice fed diets with the lowest ratio. There was no significant difference in mRNA and protein levels of MSR1, SR-B1 and CD36. It has been reported that ABCA1 expression is upregulated in response to cholesterol enrichment in Mϕ and fibroblasts in vitro [33, 34] and in mouse Mϕ in vivo [35]. Consistent with these observations, in the current study, ABCA1 mRNA and protein levels were higher in elicited peritoneal Mϕ isolated from the HSF ω-6 and highest ω-6:EPA+DHA ratio diet fed LDLr-/- mice, which had the highest Mϕ cholesterol content. Some studies have shown that ABCA1 expression is regulated by liver-X-receptors (LXR) at a transcriptional level [36-38]. We speculate that the higher ω-6:EPA+DHA ratio diets resulted in increased production of LXR ligands in Mϕ, such as 24(S),25-epoxycholesterol and 27-hydroxycholesterol, which would have activated LXR. Ligand activated-LXR can upregulate ABCA1 gene expression and enhance ABCA1-mediated cholesterol efflux from elicited peritoneal Mϕ [39, 40]. Since each mouse had limited number of elicited Mϕ accumulated in peritoneal cavity, we could not test the above hypothesis in this study. These observations suggest that the greater cholesterol accumulation in the elicited peritoneal Mϕ isolated from mice fed the HSF ω-6 diet is a result of higher plasma TC and non HDL-C concentrations rather than by elicited peritoneal Mϕ ABCA1, SR-B1 or CD36 expression.
PUFA regulate Mϕ ABCA1 protein amounts at both transcription and post-translation levels. In our study, EPA and DHA content was higher in elicited peritoneal Mϕ isolated from mice fed lower ω-6:EPA+DHA ratio diets. Previous data have shown that PUFA, including EPA and DHA, lower ABCA1 protein levels in murine Mϕ through increasing ABCA1 protein degradation [41]. EPA, DHA and their metabolites are ligands for PPAR. The ligand-activated PPAR can prevent LXR from binding to the ABCA1 promoter, and lower ABCA1 expression [39, 40, 42-44]. More work is required to compare the effect of arachidonic acids (AA) and linoleic acids (LA), two major ω-6 PUFA in elicited peritoneal Mϕ, to EPA and DHA on ABCA1 expression through PPAR-LXR pathway.
The inflammatory process plays a major role in the development of atherosclerotic plaque [4, 45]. IL-6, MCP-1 and TNFα are important inflammatory biomarkers for atherosclerosis [7, 46, 47]. Epidemiological observations have suggested a positive association between Western type diets, and plasma MCP-1, IL-6 and TNFα concentrations [48-50], and an inverse association between dietary EPA+DHA and plasma concentrations of C-reactive protein and IL-6 [51-53]. Manipulating the ω-6:EPA+DHA ratios had variable effects on markers of inflammation both in plasma concentrations and as secreted by elicited peritoneal Mϕ. Mice fed the HSF R=1:1 diet tended to have lower plasma IL-6, MCP-1 and TNFα concentrations than mice fed the HSF ω-6 diet, although the later two factors did not reach statistical significance. In contrast, stimulated elicited peritoneal Mϕ had secretion rates and mRNA levels of IL-6 and TNFα indistinguishable among diet groups. Taken together, these data suggest that the major source of plasma IL-6 and TNFα is other than that produced by Mϕ, perhaps released from endothelial cells, adipocytes or T cells.
MCP-1, a major inflammatory factor released from Mϕ, functions to recruit monocytes into the arterial intima [54]. Overexpression of MCP-1 has been positively related to monocyte accumulation in fatty streaks [9, 55]. MCP-1 and LDL receptor double null mice had lower monocyte and vessel wall lipid accumulation compared to LDLr-/- mice [56, 57]. In the present study, we found that both MCP-1 mRNA levels in elicited peritoneal Mϕ and MCP-1 secretion from stimulated elicited peritoneal Mϕ were significantly lower in mice fed the HSF R=1:1 diet than the HSF ω-6 diet, with intermediate levels in the other two diet groups. This is consistent with in vitro studies showing that EPA and DHA decrease MCP-1 mRNA levels and secretion in/from endothelial cells and human kidney cells [58, 59]. In addition to Mϕ, MCP-1 is also produced by endothelial cells [60], smooth muscle cells [61] and adipocytes [62]. It has been reported that EPA upregulates MCP-1 mRNA levels compared to arachidonic acid (C20:4, ω-6) in endothelial cells, and might further increase MCP-1 secretion [60]. This may cause a smaller difference in plasma MCP-1 concentrations and explain the disparity between plasma MCP-1 concentrations and elicited peritoneal Mϕ secretion. The data suggest that diets with lower ratios of ω-6:EPA+DHA result in lower plasma MCP-1 concentrations and its secretion from elicited peritoneal Mϕ. EPA and DHA may downregulate MCP-1 expression through inhibiting nuclear factor-kappa B activation via an activated PPAR-dependent pathway [59].
A limitation of this work is that we could not address questions related to the absolute level of EPA and DHA in the diet and subsequent effects on the outcome variables assessed. The study was designed to assess the effect of an extreme range of ω-6:EPA+DHA ratios on atherogenesis and inflammatory response, not chronic exposure as might be experienced by humans during a lifetime. This study was not designed to assess the effects of linoleic acid or α-linolenic acid on atherogenesis. These fatty acids have been shown, under a variety of conditions, to be associated with decreased CVD in humans. The LDLr-/- mouse is a good model for human atherosclerosis, allowing for an assessment of aortic lesion formation. However, plasma lipid and lipoprotein profiles and the composition of the lesion itself were not similar to that observed in humans. This is likely due to the lack of cholesteryl ester transfer protein in mice. No animal model perfectly mimics humans, including inflammatory response and plasma lipid and lipoprotein profiles, in response to the Western diet, fish oil, or one modified in fatty acid profile.
In summary, LDLr-/- mice fed diets with the lowest ratio of ω-6:DHA+EPA (HSF R=1:1) had the lowest concentrations of serum atherogenic lipids and plasma IL-6, and the least elicited peritoneal Mϕ cholesterol accumulation and MCP-1 expression, which in turn were associated with the lowest aortic lesion formation. These data suggest that the effect of different ratios of ω-6:DHA+EPA on atherogenesis is multifactorial.
Acknowledgments
The authors would like to thank Drs. Donald Smith and Mohsen Meydani for sharing their technical expertise, Drs. Julian Marsh and Alice Dillard for their thoughtful critical review of the manuscript and Omega Protein Inc. (Houston, TX) for providing the fish oil for this study.
Source of Support: T32 HL69772-01A1 (S.W., J.L.), RO1 HL 54727 and USDA agreement 588-1950-9-001. Any opinions, findings, conclusions or recommendations expressed in this publication are those of authors, and do not necessarily reflect the view of USDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Kannel WB, Giordano M. Long-term cardiovascular risk with protease inhibitors and management of the dyslipidemia. Am J Cardiol. 2004;94:901–906. doi: 10.1016/j.amjcard.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 3.Sanders TA, Lewis F, Slaughter S, Griffin BA, Griffin M, Davies I, Millward DJ, Cooper JA, Miller GJ. Effect of varying the ratio of n-6 to n-3 fatty acids by increasing the dietary intake of alpha-linolenic acid, eicosapentaenoic and docosahexaenoic acid, or both on fibrinogen and clotting factors vii and xii in persons aged 45-70 y: The optilip study. Am J Clin Nutr. 2006;84:513–522. doi: 10.1093/ajcn/84.3.513. [DOI] [PubMed] [Google Scholar]
- 4.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 5.Moore KJ, Freeman MW. Scavenger receptors in atherosclerosis: Beyond lipid uptake. Arterioscler Thromb Vasc Biol. 2006;26:1702–1711. doi: 10.1161/01.ATV.0000229218.97976.43. [DOI] [PubMed] [Google Scholar]
- 6.Baranova I, Vishnyakova T, Bocharov A, Chen Z, Remaley AT, Stonik J, Eggerman TL, Patterson AP. Lipopolysaccharide down regulates both scavenger receptor b1 and atp binding cassette transporter a1 in raw cells. Infect Immun. 2002;70:2995–3003. doi: 10.1128/IAI.70.6.2995-3003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiotti N, Giansante C, Ponte E, Delbello C, Calabrese S, Zacchi T, Dobrina A, Guarnieri G. Atherosclerosis and inflammation. Patterns of cytokine regulation in patients with peripheral arterial disease. Atherosclerosis. 1999;145:51–60. doi: 10.1016/s0021-9150(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 8.Haddy N, Sass C, Droesch S, Zaiou M, Siest G, Ponthieux A, Lambert D, Visvikis S. Il-6, tnf-alpha and atherosclerosis risk indicators in a healthy family population: The stanislas cohort. Atherosclerosis. 2003;170:277–283. doi: 10.1016/s0021-9150(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 9.Vita JA, Keaney JF, Jr, Larson MG, Keyes MJ, Massaro JM, Lipinska I, Lehman BT, Fan S, Osypiuk E, Wilson PW, Vasan RS, Mitchell GF, Benjamin EJ. Brachial artery vasodilator function and systemic inflammation in the framingham offspring study. Circulation. 2004;110:3604–3609. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- 10.Seierstad SL, Seljeflot I, Johansen O, Hansen R, Haugen M, Rosenlund G, Froyland L, Arnesen H. Dietary intake of differently fed salmon; the influence on markers of human atherosclerosis. Eur J Clin Invest. 2005;35:52–59. doi: 10.1111/j.1365-2362.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- 11.Meydani M. Omega-3 fatty acids alter soluble markers of endothelial function in coronary heart disease patients. Nutr Rev. 2000;58:56–59. doi: 10.1111/j.1753-4887.2000.tb07812.x. [DOI] [PubMed] [Google Scholar]
- 12.Napoli C, Williams-Ignarro S, De Nigris F, Lerman LO, Rossi L, Guarino C, Mansueto G, Di Tuoro F, Pignalosa O, De Rosa G, Sica V, Ignarro LJ. Long-term combined beneficial effects of physical training and metabolic treatment on atherosclerosis in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 2004;101:8797–8802. doi: 10.1073/pnas.0402734101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu D, Marko M, Claycombe K, Paulson KE, Meydani SN. Ceramide-induced and age-associated increase in macrophage cox-2 expression is mediated through up-regulation of nf-kappa b activity. J Biol Chem. 2003;278:10983–10992. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- 14.Rudel LL, Kelley K, Sawyer JK, Shah R, Wilson MD. Dietary monounsaturated fatty acids promote aortic atherosclerosis in ldl receptor-null, human apob100-overexpressing transgenic mice. Arterioscler Thromb Vasc Biol. 1998;18:1818–1827. doi: 10.1161/01.atv.18.11.1818. [DOI] [PubMed] [Google Scholar]
- 15.Matthan NR, Giovanni A, Schaefer EJ, Brown BG, Lichtenstein AH. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in cad patients with low hdl. J Lipid Res. 2003;44:800–806. doi: 10.1194/jlr.M200439-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Dorfman SE, Wang S, Vega-Lopez S, Jauhiainen M, Lichtenstein AH. Dietary fatty acids and cholesterol differentially modulate hdl cholesterol metabolism in golden-syrian hamsters. J Nutr. 2005;135:492–498. doi: 10.1093/jn/135.3.492. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein AH, Matthan NR, Jalbert SM, Resteghini NA, Schaefer EJ, Ausman LM. Novel soybean oils with different fatty acid profiles alter cardiovascular disease risk factors in moderately hyperlipidemic subjects. Am J Clin Nutr. 2006;84:497–504. doi: 10.1093/ajcn/84.3.497. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. N-3 fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 20.Hamer M, Steptoe A. Influence of specific nutrients on progression of atherosclerosis, vascular function, haemostasis and inflammation in coronary heart disease patients: A systematic review. Br J Nutr. 2006;95:849–859. doi: 10.1079/bjn20061741. [DOI] [PubMed] [Google Scholar]
- 21.Harper CR, Jacobson TA. Usefulness of omega-3 fatty acids and the prevention of coronary heart disease. Am J Cardiol. 2005;96:1521–1529. doi: 10.1016/j.amjcard.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 22.Harris WS, Assaad B, Poston WC. Tissue omega-6/omega-3 fatty acid ratio and risk for coronary artery disease. Am J Cardiol. 2006;98:19i–26i. doi: 10.1016/j.amjcard.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Furbee JW, Jr, Parks JS. Transgenic overexpression of human lecithin: Cholesterol acyltransferase (lcat) in mice does not increase aortic cholesterol deposition. Atherosclerosis. 2002;165:89–100. doi: 10.1016/s0021-9150(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 24.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. J Nutr. 2007;137:1776–1782. doi: 10.1093/jn/137.7.1776. [DOI] [PubMed] [Google Scholar]
- 25.Zampolli A, Bysted A, Leth T, Mortensen A, De Caterina R, Falk E. Contrasting effect of fish oil supplementation on the development of atherosclerosis in murine models. Atherosclerosis. 2006;184:78–85. doi: 10.1016/j.atherosclerosis.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita T, Oda E, Sano T, Yamashita T, Ijiru Y, Giddings JC, Yamamoto J. Varying the ratio of dietary n-6/n-3 polyunsaturated fatty acid alters the tendency to thrombosis and progress of atherosclerosis in apoe-/- ldlr-/- double knockout mouse. Thromb Res. 2005;116:393–401. doi: 10.1016/j.thromres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Cohen SL, Moore AM, Ward WE. Flaxseed oil and inflammation-associated bone abnormalities in interleukin-10 knockout mice. J Nutr Biochem. 2005;16:368–374. doi: 10.1016/j.jnutbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Riediger ND, Othman R, Fitz E, Pierce GN, Suh M, Moghadasian MH. Low n-6:N-3 fatty acid ratio, with fish- or flaxseed oil, in a high fat diet improves plasma lipids and beneficially alters tissue fatty acid composition in mice. Eur J Nutr. 2008;47:153–160. doi: 10.1007/s00394-008-0709-8. [DOI] [PubMed] [Google Scholar]
- 29.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class a-i/ii and cd36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 30.Ludewig B, Laman JD. The in and out of monocytes in atherosclerotic plaques: Balancing inflammation through migration. Proc Natl Acad Sci U S A. 2004;101:11529–11530. doi: 10.1073/pnas.0404612101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayek MG, Mura C, Wu D, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159:2445–2451. [PubMed] [Google Scholar]
- 32.Wu D, Mura C, Beharka AA, Han SN, Paulson KE, Hwang D, Meydani SN. Age-associated increase in pge2 synthesis and cox activity in murine macrophages is reversed by vitamin e. Am J Physiol. 1998;275:C661–668. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- 33.Langmann T, Klucken J, Reil M, Liebisch G, Luciani MF, Chimini G, Kaminski WE, Schmitz G. Molecular cloning of the human atp-binding cassette transporter 1 (habc1): Evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 1999;257:29–33. doi: 10.1006/bbrc.1999.0406. [DOI] [PubMed] [Google Scholar]
- 34.Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF. The tangier disease gene product abc1 controls the cellular apolipoprotein-mediated lipid removal pathway. J Clin Invest. 1999;104:R25–31. doi: 10.1172/JCI8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singaraja RR, James ER, Crim J, Visscher H, Chatterjee A, Hayden MR. Alternate transcripts expressed in response to diet reflect tissue-specific regulation of abca1. J Lipid Res. 2005;46:2061–2071. doi: 10.1194/jlr.M500133-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P. Lxrs control lipid-inducible expression of the apolipoprotein e gene in macrophages and adipocytes. Proc Natl Acad Sci U S A. 2001;98:507–512. doi: 10.1073/pnas.021488798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-hydroxycholesterol is an endogenous ligand for liver x receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 38.Beyea MM, Heslop CL, Sawyez CG, Edwards JY, Markle JG, Hegele RA, Huff MW. Selective up-regulation of lxr-regulated genes abca1, abcg1, and apoe in macrophages through increased endogenous synthesis of 24(s),25-epoxycholesterol. J Biol Chem. 2007;282:5207–5216. doi: 10.1074/jbc.M611063200. [DOI] [PubMed] [Google Scholar]
- 39.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, Brewer HB, Fruchart JC, Clavey V, Staels B. Ppar-alpha and ppar-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the abca1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 40.Schmitz G, Langmann T. Transcriptional regulatory networks in lipid metabolism control abca1 expression. Biochim Biophys Acta. 2005;1735:1–19. doi: 10.1016/j.bbalip.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Oram JF. Unsaturated fatty acids phosphorylate and destabilize abca1 through a phospholipase d2 pathway. J Biol Chem. 2005;280:35896–35903. doi: 10.1074/jbc.M506210200. [DOI] [PubMed] [Google Scholar]
- 42.Andersson C, Zaman MM, Jones AB, Freedman SD. Alterations in immune response and ppar/lxr regulation in cystic fibrosis macrophages. J Cyst Fibros. 2007 doi: 10.1016/j.jcf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Uehara Y, Engel T, Li Z, Goepfert C, Rust S, Zhou X, Langer C, Schachtrup C, Wiekowski J, Lorkowski S, Assmann G, von Eckardstein A. Polyunsaturated fatty acids and acetoacetate downregulate the expression of the atp-binding cassette transporter a1. Diabetes. 2002;51:2922–2928. doi: 10.2337/diabetes.51.10.2922. [DOI] [PubMed] [Google Scholar]
- 44.Uehara Y, Miura S, von Eckardstein A, Abe S, Fujii A, Matsuo Y, Rust S, Lorkowski S, Assmann G, Yamada T, Saku K. Unsaturated fatty acids suppress the expression of the atp-binding cassette transporter g1 (abcg1) and abca1 genes via an lxr/rxr responsive element. Atherosclerosis. 2007;191:11–21. doi: 10.1016/j.atherosclerosis.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Paoletti R, Gotto AM, Jr, Hajjar DP. Inflammation in atherosclerosis and implications for therapy. Circulation. 2004;109:III20–26. doi: 10.1161/01.CIR.0000131514.71167.2e. [DOI] [PubMed] [Google Scholar]
- 46.Brueckmann M, Bertsch T, Lang S, Sueselbeck T, Wolpert C, Kaden JJ, Jaramillo C, Huhle G, Borggrefe M, Haase KK. Time course of systemic markers of inflammation in patients presenting with acute coronary syndromes. Clin Chem Lab Med. 2004;42:1132–1139. doi: 10.1515/CCLM.2004.232. [DOI] [PubMed] [Google Scholar]
- 47.Hoogeveen RC, Morrison A, Boerwinkle E, Miles JS, Rhodes CE, Sharrett AR, Ballantyne CM. Plasma mcp-1 level and risk for peripheral arterial disease and incident coronary heart disease: Atherosclerosis risk in communities study. Atherosclerosis. 2005;183:301–307. doi: 10.1016/j.atherosclerosis.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Pawlak K, Pawlak D, Mysliwiec M. Inflammation but not oxidative stress is associated with beta-chemokine levels and prevalence of cardiovascular disease in uraemic patients. Cytokine. 2006;35:258–262. doi: 10.1016/j.cyto.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Baer DJ, Judd JT, Clevidence BA, Tracy RP. Dietary fatty acids affect plasma markers of inflammation in healthy men fed controlled diets: A randomized crossover study. Am J Clin Nutr. 2004;79:969–973. doi: 10.1093/ajcn/79.6.969. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80:1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 51.Basu A, Devaraj S, Jialal I. Dietary factors that promote or retard inflammation. Arterioscler Thromb Vasc Biol. 2006;26:995–1001. doi: 10.1161/01.ATV.0000214295.86079.d1. [DOI] [PubMed] [Google Scholar]
- 52.Lopez-Garcia E, Schulze MB, Manson JE, Meigs JB, Albert CM, Rifai N, Willett WC, Hu FB. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004;134:1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 53.Madsen T, Skou HA, Hansen VE, Fog L, Christensen JH, Toft E, Schmidt EB. C-reactive protein, dietary n-3 fatty acids, and the extent of coronary artery disease. Am J Cardiol. 2001;88:1139–1142. doi: 10.1016/s0002-9149(01)02049-5. [DOI] [PubMed] [Google Scholar]
- 54.Sheikine Y, Hansson GK. Chemokines and atherosclerosis. Ann Med. 2004;36:98–118. doi: 10.1080/07853890310019961. [DOI] [PubMed] [Google Scholar]
- 55.Ballantyne CM, Nambi V. Markers of inflammation and their clinical significance. Atheroscler Suppl. 2005;6:21–29. doi: 10.1016/j.atherosclerosissup.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Ishibashi M, Egashira K, Zhao Q, Hiasa K, Ohtani K, Ihara Y, Charo IF, Kura S, Tsuzuki T, Takeshita A, Sunagawa K. Bone marrow-derived monocyte chemoattractant protein-1 receptor ccr2 is critical in angiotensin ii-induced acceleration of atherosclerosis and aneurysm formation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2004;24:e174–178. doi: 10.1161/01.ATV.0000143384.69170.2d. [DOI] [PubMed] [Google Scholar]
- 57.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, Rollins BJ. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 58.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. Epa and dha reduce lps-induced inflammation responses in hk-2 cells: Evidence for a ppar-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 59.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit nf-kappab activation via a pparalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–1627. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 60.Shaw DI, Hall WL, Jeffs NR, Williams CM. Comparative effects of fatty acids on endothelial inflammatory gene expression. Eur J Nutr. 2007;46:321–328. doi: 10.1007/s00394-007-0669-4. [DOI] [PubMed] [Google Scholar]
- 61.Lopez-Franco O, Hernandez-Vargas P, Ortiz-Munoz G, Sanjuan G, Suzuki Y, Ortega L, Blanco J, Egido J, Gomez-Guerrero C. Parthenolide modulates the nf-kappab-mediated inflammatory responses in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:1864–1870. doi: 10.1161/01.ATV.0000229659.94020.53. [DOI] [PubMed] [Google Scholar]
- 62.Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007;581:4389–4396. doi: 10.1016/j.febslet.2007.07.082. [DOI] [PubMed] [Google Scholar]