Abstract
Purpose
Cell-based therapy rescues retinal structure and function in rodent models of retinal disease, but translation to clinic will require more information about consequences of transplantation in an eye closely resembling the human eye. Therefore we explored donor cell behavior using human cortical neural progenitor cells (hNPCctx) introduced into the subretinal space of normal rhesus macaques.
Methods
hNPCctx transduced with Green Fluorescent Protein (hNPCctx-GFP) were delivered bilaterally into the subretinal space of six normal adult rhesus macaques under conditions paralleling those of the human operating room. Outcome measures included clinical parameters of surgical success, multifocal electroretinogram (mfERG) and histopathological analyses performed between 3 and 39 days post-engraftment. To test the effects of GFP transduction on cell bioactivity, hNPCctx –GFP from the same batch were also injected into RCS rats and compared with non-labeled hNPCctx.
Results
Studies using RCS rats indicated that GFP transduction did not alter the ability of the cells to rescue vision. After cells were introduced into the monkey subretinal space by a pars plana transvitreal approach, the resulting detachment was rapidly resolved and retinal function showed little or no disturbance in mfERG recordings. Retinal structure was unaffected and no signs of inflammation or rejection were seen. Donor cells survived as a single layer in the subretinal space and no cells migrated into the inner retina.
Conclusions
Human neural progenitor cells can be introduced into a primate eye without complication, using an approach that would be suitable for extrapolation to human patients.
Introduction
Engraftment of several cell types into the subretinal space has been shown to slow the rate of photoreceptor degeneration and sustain a substantial level of visual function in the Royal College of Surgeons (RCS) rat, a rodent model of retinal degenerative disease.1-4 This cell-based therapy may prove efficacious for several currently-untreatable conditions including retinitis pigmentosa, Stargardt macular dystrophy and atrophic dry age-related macular degeneration (AMD).
Prior to clinical trials, several critical issues must be resolved regarding the best way to introduce cells into the human eye, including the best surgical approach, the ideal cell dosage, and the number and location of injections. In addition, safety, biodistribution and the requirement for immunosuppression must be evaluated. The structural and size differences between rodent and human eyes limit the use of these small animals to address such questions. In contrast, the rhesus monkey eye closely resembles its human counterpart in almost all respects, critically including the presence of a macula and fovea, making it optimal for preclinical testing.
Recent studies demonstrated that forebrain-derived human cortical neural progenitor cells (hNPCctx) survived transplantation to the subretinal space of dystrophic RCS rats for prolonged periods and produced significant sustained preservation of photoreceptors and visual function.4, 5 Here we used approaches that would be compatible with human implantation to explore the feasibility of introduction of these cells to the subretinal space of normal macaque monkeys and to assess their effects on retinal structure and function. Because the available human cell markers4 could not differentiate human and nonhuman primate tissues, cells were first transduced with a gene for Green Fluorescent Protein (GFP) to allow visualization and identification of cells after transplantation. To confirm that bioactivity was not impaired by the presence of GFP, we first conducted an efficacy study in RCS rats and compared the results with those obtained with untransduced cells.
Materials and Methods
This specific study and all procedures were first approved by the Institutional Animal Care and Use Committee and Institutional Biosafety Committee of Oregon Health and Science University, and conformed to NIH guidelines and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Preparation of fluorescently-labeled human cortical neural progenitor cells (hNPCctx-GFP)
Human cortical neural progenitor cells (hNPCctx) were isolated and prepared in accordance with NIH guidelines from 94 day post-conception fetal cortical brain tissue and cultured as neurospheres as previously described (Fig. 1A).6 A lentiviral construct (LV-CMV-eGFP)7 containing a cytomegalovirus internal promoter driving the eGFP gene was used to generate a parallel culture of eGFP-expressing hNPCctx neurospheres (Fig. 1B). Both hNPCctx and hNPCctx-GFP neurospheres were dissociated for 10 minutes in Accutase (1 ml/10 million cells) followed by inactivation with an equal volume of 0.2% trypsin inhibitor. Neurosphere cultures (passage 34-41) were washed twice with 10 ml of medium, gently triturated into a single cell suspension, and counted on a hemocytometer. Cell suspensions were diluted to a final concentration in balanced salt solution and kept on ice for 2-4 hours until transplantation. Trypan blue dye exclusion was performed on cell suspensions prior to and immediately following each transplantation session and showed greater than 95% cell survival.
Figure 1.
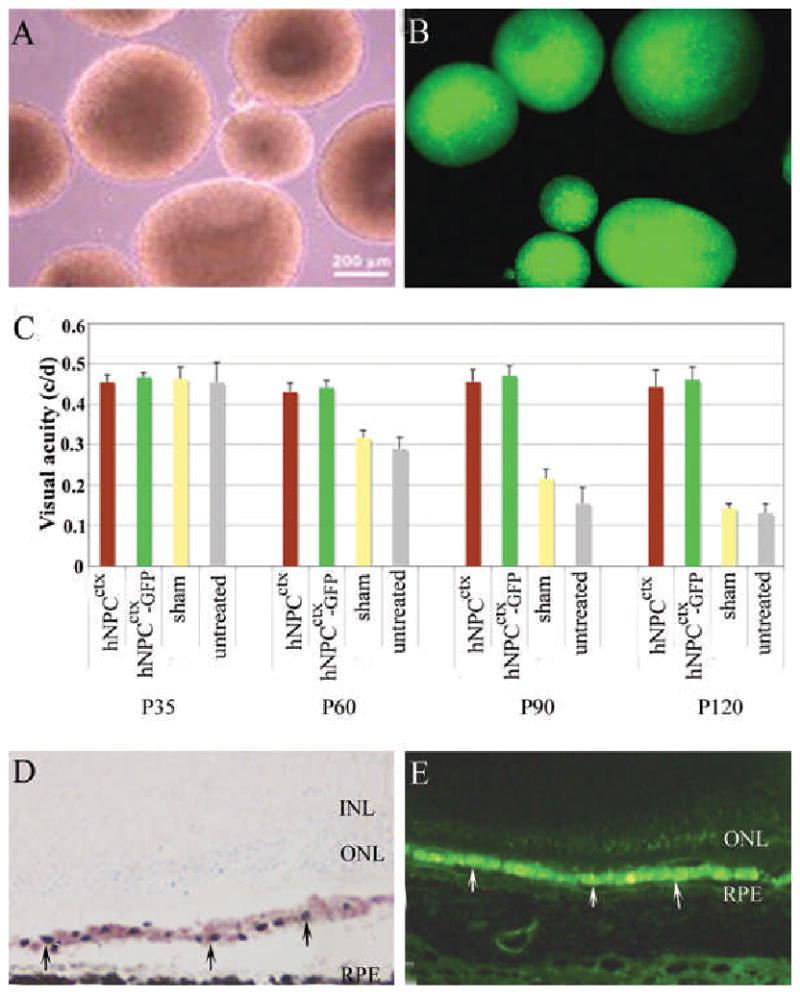
A: Light microscopy of human prenatal cortical progenitor cells (hNPCctx) cultured as neurospheres; B: fluorescence microscopy of GFP transduced hNPCctx; C: Visual thresholds from native hNPCctx, prelabeled hNPCctx-GFP, sham and untreated eyes from RCS rats (n=10/group) tested at four time points. There is no difference between native hNPCctx and hNPCctx-GFP treated groups (p>0.05); however, significantly different between cell-injected and shame/untreated controls (p<0.01). Two weeks after subretinal injection of hNPCctx, the distribution of donor cells (arrowed) is similar, as shown in D (retinal cross section with donor cells stained with human nuclear marker on cresyl violet background) and E, (hNPCctx –GFP shown green). No donor cells were seen in the sensory retina at any time.
Rodent studies: Comparison of hNPCctx-GFP and hNPCctx
Cell preparation and transplantation
To ensure that the lentiviral transduction and GFP expression did not alter the ability of the cells to rescue photoreceptors, a preliminary study was undertaken in the RCS rat (rdy+, p+) with naturally-occurring retinal degeneration, comparing efficacy of hNPCctx-GFP and non-transfected hNPCctx at a similar passage. At 22 days post-natal (P22), RCS rats received unilateral trans-scleral subretinal injections of hNPCctx-GFP (20,000 cells/2μl/eye; n=10), hNPCctx (20,000 cells/2μl/eye; n=10), or carrier medium alone (sham, n=10) using techniques described previously.4 For each animal included in this study, fellow eyes served as untreated, internal controls. All animals were maintained on cyclosporine A (210 mg/L; Novartis, East Hanover, NJ) from one day prior to transplantation until they were sacrificed, and received daily dexamethasone injections (1.6 mg/kg, i.p., American Regent, Shirley, NY) for 2 weeks starting on the day of transplantation.
Visual acuity thresholds obtained by measuring optomotor responses
Animals were tested for spatial visual acuity at P35, P60, P90 and P120 using an Optomotry testing apparatus (CerebralMechanics, Lethbridge, Canada).8 Briefly, the device comprised four computer monitors arranged in a square displaying vertical sine wave gratings. The gratings were projected as a virtual rotating cylinder in three dimensional coordinate spaces and moved horizontally at 12 degrees per second. Unrestrained rats were placed on a platform in the center of the square and observed by an experimenter who judged whether they tracked the grating with reflexive head movements. The viewing distance was held constant by repeatedly recentering the ‘cylinder’ with respect to the head of the test subject. Acuity was quantified by increasing the spatial frequency of the grating using a staircase progression until the optokinetic reflex was lost, thereby obtaining a maximum acuity threshold. Statistical analyses were performed using GraphPad Prism version 5.01 for Windows. Data are presented as mean±standard error (SEM). Statistical analyses were made using analysis of variance (ANOVA); Newman-Keuls procedure was used for post hoc multiple comparison analysis. Differences were considered to be significant at p<0.05.
Rat retinal histology
Rats were euthanized at several time points with an overdose of sodium pentobarbital (Sigma) and perfused with phosphate buffered saline (PBS). The superior pole of each eye was marked with a suture to maintain orientation. The eyes were then removed, immersed in 4% paraformaldehyde for one hour, infiltrated with sucrose, embedded in OCT and cut into 10μm horizontal sections on a cryostat for cresyl violet staining. A human nuclear marker (MAB 1281, Chemicon, Billerica, MA) was used to identify donor cells.
Nonhuman primate subretinal cell delivery
The subjects were 6 female rhesus monkeys captive-bred at the Oregon National Primate Research Center and included one juvenile and five adults, 8-13 years of age. All had normal retinal appearance and no history of ophthalmic abnormalities. Surgeries were performed in the dedicated operating rooms of the Oregon National Primate Research Center using full sterile procedures. Each surgery was performed by a trained retina surgeon with another assisting. An operating room nurse, two circulators and an individual dedicated to cell injection were present together with veterinary anesthesiology personnel. Anesthesia was induced with tiletamine/zolazepam (Telazol, 3-5 mg/kg IM, Fort Dodge Animal Health, Fort Dodge, IA) or ketamine (10-20 mg/kg IM, Bioniche Pharma, Lake Forest, IL). Following induction, an endotracheal tube was placed and surgical plane anesthesia was maintained by inhalation of isoflurane (1-3%, Hospira, Lake Forest, IL) in 100% oxygen. One drop each of tropicamide 1% (Tropicacyl, Akorn, Buffalo Grove, IL) and phenylephrine 2.5% (Bausch and Lomb, Tampa, FL) was administered to both eyes two times at approximately 10 minute intervals, with additional drops administered until full dilation was achieved.
With the animal supine and the surgeon positioned at the head (as standard for human ocular surgery), drops of 5% povidone-iodine solution (Betadine, Purdue, Stamford, CT) solution were administered into the ocular fornix and 10% povidone-iodine solution applied to the adnexa, eyelids and surrounding surgical field. The eye was draped with a sterile drape and a lid speculum placed. With the use of a standard ophthalmic surgical operating microscope (Zeiss, Dublin, CA, Universal S3B), limited peritomies were performed to allow two 20-gauge pars plana sclerotomies to be made. The episclera was treated with cautery, as necessary. Using a sterile contact lens coupling solution, a sterile Machemer fundus contact lens was placed into position. The retina was approached with a 39/21-gauge curved Synergetics (O'Fallon, MO) subretinal cannula connected to a 1ml tuberculin syringe with screw plunger containing Balanced Salt solution (BSS, Alcon, Fort Worth, TX). The BSS was injected slowly to create a retinotomy and then a small subretinal bleb was raised. This procedure minimized retinal trauma. The cannula was introduced through the retinotomy and the BSS injection restarted and continued to expand the bleb to the correct volume. A process of gentle retinal massage was used to release the tension in the bleb. The Synergetics cannula was then removed and a 30-gauge curved Hurricane Instruments (San Francisco, CA) cannula, connected to sterile tubing and a Hamilton syringe (Reno, NV) preloaded with cells, was introduced. This cannula was found not to affect cell viability by trypsin dye exclusion in preliminary experiments (data not shown). The cells were infused over approximately one minute under direct viewing to ensure correct cannula positioning, and the cannula was held in position for an additional minute to avoid reflux. The sclerotomies and conjunctival periotomies were then repaired with interrupted 6-0 vicryl S-29 and 6-0 plain gut sutures respectively. Subconjunctival injections of 125 mg/1ml cefuroxime (West-Ward, Eatontown, NJ) and 10 mg/1ml dexamethasone (American Regent, Shirley, NY) were delivered to each eye, and erythromycin ophthalmic ointment (Fougera, Melville, NY) was applied topically.
Number and location of blebs and cell doses
Subretinal blebs were created in each eye either within the macular or in an extra/juxta- macular position, as summarized in Table 1. Four animals received 100,000 cells per eye, with either the entire dose delivered in a single bleb or divided between two blebs receiving 50,000 cells each. Two subsequent animals received higher doses ranging from 480,000 – 600,000 cells per bleb with 1 – 3 blebs per eye.
Table 1.
Details of procedures for rhesus monkeys included in the study. All blebs had a volume of 100μl. The presence of hNPCctx-GFP cells in the subretinal space was confirmed postmortem in all cases.
| Monkey # | Right eye: blebs, cell doses | Left eye: blebs, cell doses | Multifocal ERGs | Fundus photography | Fluorescein angiography | Cyclosporine immuno-suppression | Postoperative survival (days) |
|---|---|---|---|---|---|---|---|
| 1 | 2 extramacular blebs 50,000 cells/bleb | 1 submacular bleb 100,000 cells/bleb | - | - | - | No | 3 |
| 2 | 1 submacular bleb 100,000 cells/bleb | 2 submacular blebs 50,000 cells/bleb | - | - | - | No | 7 |
| 3 | 1 submacular bleb 100,000 cells/bleb | 2 extramacular blebs 50,000cells/bleb | - | Preop, 7 days postop | 7 days postop | No | 14 |
| 4 | 1 juxtamacular & 1 extramacular bleb 50,000 cells/bleb | 1 submacular bleb 100,000 cells/bleb | Preop, 31 days postop | Preop, 7 & 31 days postop | 7 days postop | No | 31 |
| 5 | 1 submacular & 1 extramacular bleb 600,000 cells/bleb | 1 submacular & 2 extramacular blebs 480,000 cells/bleb | Preop, 31 days postop | Preop, 7 & 34 days postop | 7 & 34 days postop | Yes | 39 |
| 6 | 3 extramacular blebs 480,000 cells/bleb | 3 extramacular blebs 480,000 cells/bleb | Preop, 31 days postop | Preop, 7 & 34 days postop | 7 & 34 days postop | Yes | 39 |
Immunosuppression and anti-inflammatory treatment
In addition to postoperative subconjuctival dexamethasone, all animals received topical steroid (prednisolone 1%, Falcon, Fort Worth, TX) and antibiotic (ofloxacin 0.3%, Falcon, Fort Worth, TX) eyedrops in both eyes twice daily for 5 days starting on postoperative day 3. Two animals received oral cyclosporine (Novartis, East Hanover, NJ) at 35 mg/kg for two days preoperatively and then continuously until euthanized.
Fundus photography and fluorescein angiography
Monkeys were sedated with a 1:1 combination of tiletamine and zolazepam (Telazol, Fort Dodge Animal Health, Fort Dodge, IA) each at 1.75 mg/kg, intubated, and maintained under 1-3% isoflurane anesthesia (Hospira, Lake Forest, IL) in 100% oxygen. Pupils were dilated with 2-3 applications of tropicamide 1% (Tropicacyl, Acorn, Buffalo Grove, IL) plus phenylephrine 2.5%. Color stereo retinal fundus photographs were taken with a Zeiss (Dublin, CA) F3 retinal camera system, followed by red-free photographs. For fluorescein angiography, a catheter was inserted in the saphenous vein and 0.06 mg/kg of sodium fluorescein (Akorn, Buffalo Grove, IL) was injected, followed immediately by a standard series of angiographic photographs.
Multifocal electroretinography
Monkeys were anesthetized via intramuscular injection of ketamine (Bioniche Pharma, Lake Forest, IL), xylazine (Lloyd, Shenandoah, IA) and atropine (10:1:0.4 mg/kg, Abraxis, Schaumberg, IL). Anesthesia was maintained with the same drug combination at 5:0.5:0.4 mg/kg given at 30-50 min intervals as required. Supplemental oxygen was delivered via nasal cannula at 0.5 L/min. Core body temperature was maintained between 37.0°C and 38.8°C by water-circulating heated pads placed on both sides of the animal. Heart rate and O2 saturation were monitored by pulse oximetry. Prior to recording, pupils were dilated with 2-3 applications of tropicamide 1% (Tropicacyl, Akorn, Buffalo Grove, IL) and phenylephrine 2.5% (Bausch and Lomb, Tampa, FL). The cornea was anesthetized with proparacaine 1% (Akorn, Buffalo Grove, IL) and lubricated with methylcellulose 1% (Murocel, Bausch and Lomb, Tampa, FL) prior to insertion of a bipolar Burian-Allen electrode (Hansen Ophthalmic, Coralville, IA). The electrode was fitted with a +3D contact lens to allow approximate focus at the 40 cm stimulus distance. A subdermal needle electrode placed in the back served as ground. Multifocal electroretinograms (mfERGs) were recorded using a commercial electrophysiology recording system (VERIS, EDI, San Mateo, CA). The mfERG stimulus was presented on a 20″ monochrome monitor with a 75 Hz refresh rate. Mean screen luminance was 100cd/m2 and field size was approximately 40° at the 40 cm viewing distance. Before recording mfERG data, the macula was aligned with the central stimulus hexagon using a reversible ophthalmoscope. Subsequent 2 min trials were used to refine alignment such that the foveal response was centered in the mfERG response array. mfERG recordings then were obtained from each eye using stimuli with both 103 and 241 unscaled hexagon elements. The luminance of the hexagons was modulated between dark (<1 cd/m2) and light (200 cd/m2) using a pseudorandom binary m-sequence with a base interval of 13.3msec. Once the eye was aligned with the stimulus, data recordings lasting 8 minutes, were obtained in 1-minute epochs for each stimulus pattern. ERG signals were amplified (100,000) and filtered (-3dB at 10Hz & 300 Hz; notch filter at 60Hz), sampled at 1.2 KHz and stored for off-line analysis. Student's two-tailed paired t-tests or repeated measures analyses of variance (ANOVA) were used to test for differences in response densities (amplitudes) before and after subretinal injections, using GraphPad Prism version 3.02 for Windows (GraphPad Software, San Diego California USA).
Rhesus retinal histology
At 3 - 39 days after surgery, animals were overdosed with sodium pentobarbital; eyes were rapidly enucleated and a cut was made in the cornea to facilitate fixative penetration. One eye from each animal was immersed for 2 hours in 4% paraformaldehyde in sodium phosphate buffer, infiltrated with sucrose, embedded in OCT, cut as frozen sections on a cryostat and used to analyze cell survival, location and morphology as described below. The second eye was immersed for 2-3 days in 10% formalin and embedded in paraffin for histological analysis and to confirm retinal reattachment. A series of sections was stained with cresyl violet for general retinal organization and a second set were left unstained to allow visualization of fluorescent cells.
Results
Comparison of hNPCctx-GFP and hNPCctx in RCS rats
Optomotor acuity testing was performed for groups of ten rats in each experimental group at P35, P60, P90 and P120 (Figure1C). At all the time points after P35, both cell-injected groups showed significantly superior visual acuity than controls (p<0.01), as found previously for unlabeled cells.4, 5 No significant difference was found between the hNPCctx-GFP and hNPCctx groups. Similarly, histological examination of cresyl violet-stained sections showed comparable levels of photoreceptor rescue using the labeled and unlabeled cells at all the time points studied (data not shown). A human nuclear marker (Mab 1281) was used to identify native hNPCctx and hNPCctx-GFP, and the latter were also visualized with fluorescence microscopy. Both types of cells showed the same pattern of distribution, with cells forming a nearly continuous distinct subretinal layer lying between the host RPE and photoreceptors (Figure 1 D&E). No cells of either type were identified in the neurosensory retina, choroid or vitreous cavity in any case. The absence of cells within the retina was at variance with previous observations,4, 5 but may relate to the late passage cells used here.
The similarity in efficacy of the two cell preparations showed that GFP transduction did not affect the ability of the donor cells to rescue visual function. Therefore GFP labeling should not have compromised results obtained from transduced cells in primates.
Nonhuman primate studies
General observations
No perioperative or significant postoperative complications were noted at any cell dose. Specifically, there were no instances of retinal detachment, subretinal or intravitreal hemorrhage, endophthalmitis or intraocular inflammation, wound leaks or cataract formation/lens opacification. Vitrectomy was not performed in these surgeries because previous experiments using different cells in which core vitreous removal was undertaken had shown no advantage or difference in complication rates for these short term experiments (data not shown). Despite introducing the cannula tip inside the bleb and occluding the retinotomy with the cannula shaft, some reflux of cells into the vitreous cavity was noted in some cases. No complications relating to this reflux were noted. Following the procedure, retinal reattachment was observed in all instances within 24 hours and was confirmed by retinal photography. Figure 2 provides representative examples of fluorescein angiography after subretinal retinal engraftment of cells. There were no cases of subsequent retinal detachment or proliferative vitreoretinopathy as confirmed by postmortem histopathology.
Figure 2.
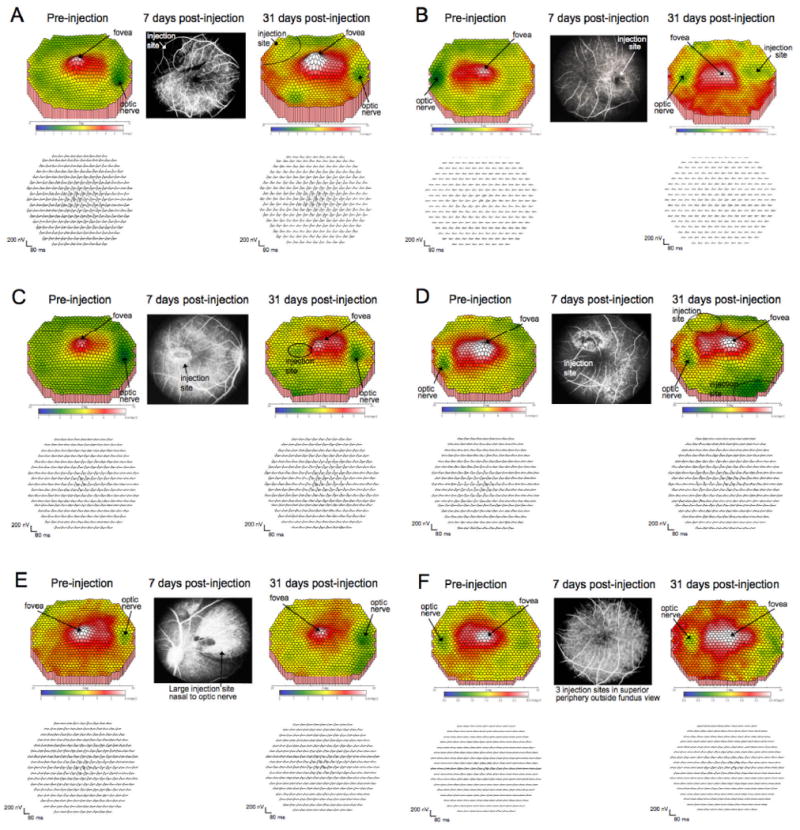
Central retinal function as assessed with multifocal ERGs in monkeys before and after subretinal cell injections. A: monkey 4 OD; B: monkey 4 OS; C: monkey 5 OD; D: monkey 5 OS; E: monkey 6 OD; F: monkey 6 OS. Left panels in each case show central macula multifocal ERG results before injection, and right panels show results at 31 days after injections; top: false color map of scalar product response amplitudes in the central 40°; bottom: the corresponding response traces for each stimulus hexagon. Central panel: fluorescein angiograms showing the injection sites within the central 40°, illustrating undisturbed vascular patterns and persistence of injection bleb boundaries; arrows indicate the positions of the fovea and optic nerve and the injection sites. In monkey 4 right eye and both eyes of 5, other injections were placed outside the central 40°; in monkey 6, all injection sites were outside the central 40°. Retinal function remained intact within the macula and showed only minor disturbance even within the injection sites.
Retinal function by multifocal ERG
In the three monkeys with longer survival times (Table 1, animals 4-6), multifocal retinal electrophysiology was performed prior to surgery and 31 days postoperatively. For the six eyes measured, post-operative mean amplitudes (mean±SEM nV/deg2) from the foveal and parafoveal rings (31.9±5.4 and 27.3±4.6 respectively) were not different from the corresponding pre-operative values (31.7±4.5 and 25.2±3.4). For 3 of the 4 eyes in which injections occurred inside the retinal arcades (monkeys 4 and 5), small depressions in the mfERG amplitude plot were observed postoperatively at the location of the bleb (Figure 2A vs. 2C; 2D vs. 2E). However, these depressions were subtle, and a functioning retina was still present within the region of bleb formation. No loss of the foveal peak was seen in any of these cases. In monkey 6, the injections were outside the central 40° evaluated by the multifocal electroretinography method and no changes in function were seen. Thus, the mfERG results indicated no significant functional loss following subretinal injections.
Retinal histology
Apart from the area immediately surrounding the retinotomy made to create the subretinal bleb, the architecture of the retina overlying the subretinal graft remained undisturbed, with normal outer nuclear layer thickness and morphology of photoreceptors including outer segments. Fluorescence microscope examination revealed that hNPCctx-GFP donor cells formed a semi-continuous subretinal layer between host RPE and photoreceptors at time points ranging from 3 days to 39 days post-injection (Figure 3, A-H). Even at 3 days (Figure 3A), hNPCctx-GFP had already formed a single layer. Donor cells lay in close proximity to outer segments (Figure 3B-H), as seen at 7 day time point (Figure 3B&G), 14 day time point (Figure 3C) and 31 day time point (Figure 3D-F&H). No inflammatory cell infiltrates were noted and no hNPCctx-GFP cells were identified in other layers of the sensory retina. In addition, the overlying retinal architecture appeared normal with normal outer nuclear layer thickness and no abnormality in host RPE and choroidal structures (Figure 3G).
Figure 3.
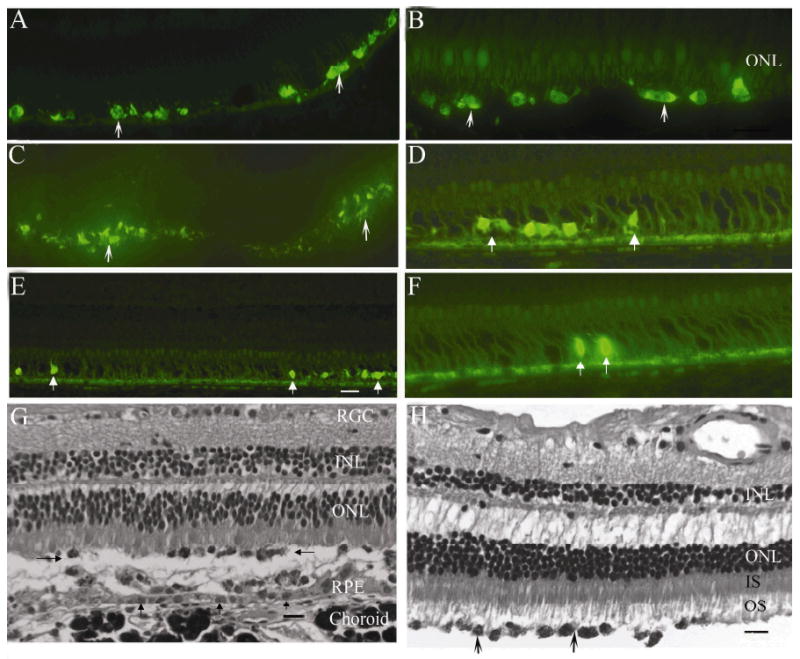
Immunofluorescence images of hNPCctx-GFP cells injected into the subretinal space of primate eyes at day 3 (A), 7 (B &G), 14 (C) and 31 (D, E, F &H) after injection (monkeys 1, 2, 3 and 4, respectively). G and H are black and white images of cresyl violet stained sections at day 7 (monkey 2) and day 31 (monkey 4) after injection, respectively. hNPCctx-GFP (white arrows in A-F, black arrows in H) were distributed in the subretinal space and in contact with host outer segments. G: donor cells are indicated by left and right pointing arrows, RPE layer by vertical arrows; scale bars equal 20μm). H: the process of tissue fixation has resulted in artefactual detachment of the RPE layer and enlargement of spaces between inner and outer nuclear layers. At seven days after injection, photoreceptor outer segments were shortened (B & G) but by day 31 (H), outer segments were well formed and donor cells appeared closely apposed.
Discussion
In this study, we report survival of bioactive human neural progenitor cells in the subretinal space of normal nonhuman primates for more than one month without evident complication and with preservation of retinal function in the transplant area. The work shows that introduction of cells into an eye with close similarity to the human eye9,10 is feasible with a pars plana surgical approach that would be appropriate to the human clinical situation (with additional vitrectomy).
The cells, suspended in non-nutrient medium, were infused through a retinotomy and formed a continuous single sheet of cells in the subretinal space. The surgical method was low cost, clinically acceptable and scalable for the treatment of a significant number of patients. The procedure and continued presence of the grafts failed to elicit any deleterious manifestations in normal retinas, at any of the doses tested, when evaluated clinically, physiologically and histologically.
Surgeries were performed in healthy adult retinas since no validated models of retinal degeneration exist in nonhuman primates. This model provides information regarding dosage, location of cell engraftment and surgical technique that cannot be addressed in small rodent eyes. Retinas with advanced and widespread disease (e.g., in retinitis pigmentosa) may behave differently, for example with regard to subretinal bleb formation. However, the model is pertinent to early disease states, before marked degenerative changes have developed, and to macular degenerations such as AMD and Stargardt disease where juxtamacular delivery of cells to the surrounding more ‘normal’ retina might be employed.
Autologous RPE grafts introduced into the subretinal space of advanced patients with neovascular or “wet” AMD11, 12 appear to have little positive impact on vision and are associated with a high rate of significant complications including retinal detachment and hemorrhage affecting the graft. These disappointing findings are likely related to the complexity of performing this particular surgery as well as the advanced stage of the disease.13, 14 Indeed, creation of a retinal detachment by subretinal injection can lead to reduced visual function in the area of the detachment without subsequent recovery. Previous work has shown that introduction of growth factors such as brain-derived and glial-derived neurotrophic factors (BDNF and GDNF) can diminish this detachment effect.15, 16 Whether factors released by these neural progenitor cells may provide similar protection was not explored explicitly here but would be consistent with the preservation of mfERG responses in retinal areas where subretinal blebs had been elevated.
An issue of some importance is whether cells migrate from their site of engraftment. This question is critical to future use of cell therapy for AMD, given the need to introduce cells as close to the macula as possible without directly compromising macular vision. Indeed it would be advantageous if the cells could be delivered to an extra-macular location and then would migrate in the subretinal space under the fovea and throughout the retina. The degree of lateral migration or the retinal area covered by the cells could not be quantified in the present study, because it was not possible to define the border of the bleb in the post-mortem histology. Therefore, the potential area over which cells introduced into the bleb might have spread by active migration is unknown in the present study. Further work will address this issue.
The question of whether precautions are needed to prevent immune rejection, beyond the steroid application used here, also deserves more attention. Previous work in RCS rats has shown that even allogeneic grafts can undergo rejection and lead to loss of vision, while syngeneic grafts can survive when introduced under similar conditions.3, 17 In pigs, triple immune suppression with prednisone, cyclosporine and azathioprine was still not sufficient to sustain RPE graft survival.18 In the present study, cells survived up to 5 weeks even in the monkey treated postsurgically only with 5 days of topical steroids but not with systemic cyclosporine. Therefore it is possible that the donor cells used are less immunogenic than the RPE cells used in previous work. Two studies have suggested that human fetal RPE cells can survive in the primate subretinal space for significant periods.9, 19 It has also been suggested that the primate eye shows a stronger level of immune privilege than other species,20, 21 or that the pars plana approach avoids overt graft rejection, at least to 5 weeks, the longest time point studied here without cyclosporine.
Our study shows that human embryonic tissue-derived progenitor cells can survive transplantation into the subretinal space of non-human primates for at least five weeks. A more extensive and longer study is required to address issues (e.g. biodistribution, safety) that must be resolved before the application of cell-based therapies to the treatment of human retinal disorders including AMD.
Acknowledgments
The authors thank Dr Andrea Bauman for organizational and administrative support and for assistance with manuscript preparation, and Elizabeth Capowski, Jie Duan, Thea Burke, Noelle Landauer and Laurie Renner for technical assistance. The GFP-hNPCctx were kindly provided by Dr. Clive Svendsen, U. Wisconsin-Madison.
Grant sponsors: Lincy Foundation, The Foundation Fighting Blindness, Owings Mills, MD (MN, RDL, PJF, DMG, SW); National Institutes of Health K08EY015138 (DMG); Research to Prevent Blindness, New York, NY (PJF, Career Development Award, DMG Robert E. McCormick Scholar Award); the Clayton Foundation (JTS); the Walsh Foundation (RDL, DMG); Hear See Hope Foundation (PJF, MN).
References
- 1.Lund RD, Wang S, Klimanskaya I, et al. Human embryonic stem cell-derived cells rescue visual function in dystrophic RCS rats. Cloning Stem Cells. 2006;8:189–199. doi: 10.1089/clo.2006.8.189. [DOI] [PubMed] [Google Scholar]
- 2.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25:602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 3.McGill TJ, Lund RD, Douglas RM, et al. Syngeneic Schwann cell transplantation preserves vision in RCS rat without immunosuppression. Invest Ophthalmol Vis Sci. 2007;48:1906–1912. doi: 10.1167/iovs.06-1117. [DOI] [PubMed] [Google Scholar]
- 4.Gamm DM, Wang S, Lu B, et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE. 2007;2:e338. doi: 10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang S, Girman S, Lu B, et al. Long-term vision rescue by human neural progenitors in a rat model of photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2008;49:3201–3206. doi: 10.1167/iovs.08-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Svendsen CN, Borg MG, Armstrong RJ, et al. A new method for the rapid and long term growth of human neural precursor cells. J Neurosci Methods. 1998;85:141–152. doi: 10.1016/s0165-0270(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 7.Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–390. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- 9.Sheng Y, Gouras P, Cao H, et al. Patch transplants of human fetal retinal-pigment epithelium in rabbit and monkey retina. Invest Ophthalmol Vis Sci. 1995;36:381–390. [PubMed] [Google Scholar]
- 10.Warfvinge K, Kiilgaard JF, Klassen H, et al. Retinal progenitor cell xenografts to the pig retina: immunological reactions. Cell Transplant. 2006;15:603–612. doi: 10.3727/000000006783981594. [DOI] [PubMed] [Google Scholar]
- 11.Stanga PE, Kychenthal A, Fitzke FW, et al. Retinal pigment epithelium translocation and central visual function in age related macular degeneration: preliminary results. Int Ophthalmol. 2001;23:297–307. doi: 10.1023/a:1014482025960. [DOI] [PubMed] [Google Scholar]
- 12.Tezel TH, Del Priore LV, Berger AS, Kaplan HJ. Adult retinal pigment epithelial transplantation in exudative age-related macular degeneration. Am J Ophthalmol. 2007;143:584–595. doi: 10.1016/j.ajo.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 13.MacLaren RE, Uppal GS, Balaggan KS, et al. Autologous transplantation of the retinal pigment epithelium and choroid in the treatment of neovascular age-related macular degeneration. Ophthalmology. 2007;114:561–570. doi: 10.1016/j.ophtha.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 14.Joussen AM, Heussen FM, Joeres S, et al. Autologous translocation of the choroid and retinal pigment epithelium in age-related macular degeneration. Am J Ophthalmol. 2006;142:17–30. doi: 10.1016/j.ajo.2006.01.090. [DOI] [PubMed] [Google Scholar]
- 15.Lewis GP, Linberg KA, Geller SF, Guerin CJ, Fisher SK. Effects of the neurotrophin brain-derived neurotrophic factor in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 1999;40:1530–1544. [PubMed] [Google Scholar]
- 16.Wu WC, Lai CC, Chen SL, et al. Gene therapy for detached retina by adeno-associated virus vector expressing glial cell line-derived neurotrophic factor. Invest Ophthalmol Vis Sci. 2002;43:3480–3488. [PubMed] [Google Scholar]
- 17.Zhang X, Bok D. Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest Ophthalmol Vis Sci. 1998;39:1021–1027. [PubMed] [Google Scholar]
- 18.Del Priore LV, Ishida O, Johnson EW, et al. Triple immune suppression increases short-term survival of porcine fetal retinal pigment epithelium xenografts. Invest Ophthalmol Vis Sci. 2003;44:4044–4053. doi: 10.1167/iovs.02-1175. [DOI] [PubMed] [Google Scholar]
- 19.Berglin L, Gouras P, Sheng Y, et al. Tolerance of human fetal retinal pigment epithelium xenografts in monkey retina. Graefes Arch Clin Exp Ophthalmol. 1997;235:103–110. doi: 10.1007/BF00941738. [DOI] [PubMed] [Google Scholar]
- 20.Niederkorn JY. Immune privilege and immune regulation in the eye. Adv Immunol. 1990;48:191–226. doi: 10.1016/s0065-2776(08)60755-5. [DOI] [PubMed] [Google Scholar]
- 21.Tompsett E, Abi-Hanna D, Wakefield D. Immunological privilege in the eye: a review. Curr Eye Res. 1990;9:1141–1145. doi: 10.3109/02713689009003470. [DOI] [PubMed] [Google Scholar]


