Abstract
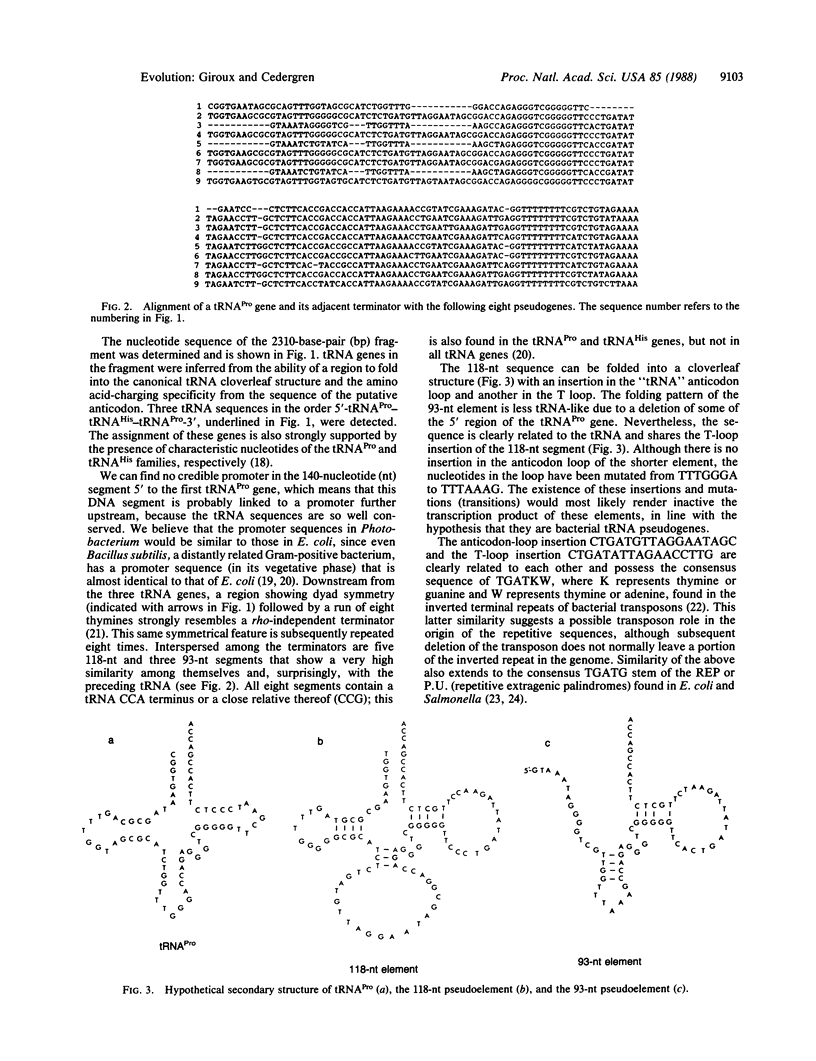
A region distal to three tRNA genes in Photobacterium phosphoreum, a Gram-negative eubacterium, unexpectedly contains a high number of repeated DNA segments that are closely related to the adjacent tRNAPro gene. The 5' to 3' order of this cluster is tRNAPro-tRNAHis-tRNAPro followed by eight tRNAPro-like structures interspersed by rho-independent terminators. The two tRNAPro genes, which are identical, and the tRNAHis gene have 86% and 87% positional identity, respectively, to their counterparts in the argT operon of Escherichia coli. The facts that these tRNA-like structures are not transcribed, in contrast to the tRNA retropseudogenes of eukaryotes, and that these structures are clustered near their progenitor suggest they are an unusual class of tRNA pseudogenes that arose by tandem duplication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. P., Roth J. R. Tandem genetic duplications in phage and bacteria. Annu Rev Microbiol. 1977;31:473–505. doi: 10.1146/annurev.mi.31.100177.002353. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Cedergren R., Lang B. F. Probing fungal mitochondrial evolution with tRNA. Biosystems. 1985;18(3-4):263–267. doi: 10.1016/0303-2647(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Duester G., Campen R. K., Holmes W. M. Nucleotide sequence of an Escherichia coli tRNA (Leu 1) operon and identification of the transcription promoter signal. Nucleic Acids Res. 1981 May 11;9(9):2121–2139. doi: 10.1093/nar/9.9.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan J., Landy A. Structural analysis of the tRNA1Tyr gene of Escherichia coli. A 178 base pair sequence that is repeated 3.14 times. J Biol Chem. 1978 May 25;253(10):3607–3622. [PubMed] [Google Scholar]
- Field K. G., Olsen G. J., Lane D. J., Giovannoni S. J., Ghiselin M. T., Raff E. C., Pace N. R., Raff R. A. Molecular phylogeny of the animal kingdom. Science. 1988 Feb 12;239(4841 Pt 1):748–753. doi: 10.1126/science.3277277. [DOI] [PubMed] [Google Scholar]
- Flores M., González V., Brom S., Martínez E., Piñero D., Romero D., Dávila G., Palacios R. Reiterated DNA sequences in Rhizobium and Agrobacterium spp. J Bacteriol. 1987 Dec;169(12):5782–5788. doi: 10.1128/jb.169.12.5782-5788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Clément J. M., Brutlag D., Hofnung M. A family of dispersed repetitive extragenic palindromic DNA sequences in E. coli. EMBO J. 1984 Jun;3(6):1417–1421. doi: 10.1002/j.1460-2075.1984.tb01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Osawa S. Origin and evolution of organisms as deduced from 5S ribosomal RNA sequences. Mol Biol Evol. 1987 Sep;4(5):445–472. doi: 10.1093/oxfordjournals.molbev.a040455. [DOI] [PubMed] [Google Scholar]
- Hsu L. M., Klee H. J., Zagorski J., Fournier M. J. Structure of an Escherichia coli tRNA operon containing linked genes for arginine, histidine, leucine, and proline tRNAs. J Bacteriol. 1984 Jun;158(3):934–942. doi: 10.1128/jb.158.3.934-942.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. C., Moran C. P., Jr, Losick R. Two RNA polymerase sigma factors from Bacillus subtilis discriminate between overlapping promoters for a developmentally regulated gene. Nature. 1983 Apr 28;302(5911):800–804. doi: 10.1038/302800a0. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Transposable elements in prokaryotes. Annu Rev Genet. 1981;15:341–404. doi: 10.1146/annurev.ge.15.120181.002013. [DOI] [PubMed] [Google Scholar]
- MacDonell M. T., Swartz D. G., Ortiz-Conde B. A., Last G. A., Colwell R. R. Ribosomal RNA phylogenies for the vibrio-enteric group of eubacteria. Microbiol Sci. 1986 Jun;3(6):172-5, 178. [PubMed] [Google Scholar]
- Nicoghosian K., Bigras M., Sankoff D., Cedergren R. Archetypical features in tRNA families. J Mol Evol. 1987;26(4):341–346. doi: 10.1007/BF02101153. [DOI] [PubMed] [Google Scholar]
- Orozco E. M., Jr, Rushlow K. E., Dodd J. R., Hallick R. B. Euglena gracilis chloroplast ribosomal RNA transcription units. II. Nucleotide sequence homology between the 16 S--23 S ribosomal RNA spacer and the 16 S ribosomal RNA leader regions. J Biol Chem. 1980 Nov 25;255(22):10997–11003. [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Reed R. E., Altman S. Repeated sequences and open reading frames in the 3' flanking region of the gene for the RNA subunit of Escherichia coli ribonuclease P. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5359–5363. doi: 10.1073/pnas.80.17.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. E., Baer M. F., Guerrier-Takada C., Donis-Keller H., Altman S. Nucleotide sequence of the gene encoding the RNA subunit (M1 RNA) of ribonuclease P from Escherichia coli. Cell. 1982 Sep;30(2):627–636. doi: 10.1016/0092-8674(82)90259-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Mechanisms of DNA reorganization in bacteria. Int Rev Cytol. 1985;93:25–56. doi: 10.1016/s0074-7696(08)61371-6. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- Vold B. S. Structure and organization of genes for transfer ribonucleic acid in Bacillus subtilis. Microbiol Rev. 1985 Mar;49(1):71–80. doi: 10.1128/mr.49.1.71-80.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]