Abstract
In spite of the eradication of smallpox over 30 years ago; orthopox viruses such as smallpox and monkeypox remain serious public health threats both through the possibility of bioterrorism and the intentional release of smallpox and through natural outbreaks of emerging infectious diseases such as monkeypox. The eradication effort was largely made possible by the availability of an effective vaccine based on the immunologically cross-protective vaccinia virus. Although the concept of vaccination dates back to the late 1800s with Edward Jenner, it is only in the past decade that modern immunologic tools have been applied toward deciphering poxvirus immunity. Smallpox vaccines containing vaccinia virus elicit strong humoral and cellular immune responses that confer cross-protective immunity against variola virus for decades after immunization. Recent studies have focused on: establishing the longevity of poxvirus-specific immunity, defining key immune epitopes targeted by T and B cells, developing subunit-based vaccines, and developing genotypic and phenotypic immune response profiles that predict either vaccine response or adverse events following immunization.
Introduction
Variola virus, the causative agent of smallpox, can be found throughout human history and probably developed alongside human civilization [1]. In 1798 Edward Jenner advanced the concept of using cowpox as a prophylactic agent against smallpox. Early practitioners used a wide variety of pox viruses taken from or grown on cows, sheep, horses, goats, pigs, and buffaloes [1]. Vaccination quickly became widespread and by the 20th century almost all vaccines contained yet another orthopoxvirus: vaccinia virus (VACV) [1]. In spite of its indefinite origins vaccinia virus was the basis for extremely effective vaccines that, together with surveillance and monitoring led to the eradication of smallpox in 1980. Rare, but potentially life-threatening adverse events, led to the cessation of vaccine use among the general public, and recent vaccination programs have highlighted the risk of cardiovascular adverse events [2, 3]. During the height of the eradication effort in the 1960s research efforts focused on humoral immunity, although the importance of cellular responses was predicted. In fact, there are two historical definitions of ‘protection’ that, while not mutually exclusive, do rely on humoral and cellular immune responses, respectively. These are (1) serum neutralizing antibody titer > 1:32 [4] and (2) the formation of a ‘take’ or vesicle at the vaccination site due to cellular immune responses to the local infection [1].
Terrorist activities in the early 21st century as well as imported outbreaks of monkeypox in the USA spurred renewed interest in biodefense countermeasures for these public health threats [5, 6]. Faced with inadequate stocks of smallpox vaccine, an outdated vaccine production method, an increasing unvaccinated, and hence susceptible population, as well as a growing number of both immunosuppressed individuals and people with vaccine contraindications (heart conditions, cancer patients, organ transplant recipients, skin diseases such as eczema); research efforts focused on increasing our understanding of poxvirus immunity in order to develop safe and effective next-generation vaccines. In this review we will focus on the highlights of research regarding the mechanisms of disease protection elicited by smallpox vaccines.
Vaccines used during the eradication effort (Dryvax®, APSV®, Lancy–Vaxina®, L-IVP®) are termed first-generation vaccines (last produced in the 1970s and early 1980s) and contained live vaccinia virus administered by puncturing the skin of the upper arm with a bifurcated needle (Table 1 ). Successful administration of the vaccine typically led to the development of a characteristic pustule at the vaccination site. Historically the development of this ‘take’ was considered evidence of protection [1]. Several recent studies have demonstrated that these live vaccines can be diluted 5–10-fold with no significant decreases in immunogenicity [7, 8]. These vaccines induced robust humoral immunity characterized by high antibody titers capable of neutralizing and opsonizing viral particles, fixing complement, hemagglutination, as well as participating in antibody dependent cell cytotoxicity [1, 13, 14•]. Similarly, these vaccines induced strong cellular responses capable of secreting effector cytokines such as IFNγ and lysing infected cells [1, 13, 25]. Together, these adaptive immune responses cleared the localized vaccinia infection at the immunization site and elicited long-lived memory responses capable of recognizing and clearing subsequent variola infections.
Table 1.
Smallpox vaccines and vaccine candidates
| Vaccine | Virus strain | Usage | Details |
|---|---|---|---|
| Dryvax® | NYCBOH | Widespread use | Used in US during eradication. Highly effective. Lyophilized stock. |
| APSV® | NYCBOH | Widespread use | Used in US during eradication. Highly effective. Frozen liquid preparation. |
| Lancy–Vaxina | Lister | Widespread use | Used world-wide during eradication. Highly effective. |
| EM-63 | NYCBOH | Widespread use | Used in Russia during eradication. Highly effective. |
| Temple of Heaven | Tian Tian | Widespread use | Used in China during eradication. Highly effective. Greater number of adverse events compared to NYCBOH and Lister vaccines. |
| ACAM1000 | NYCBOH | Clinical trials | Tissue culture (MRC-5 cells). Equivalent immunogenicity to Dryvax®. |
| ACAM2000 | NYCBOH | Clinical trials | Tissue culture (Vero cells). Equivalent immunogenicity to Dryvax®. FDA approved in 2008. Part of US National Stockpile. |
| CCSV | NYCBOH | Clinical trials | Tissue culture vaccine. Equivalent immunogenicity to Dryvax®. |
| Elstree-BN | Lister | Clinical trials | Tissue culture vaccine. Replacement for early Lister vaccines. |
| MVA | Ankara | Limited use | Lost 15% of genome through serial passage in chick embryo fibroblasts. Cannot replicate in human cells. Used in Germany with fewer adverse events. Immunogenicity may not be equal to replication-competent vaccines. |
| ACAM3000 | Ankara | Clinical trials | Next-generation MVA-based vaccine. |
| IMVAMUNE | Ankara | Clinical trials | Next-generation MVA-based vaccine. |
| TBC-MVA | Ankara | Clinical trials | Next-generation MVA-based vaccine. |
| NYVAC | Copenhagen | Clinical trials | 18 ORFs deleted. Improved safety profile. Not widely tested. Immunogenicity may not be equal to unattenuated live vaccines. |
| LC16m8 | Lister | Limited use | Attenuated vaccine based on Lister strain. Used in Japan with good safety record. No efficacy data. Immunogenicity may not be equal to unattenuated live vaccines. |
| dVV-L | Lister | Clinical trials | Lister-based vaccine with UDG gene deleted to improve safety. No efficacy data. Immunogenicity may not be equal to unattenuated live vaccines. |
| Subunit | Various | R&D | DNA or protein-based subunit vaccines |
The second-generation vaccines, produced in the past 5–10 years, contain replication competent viruses produced in tissue culture and are designed as replacements for these early vaccines [5]. These replacement vaccines were commonly compared to Dryvax® and were designed to elicit similar levels of immunity [9]. Third generation vaccine formulations have focused on attenuated vaccinia strains (LC16m8, MVA, NYVAC, dVVL) with the hope that they have better safety profiles [9] (see Table 2 for adverse events associated with smallpox vaccines). Next-generation vaccine development is now focusing on a variety of subunit (protein and DNA-based) in order to create safer, yet still efficacious smallpox vaccines. This review will focus primarily on the immune responses generated by first-generation and second-generation vaccines.
Table 2.
Adverse events associated with live smallpox vaccines
| Adverse event | Rate of occurrence |
|---|---|
| Serious and/or life-threatening | (per million vaccinees) |
| Death | 1–2 |
| Postvaccinal Encephalitis | 3–9 |
| Progressive Vaccinia | 1–7 |
| Eczema Vaccinatum | 2–35 |
| Moderate | (per million vaccinees) |
| Generalized vaccinia | 40–200 |
| Myopericarditis | 100 |
| Accidental inoculation | 100–600 |
| Bacterial Infection | Unknown |
| Non-infectious rashes | 1–5% |
| Mild (these are far more common, affecting 5–80% of vaccinees) | |
| Itching | |
| Fever | |
| Lymphadenopathy | |
| Headache | |
| Nausea | |
| Pain at vaccination site | |
| Fatigue | |
| Muscle aches | |
| Chills | |
We focus here on adaptive immune responses due to vaccination, but the crucial role that innate immunity plays in poxvirus pathogenesis should not be overlooked. Moulton et al. recently reported that mice lacking components of the complement system suffer from increased disease severity and mortality when challenged with ectromelia virus (ECTV) [10•]. Poxviruses possess a number of crucial virulence factors that act as immunomodulatory proteins targeting key innate pathways such as interferons, chemokines, inflammatory cytokines, complement, and the toll-like receptor (TLR) family of pattern recognition receptors [11]. These innate responses, including chemokines, inflammatory cytokines, as well as pattern recognition receptors and their associated pathways (Figure 1 ) initiate the more robust adaptive immune responses, as is evidenced by a recent report showing that TLR signaling is crucial to the development of CD8 T cell memory following vaccinia infection [12•].
Figure 1.
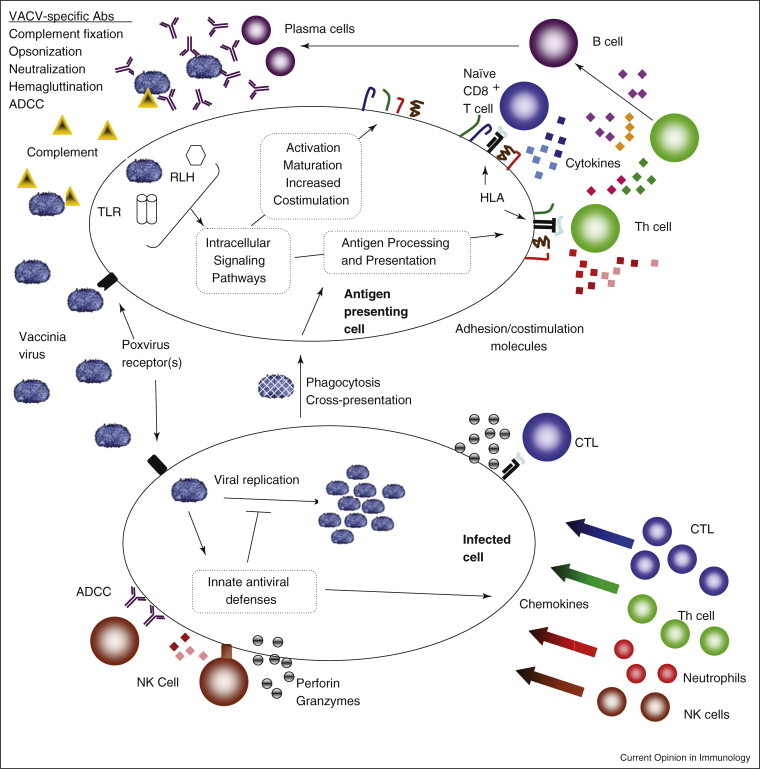
Immune response pathways activated by smallpox vaccines. Immunization with the smallpox vaccines elicits a cascading network of integrated immune pathways. Non-specific innate responses activated by pattern recognition receptors serve to inhibit initial viral replication and to activate antigen presenting cells in order to properly initiate adaptive immunity. Innate inflammatory cytokines and chemokines then attract effector lymphocytes into infected tissues. T helper cells supply necessary cytokines (IL-4, IL-5) and costimulatory signals (CD40L) for the B cell maturation, replication and isotype switching. T cell help (IL-2, IFNg) also promotes CTL activation, clonal expansion and effector function. VACV-specific T helper cells can also have direct lytic activity. B cells produce antibodies that agglutinate, opsonize, and neutralize viral particles, fix complement and allow for antibody dependent cell cytotoxicity (ADCC). Activated CD8 T cells lyse infected cells through perforin, granzymes, and through death receptors such as FasL. Cytokine secretion (IFNg, TNFa) by T lymphocytes can also have direct antiviral activity. Together humoral and adaptive responses halt viral replication, lyse infected cells, and remove viral particles from the host. Virus-specific lymphocyte numbers then contract to a small, long-lived memory population capable of rapidly responding to subsequent infection with VACV and more. Electron micrograph of vaccinia virus adapted from the Centers for Disease Control and Prevention Public Health Image Library, image #2143.
B cell responses
Smallpox vaccine induces strong humoral responses that play a crucial role in protection against disease [13, 14•]. A prospective study by Mack et al., found that neutralizing serum antibody titers > 1:32 were associated with protective immunity against smallpox disease [4]. Vaccinia Immune Globulin (VIG), prepared from serum of recent vaccinees, prevents infection of close contacts of smallpox victims and treats vaccine-related complications [15]. Defects in humoral immunity have severe consequences during poxvirus infection. B cell deficient mice are unable to clear ectromelia infection in spite of detectable levels of anti-viral CD8+ T cell activity [16]. Similarly, a study infecting Rhesus macaques with monkeypox virus demonstrated that vaccinia-specific B cell responses are essential for protection [17]. Recent data demonstrate that vaccinia-specific antibody levels (both total IgG and neutralizing antibody) persist for decades and that vaccinia-specific memory B cells are functional, maintained for more than 50 years, and are able to mount a vigorous antibody response upon re-vaccination with Dryvax® [18]. Moreover, the Baltimore Longitudinal Study of Aging, using 209 individuals who had been vaccinated as far back as 88 years prior, indicated that nearly 97% of individuals maintain both vaccinia-specific IgG and neutralizing antibodies at protection levels against smallpox [19].
Recently, protein microarrays have been used to characterize humoral immune response profiles to smallpox vaccines [20•, 21•]. These studies have shown that antibody responses in humans display considerable interindividual variation and are directed against multiple vaccinia virus proteins (see Table 3 ). The proteins targeted by humoral responses are predominantly viral structural, and membrane proteins, although responses to core proteins and proteins expressed only in infected cells have also been observed [22•]. Recent reports have shown that specific antibodies against both the intracellular mature (IM) and extracellular enveloped (EE) virions of vaccinia (and variola) are essential for optimal protective immunity induced by vaccination [23].
Table 3.
Proteins targeted by T and B cells
| Epitope typea | # of Epitopes recognizedb | Target protein characteristicsc |
|---|---|---|
| B cell | 9–15 ORFs (# of ORFs per subject not ascertained) | Predominantly proteins with late or early/late promoters. Almost exclusively membrane and core proteins |
| CD4+ T cell | >130 ORFs (∼0–20 ORFs per subject) | Early, intermediate and late proteins, predominantly structural and membrane proteins as well as DNA replication enzymes. |
| CD8+ T cell | >190 ORFs epitope diversity within individuals is not well studied. Most subjects recognized more than 1 epitope. | Predominantly early proteins. Multiple functional categories (virulence factors, viral replication enzymes, transcription factors, structural proteins) are targeted by CTL. |
Immune epitopes from VACV.
Lymphocyte subset recognizing each group of epitopes.
Top number represents the total # of ORFs for which epitopes have been identified. The number(s) in parentheses indicate the extent or diversity of antigenic recognition on a per subject basis.
Newer studies of humoral immune response after smallpox vaccination seek to identify the repertoire of antigenic peptides recognized by vaccinia-specific B cells. For example, the viral B5R protein was found to be the main target of neutralizing antibodies in VIG [24]. Clearly defined humoral epitopes may inform development of new vaccine candidates, antibody-based therapeutics, and could provide further understanding regarding protection against smallpox.
T cell responses
Smallpox vaccine induces strong CD4+ and CD8+ T cell responses that peak at two to four weeks postimmunization and then contract to form a stable memory population of T cells that remain detectable for decades [13, 25]. Interestingly, the CD8 T cell memory population appears to decline faster than memory CD4+ T cells [26]. It has long been noted that defects in cellular immunity lead to uncontrolled vaccinia infection, indicating that T cells play an important role in protection [27]. More recent data have shown that CD4+ and CD8+ T cells can prevent mortality in B cell deficient animals challenged with VACV [1, 28]. Viral infection of CD4 deficient mice results in delayed viral clearance and increased mortality [29, 30]. By contrast, animals lacking CD8 T cells are able to clear virus normally [31]. However, in the absence of humoral immunity, CD8+ T cells can provide partial protection [30], and immunization with a single HLA class I restricted epitope can provide varying degrees of disease protection [32]. The data illustrating how effective CD8 T cell responses are in the absence of humoral immunity greatly depend on the animal and the virus used. Studies using VACV as a challenge have shown that T cells are capable of delaying and in some cases clearing infection, while in models using species-specific pathogens (ECMV in mice or MXPV in non-human primates), CD8+ T cells are far less capable of viral clearance. The requirement for CD4+ T cells in protection is clear-cut as robust poxvirus-specific antibody responses fail to develop in animals lacking CD4+ T cells [17, 30]. Similarly, CD4+ T cell help is essential for optimal CTL function and memory formation [33].
We have recently reviewed a large number of epitope mapping studies identifying well over 100 CD8+ T cell targets [34]. Additional efforts to pinpoint CD4+ T cell epitopes are underway as well [35, 36, 37•, 38].
In contrast to cellular responses to other pathogens, VACV-specific CD4+, and CD8+ T cells recognize a diverse array of viral proteins with no clear-cut patterns of immunodominance. CD8+ T cell epitopes are predominantly found in early, non-structural genes and transcription factors [39, 40•]. Proteins encoded by early genes may be synthesized and presented more efficiently than those expressed late in the viral life cycle, and CTL specific for these epitopes are more likely to lyse infected cells before progeny virions are produced. By contrast, CD4+ T cell epitopes are concentrated in late, viral membrane and structural proteins as well as in enzymes involved in viral replication, and are capable of recognizing over 68% of viral proteins [37•]. Interestingly, new evidence suggests a close linkage between B cell and CD4+ T cell epitopes to vaccinia proteins [41••]. Thus, viral proteins recognized by CD4+ T cells are also likely to be targeted by humoral responses, indicating that cognate T helper cell–B cell interactions may be required to generate robust VACV-specific antibody responses. Importantly, priming with CD4+ T cell epitopes can protect against lethal infection [41]. It is logical to assume that this protective effect may be more pronounced by the inclusion of nearby B cell epitopes, a conclusion that may account for the success of many subunit-based smallpox vaccines in animal studies [23, 42, 43, 44].
Conclusions
Smallpox vaccines induce robust T and B cell responses that target a wide array of viral proteins and provide cross-protective immunity against important human pathogens such as variola and monkeypox. Recent advances in proteome-wide immune profiling and epitope identification have provided important information regarding poxvirus immunology. These types of studies that allow for deconstructing immune responses will probably be essential to the development of safer, next-generation vaccines and anti-viral therapies.
Another important avenue of research is in the understanding of the genetic factors influencing both vaccine response and adverse events. We have recently reported that gender is significantly associated with variations in neutralizing antibody titers developing after smallpox vaccination [45]. Stanley et al. have demonstrated that specific variations in the IL-1 and IL-18 genes are associated with the development of fever following smallpox vaccination [46]. Similarly, Reif et al. have identified single nucleotide polymorphisms in two genes (MTHFR, IRF1) that are associated with development of adverse reactions to Dryvax® [47]. McKinney et al. have identified patterns of serum cytokine expression, such as granulocyte colony-stimulating factor, stem cell factor, monokine induced by IFN-γ (CXCL9), intercellular adhesion molecule-1, eotaxin, and tissue inhibitor of metalloproteinases-2, after smallpox vaccination that may play a role in systemic adverse events [48]. These studies are significant in that a population could be screened for the presence of genetic or phenotypic profiles that predict serious adverse events before vaccination. One could similarly develop an immune profile that predicts the development of protective or ineffective vaccine responses, which would allow us to tailor more appropriate vaccination plans for these individuals [49]. The continuation of these studies will also improve our understanding of poxvirus pathogenesis, and may inform newer vaccine development.
Conflict of interest statement
Dr Poland has provided consultant advice to Acambis regarding smallpox vaccine development. The other authors do not have any conflicts of interest.
References and recommended reading
Papers of particular interest, published within the past two years, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank Jessica Gunther for her editorial assistance. We acknowledge support from National Institutes of Health grant AI 40065, the National Center for Research Resources grant 1 UL1 RR024150-01 and the NIH/NIAID Regional Center of Excellence for Bio-defense and Emerging Infectious Diseases Research (RCE) Program. The authors wish to acknowledge membership within and support from the Region V ‘Great Lakes’ RCE (NIH award 1-U54-AI-057153).
References
- 1.Fenner F. World Health Organization; Geneva: 1988. Smallpox and its Eradication. [Google Scholar]
- 2.Fulginiti V.A., Papier A., Lane J.M., Neff J.M., Henderson D.A. Smallpox vaccination: a review, part II. Adverse events. Clin Infect Dis. 2003;37:251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- 3.Halsell J.S., Riddle J.R., Atwood J.E., Gardner P., Shope R., Poland G.A., Gray G.C., Ostroff S., Eckart R.E., Hospenthal D.R., et al. Myopericarditis following smallpox vaccination among vaccinia-naive US military personnel. JAMA. 2003;289:3283–3289. doi: 10.1001/jama.289.24.3283. [DOI] [PubMed] [Google Scholar]
- 4.Mack T.M., Noble J., Jr., Thomas D.B. A prospective study of serum antibody and protection against smallpox. Am J Trop Med Hyg. 1972;21:214–218. doi: 10.4269/ajtmh.1972.21.214. [DOI] [PubMed] [Google Scholar]
- 5.Artenstein A.W., Grabenstein J.D. Smallpox vaccines for biodefense: need and feasibility. Expert Rev Vaccines. 2008;7:1225–1237. doi: 10.1586/14760584.7.8.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey S.E., Couch R.B., Tacket C.O., Treanor J.J., Wolff M., Newman F.K., Atmar R.L., Edelman R., Nolan C.M., Belshe R.B. Clinical responses to undiluted and diluted smallpox vaccine. N Engl J Med. 2002;346:1265–1274. doi: 10.1056/NEJMoa020534. [DOI] [PubMed] [Google Scholar]
- 8.Rock M.T., Yoder S.M., Talbot T.R., Edwards K.M., Crowe J.E., Jr. Cellular immune responses to diluted and undiluted aventis pasteur smallpox vaccine. J Infect Dis. 2006;194:435–443. doi: 10.1086/505506. [DOI] [PubMed] [Google Scholar]
- 9.Artenstein A.W. New generation smallpox vaccines: a review of preclinical and clinical data. Rev Med Virol. 2008;18:217–231. doi: 10.1002/rmv.571. [DOI] [PubMed] [Google Scholar]
- 10•.Moulton E.A., Atkinson J.P., Buller R.M. Surviving mousepox infection requires the complement system. PLoS Pathog. 2008;4:e1000249. doi: 10.1371/journal.ppat.1000249. [DOI] [PMC free article] [PubMed] [Google Scholar]; Much of what we know regarding immunity to poxviruses comes from murine models using ectromelia (mousepox). This study found that deficiencies in complement components led to increase mortality after infection, suggesting that the complement cascade may also protect against smallpox infection.
- 11.Seet B.T., Johnston J.B., Brunetti C.R., Barrett J.W., Everett H., Cameron C., Sypula J., Nazarian S.H., Lucas A., McFadden G. Poxviruses and immune evasion. Annu Rev Immunol. 2003;21:377–423. doi: 10.1146/annurev.immunol.21.120601.141049. [DOI] [PubMed] [Google Scholar]
- 12•.Quigley M., Martinez J., Huang X., Yang Y. A critical role for direct TLR2-MyD88 signaling in CD8 T-cell clonal expansion and memory formation following vaccinia viral infection. Blood. 2009;113:2256–2264. doi: 10.1182/blood-2008-03-148809. [DOI] [PMC free article] [PubMed] [Google Scholar]; This report underscores the importance of innate immune pathways in the generation of robust adaptive immunity. Direct TLR2 signaling in CD8 T cells was necessary for CTL differentiation into memory cells. This effect was dependent on the PI3K-Akt pathway in these cells.
- 13.Amanna I.J., Slifka M.K., Crotty S. Immunity and immunological memory following smallpox vaccination. Immunol Rev. 2006;211:320–337. doi: 10.1111/j.0105-2896.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- 14•.Panchanathan V., Chaudhri G., Karupiah G. Correlates of protective immunity in poxvirus infection: where does antibody stand? Immunol Cell Biol. 2008;86:80–86. doi: 10.1038/sj.icb.7100118. [DOI] [PubMed] [Google Scholar]; An excellent review of animal studies that have focused on determining the role of antibodies in protection against poxviruses.
- 15.Hopkins R.J., Lane J.M. Clinical efficacy of intramuscular vaccinia immune globulin: a literature review. Clin Infect Dis. 2004;39:819–826. doi: 10.1086/422999. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhri G., Panchanathan V., Bluethmann H., Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006;80:6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edghill-Smith Y., Bray M., Whitehouse C.A., Miller D., Mucker E., Manischewitz J., King L.R., Robert-Guroff M., Hryniewicz A., Venzon D., et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 18.Crotty S., Felgner P., Davies H., Glidewell J., Villarreal L., Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 19.Taub D.D., Ershler W.B., Janowski M., Artz A., Key M.L., McKelvey J., Muller D., Moss B., Ferrucci L., Duffey P.L., et al. Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med. 2008;121:1058–1064. doi: 10.1016/j.amjmed.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20•.Davies D.H., Wyatt L.S., Newman F.K., Earl P.L., Chun S., Hernandez J.E., Molina D.M., Hirst S., Moss B., Frey S.E., et al. Antibody profiling by proteome microarray reveals the immunogenicity of the attenuated smallpox vaccine modified vaccinia virus ankara is comparable to that of Dryvax®. J Virol. 2008;82:652–663. doi: 10.1128/JVI.01706-07. [DOI] [PMC free article] [PubMed] [Google Scholar]; References [20•, 21•] and [22•] document the creation of protein microarrays that allow for proteome-wide interrogation of antibody responses to poxviruses. The simple and high-throughput techniques can be readily adapted to other complex microorganisms and may facilitate rapid antigen discovery for future vaccines, immunogenicity testing of current vaccines or in the development of diagnostics.
- 21•.Schmid K., Keasey S.L., Pittman P., Emerson G.L., Meegan J., Tikhonov A.P., Chen G.S.B., Ulrich R.G. Analysis of the human immune response to vaccinia by use of a novel protein microarray suggests that antibodies recognize less than 10% of the total viral proteome. Proteom Clin Appl. 2008;2:1528–1538. doi: 10.1002/prca.200780113. [DOI] [PubMed] [Google Scholar]; See reference [20•].
- 22•.Davies D.H., Molina D.M., Wrammert J., Miller J., Hirst S., Mu Y., Pablo J., Unal B., Nakajima-Sasaki R., Liang X., et al. Proteome-wide analysis of the serological response to vaccinia and smallpox. Proteomics. 2007;7:1678–1686. doi: 10.1002/pmic.200600926. [DOI] [PubMed] [Google Scholar]; See reference [20•].
- 23.Fogg C., Lustig S., Whitbeck J.C., Eisenberg R.J., Cohen G.H., Moss B. Protective immunity to vaccinia virus induced by vaccination with multiple recombinant outer membrane proteins of intracellular and extracellular virions. J Virol. 2004;78:10230–10237. doi: 10.1128/JVI.78.19.10230-10237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell E., Shamim M., Whitbeck J.C., Sfyroera G., Lambris J.D., Isaacs S.N. Antibodies against the extracellular enveloped virus B5R protein are mainly responsible for the EEV neutralizing capacity of vaccinia immune globulin. Virology. 2004;325:425–431. doi: 10.1016/j.virol.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Hammarlund E., Lewis M.W., Hansen S.G., Strelow L.I., Nelson J.A., Sexton G.J., Hanifin J.M., Slifka M.K. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 26.Amara R.R., Nigam P., Sharma S., Liu J., Bostik V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J Virol. 2004;78:3811–3816. doi: 10.1128/JVI.78.8.3811-3816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane J.M., Ruben F.L., Neff J.M., Millar J.D. Complications of smallpox vaccination, 1968: results of ten statewide surveys. J Infect Dis. 1970;122:303–309. doi: 10.1093/infdis/122.4.303. [DOI] [PubMed] [Google Scholar]
- 28.Belyakov I.M., Earl P., Dzutsev A., Kuznetsov V.A., Lemon M., Wyatt L.S., Snyder J.T., Ahlers J.D., Franchini G., Moss B., et al. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100:9458–9463. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wyatt L.S., Earl P.L., Eller L.A., Moss B. Highly attenuated smallpox vaccine protects mice with and without immune deficiencies against pathogenic vaccinia virus challenge. Proc Natl Acad Sci U S A. 2004;101:4590–4595. doi: 10.1073/pnas.0401165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu R., Johnson A.J., Liggitt D., Bevan M.J. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 31.Edghill-Smith Y., Golding H., Manischewitz J., King L.R., Scott D., Bray M., Nalca A., Hooper J.W., Whitehouse C.A., Schmitz J.E., et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 32.Drexler I., Staib C., Kastenmuller W., Stevanovic S., Schmidt B., Lemonnier F.A., Rammensee H.G., Busch D.H., Bernhard H., Erfle V., et al. Identification of vaccinia virus epitope-specific HLA-A*0201-restricted T cells and comparative analysis of smallpox vaccines. Proc Natl Acad Sci U S A. 2003;100:217–222. doi: 10.1073/pnas.262668999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J.C., Bevan M.J. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kennedy R., Poland G.A. T-Cell epitope discovery for variola and vaccinia viruses. Rev Med Virol. 2007;17:93–113. doi: 10.1002/rmv.527. [DOI] [PubMed] [Google Scholar]
- 35.Tang J., Murtadha M., Schnell M., Eisenlohr L.C., Hooper J., Flomenberg P. Human T-cell responses to vaccinia virus envelope proteins. J Virol. 2006;80:10010–10020. doi: 10.1128/JVI.00601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvo-Calle J.M., Strug I., Nastke M.D., Baker S.P., Stern L.J. Human CD4+ T cell epitopes from vaccinia virus induced by vaccination or infection. PLoS Pathog. 2007;3:1511–1529. doi: 10.1371/journal.ppat.0030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Jing L., Davies D.H., Chong T.M., Chun S., McClurkan C.L., Huang J., Story B.T., Molina D.M., Hirst S., Felgner P.L., et al. An extremely diverse CD4 response to vaccinia virus in humans is revealed by proteome-wide T-cell profiling. J Virol. 2008;82:7120–7134. doi: 10.1128/JVI.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; This reference describes a technique for identifying global CD4+ T cell responses. Individual subjects displayed diverse T helper responses to poxviruses, recognizing, on average, 30 separate ORFs.
- 38.Sirven P., Castelli F.A., Probst A., Szely N., Maillere B. In vitro human CD4+ T cell response to the vaccinia protective antigens B5R and A33R. Mol Immunol. 2009;46:1481–1487. doi: 10.1016/j.molimm.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 39.Jing L., Chong T.M., McClurkan C.L., Huang J., Story B.T., Koelle D.M. Diversity in the acute CD8 T cell response to vaccinia virus in humans. J Immunol. 2005;175:7550–7559. doi: 10.4049/jimmunol.175.11.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Terajima M., Orphin L., Leporati A.M., Pazoles P., Cruz J., Rothman A.L., Ennis F.A. Vaccinia virus-specific CD8(+) T-cell responses target a group of epitopes without a strong immunodominance hierarchy in humans. Hum Immunol. 2008;69:815–825. doi: 10.1016/j.humimm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors examined 73 previously identified CD8+ T cell epitopes and found that human CTL responses to poxviruses were extremely diverse and while some proteins were recognized more frequently than others no clear immunodominant hierarchy could be ascertained. These results parallel the work done with CD4+ T helper responses to poxviruses.
- 41••.Sette A., Moutaftsi M., Moyron-Quiroz J., McCausland M.M., Davies D.H., Johnston R.J., Peters B., Rafii-El-Idrissi Benhnia M., Hoffmann J., Su H.P., et al. Selective CD4+ T cell help for antibody responses to a large viral pathogen: deterministic linkage of specificities. Immunity. 2008;28:847–858. doi: 10.1016/j.immuni.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that a tight linkage exists between proteins targeted by antibody responses and CD4+ T cells. This information may explain why IgG responses to vaccinia are dependent upon CD4+ T cell help.
- 42.Hooper J.W., Thompson E., Wilhelmsen C., Zimmerman M., Ichou M.A., Steffen S.E., Schmaljohn C.S., Schmaljohn A.L., Jahrling P.B. Smallpox DNA vaccine protects nonhuman primates against lethal monkeypox. J Virol. 2004;78:4433–4443. doi: 10.1128/JVI.78.9.4433-4443.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berhanu A., Wilson R.L., Kirkwood-Watts D.L., King D.S., Warren T.K., Lund S.A., Brown L.L., Krupkin A.K., Vandermay E., Weimers W., et al. Vaccination of BALB/c mice with Escherichia coli-expressed vaccinia virus proteins A27L, B5R, and D8L protects mice from lethal vaccinia virus challenge. J Virol. 2008;82:3517–3529. doi: 10.1128/JVI.01854-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakhatskyy P., Wang S., Zhang C., Chou T.H., Kishko M., Lu S. Immunogenicity and protection efficacy of subunit-based smallpox vaccines using variola major antigens. Virology. 2008;371:98–107. doi: 10.1016/j.virol.2007.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kennedy R.B., Ovsyannikova I.G., Pankratz V.S., Vierkant R.A., Jacobson R.M., Ryan M.A., Poland G.A. Gender effects on humoral immune responses to smallpox vaccine. Vaccine. 2009;27:3319–3323. doi: 10.1016/j.vaccine.2009.01.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanley S.L., Jr., Frey S.E., Taillon-Miller P., Guo J., Miller R.D., Koboldt D.C., Elashoff M., Christensen R., Saccone N.L., Belshe R.B. The immunogenetics of smallpox vaccination. J Infect Dis. 2007;196:212–219. doi: 10.1086/518794. [DOI] [PubMed] [Google Scholar]
- 47.Reif D.M., McKinney B.A., Motsinger A.A., Chanock S.J., Edwards K.M., Rock M.T., Moore J.H., Crowe J.E. Genetic basis for adverse events after smallpox vaccination. J Infect Dis. 2008;198:16–22. doi: 10.1086/588670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKinney B.A., Reif D.M., Rock M.T., Edwards K.M., Kingsmore S.F., Moore J.H., Crowe J.E., Jr. Cytokine expression patterns associated with systemic adverse events following smallpox immunization. J Infect Dis. 2006;194:444–453. doi: 10.1086/505503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crowe J.E., Jr. Genetic predisposition for adverse events after vaccination. J Infect Dis. 2007;196:176–177. doi: 10.1086/518800. [DOI] [PubMed] [Google Scholar]
- 50.Metzger W., Mordmueller B.G. Vaccines for preventing smallpox. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004913.pub2. CD004913. [DOI] [PMC free article] [PubMed] [Google Scholar]


