Abstract
Objective
An abnormal superior temporal gyrus has figured prominently in schizophrenia research, and left superior temporal gyrus volume has been shown to be smaller in male subjects with schizotypal personality disorder. This is the first structural magnetic resonance imaging study to examine a group of female subjects with schizotypal personality disorder.
Method
The superior temporal gyrus was drawn on coronal images acquired from female subjects recruited from the community (schizotypal personality disorder group: N=21, comparison group: N=29).
Results
There were no gray matter volume differences in the left or right superior temporal gyrus between the subjects with schizotypal personality disorder and the comparison subjects. Within the schizotypal personality disorder group, however, there was an interaction between hemisphere and family history of mental illness. Moreover, subjects with schizotypal personality disorder did demonstrate formal thought disorder and a negative correlation between left superior temporal gyrus volume and odd speech.
Conclusions
This study of female subjects with schizotypal personality disorder showed no superior temporal gyrus volume differences, but preliminary findings indicate that among female subjects with schizotypal personality disorder, there is a left–right difference in those who have a family history of mental illness relative to those who do not. These data also suggest an association between abnormal speech and left superior temporal gyrus volume, a finding similar to that found in schizophrenia. Results from this study thus clearly reinforce the importance of studying female subjects separately.
A review of structural magnetic resonance imaging (MRI) studies in schizophrenia noted that all studies that specifically examined gray matter of the superior temporal gyrus showed volume reductions, suggesting that the superior temporal gyrus may be a fundamental abnormality in schizophrenia (1). Studies of schizophrenic subjects may be difficult to interpret, given the potential effects of neuroleptics on MRI volumes measured (2). The study of alternate subject groups free of the confounds of medications, such as subjects with schizotypal personality disorder (3, 4), has been helpful in defining important MRI abnormalities. Our laboratory previously reported smaller left superior temporal gyrus volumes in male subjects with schizotypal personality disorder (5), reinforcing the hypothesis that smaller superior temporal gyrus volumes were key to the development of schizophrenia spectrum disorders.
This MRI study examines the superior temporal gyrus in female subjects with schizotypal personality disorder recruited from the community and age-matched comparison subjects. If the hypothesis is correct that a gray matter abnormality in the superior temporal gyrus is critical to the development of schizophrenia spectrum disorders, then female subjects with schizotypal personality disorder should also exhibit this abnormality. However, if the potential ameliorating effect of gender is strong, then superior temporal gyrus abnormalities may be attenuated or not present.
Method
Subject Recruitment
Twenty-one women with schizotypal personality disorder and 29 female comparison subjects were recruited from the community. Subjects were right-handed women 18–55 years old with no history of neurologic disorder or substance abuse within the last year. For comparison subjects, additional inclusion criteria were no personal history of axis I or axis II disorders nor any history of axis I disorders in first-degree relatives. These additional criteria were used in order to help ensure that the comparison subjects had minimal psychopathology in themselves or in their family members. Nine out of the 21 subjects with schizotypal personality disorder reported a first-degree relative with either major depression (N=14) or schizophrenia (N=3) for a total of 17 affected relatives. However, none of the subjects with schizotypal personality disorder consented to having any relative contacted, so that independent confirmation was not possible. No subject had been previously exposed to neuroleptic medication per subject report. After the procedures were explained, written informed consent was obtained from all subjects. The Structured Clinical Interview for DSM-IV and the Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) were conducted by a licensed neuropsychologist and neuropsychiatrists (M.M.V., C.C.D., M.F.) who made the DSM-IV diagnoses.
Clinical/Cognitive Measures
Impairment due to odd speech, one of the nine criteria of schizotypal personality disorder derived from the SCID-II, was quantified. This symptom measure was selected because the superior temporal gyrus is thought to be involved in the processing of early auditory sensory input, a component of language processing (6). A randomly selected subgroup of subjects with schizotypal personality disorder (N=8) were given the Thought Disorder Index (7) to be consistent with our earlier publication on male subjects with schizotypal personality disorder and with our study of male schizophrenic subjects (8).
MRI Procedures
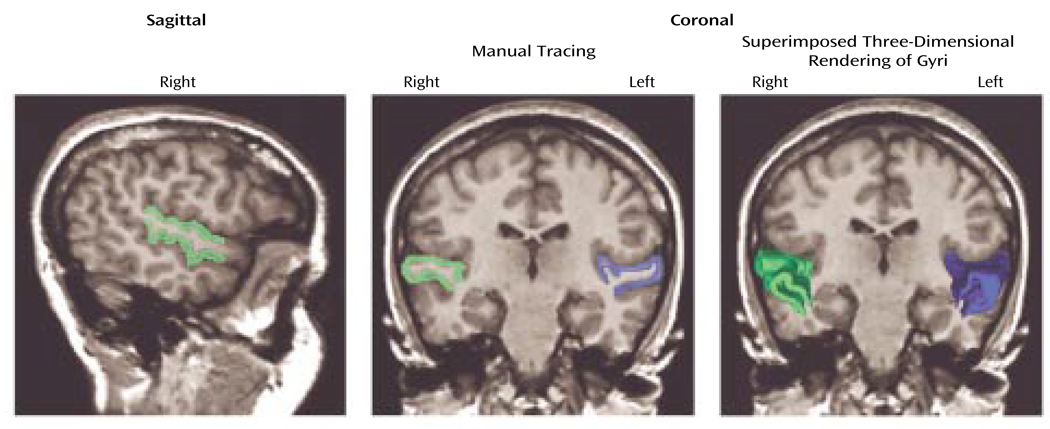
The protocol followed that of our previous publication (5), except here the acquired coronal spoiled gradient recall acquisition MRIs 1.5-mm thick were realigned (correcting for head tilt) and reformatted into images with isotropic voxels (0.9375 mm3) for manual tracing. Figure 1 shows images obtained from a subject with schizotypal personality disorder in which left (in blue) and right (in green) superior temporal gyrus gray matter is shown in the sagittal view, with coronal manual tracing, and in the coronal view with superimposed three-dimensional rendering of the gyri (as seen from left to right). The anterior boundary of the superior temporal gyrus was the temporal stem, and the posterior boundary was the complete crux of the fornix. Interrater reliability for this study was high (intraclass correlation coefficient >0.99). Mean uncorrected volumes for the left superior temporal gyrus in the schizotypal personality disorder and healthy groups were 8.22 (SD=1.5) and 8.55 (SD=1.29), respectively; for the right superior temporal gyrus, mean uncorrected volumes were 9.30 (SD=1.35) and 9.36 (SD=1.08).
FIGURE 1. Delineation of Gray Matter in the Left and Right Superior Temporal Gyrus of a Female Subject With Schizotypal Personality Disorder.
Statistical Procedures
One-way analysis of variance (ANOVA) was used to evaluate the demographic variables. Parenchyma and CSF volumes were used in a regression procedure to correct for effect of head size. Since right superior temporal gyrus data were not normally distributed for the subjects with schizotypal personality disorder (Shapiro-Wilks=0.9, df=21, p<0.05), the raw data were log transformed, and the regression procedure was reiterated. ANOVA with repeated measures of hemisphere was performed on volume measures with Greenhouse-Geisser correction. Following a question by a reviewer on family history, the group of subjects with schizotypal personality disorder was divided into those subjects with a family history of major mental illness (N=9) and those without (N=12). Region of interest volumes were compared between the two groups by using an ANOVA with a repeated measure of hemisphere. Follow-up one-way ANOVAs were implemented on each hemisphere separately. Paired t tests comparing right and left superior temporal gyrus volumes were also evaluated separately for subjects with schizotypal personality disorder with and without a family history of mental illness. Pearson correlations were used between volume and cognitive and clinical measures. For the subjects with schizotypal personality disorder, left and right superior temporal gyrus volume correlations with both Thought Disorder Index scores and schizotypal personality disorder symptoms were performed. Bonferroni corrections were not calculated because these preliminary correlations have not been executed previously on female subjects with schizotypal personality disorder. All tests were two-tailed.
Results
Subject Demographics
One-way ANOVA revealed no difference between the women with schizotypal personality disorder and the healthy women in terms of age (mean=28.4 years [SD=8.1] and 30.4 years [SD=9.2], respectively; F=0.61, df=18, p=0.44), IQ (mean=115.6 [SD=10.6] and 119.5 [SD=9.4]; F=1.83, df=1, 47, p=0.18), or parental socioeconomic status (F=0.21, df=1, 48, p=0.65). Despite the similar IQ, however, subjects with schizotypal personality disorder completed fewer years of education (mean=15.1, SD=1.9) than did the healthy subjects (mean=16.4, SD=1.6) (F=7.69, df=1, 48, p=0.008) and had a lower socioeconomic status (F=14.8, df=1, 48, p<0.0009).
Superior Temporal Gyrus Measures
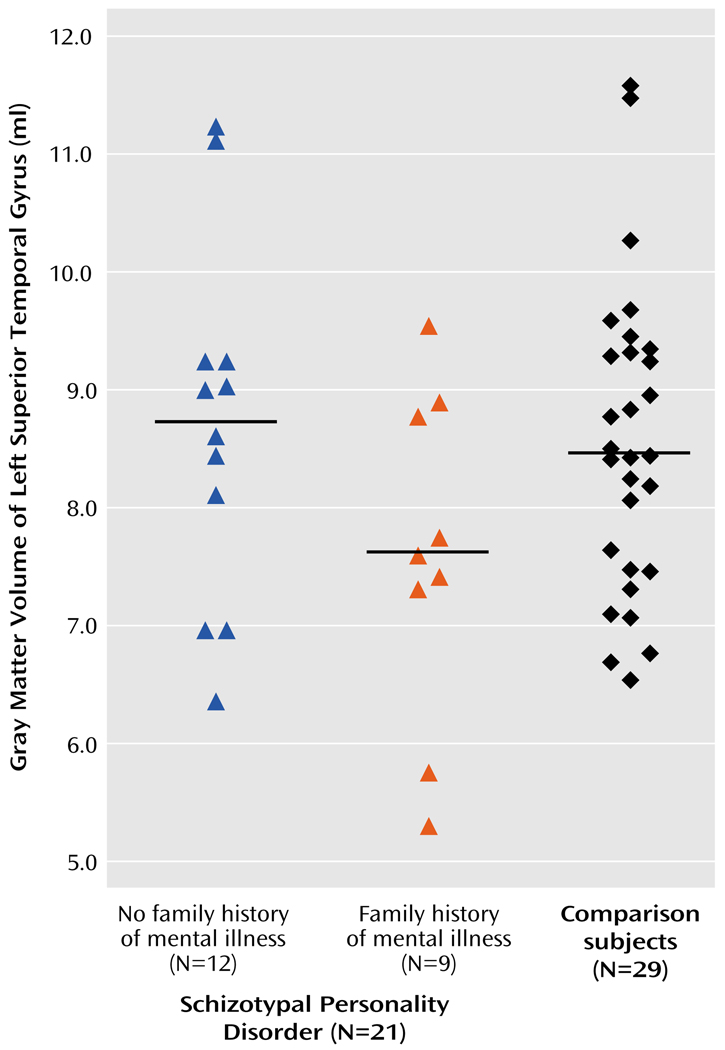
ANOVA with the repeated measure of hemisphere revealed no main effect of side (F=0.102, df=1, 48, p<0.76; effect size [η2]=0.002) or interaction effect between diagnostic group (schizotypal personality disorder versus comparison) and hemisphere (F=0.785, df=1, 48, p=0.38; η2=0.016). Within the schizotypal personality disorder group, ANOVA with repeated measures of hemisphere revealed no main effect of hemisphere (F=1.8, df=1, 19, p<0.20; η2=0.09), but there was an interaction effect for hemisphere and family history (F=5.1, df=1, 19, p<0.04; η2=0.21). Follow-up one-way ANOVA for each hemisphere separately revealed a difference that approached significance for the left superior temporal gyrus gray matter (F=3.276, df=1, 19, p<0.09; η2=0.15) but not the right superior temporal gyrus gray matter (F=0.26, df=1, 19, p<0.62; η2=0.014). Paired t tests on left–right superior temporal gyrus volumes within those subjects with schizotypal personality disorder and a family history of mental illness revealed a difference (t=−2.313, df=8, p<0.05) that was not present in those subjects without a family history (t=0.721, df=11, p<0.49) (Figure 2).
FIGURE 2. Gray Matter Volumes of the Left Superior Temporal Gyrus in Women With Schizotypal Personality Disorder, by Family History of Mental Illness, and Healthy Womena.
a Horizontal bars delineate mean uncorrected volumes (schizotypal subjects with a positive family history of mental illness: mean=7.59 [SD=1.4]; schizotypal subjects without a family history: mean=8.69 [SD=1.5]; comparison subjects: mean=8.47 [SD=1.4]). Note that corrected volumes were used for all statistical analyses.
Clinical/Cognitive Characteristics Correlated With Superior Temporal Gyrus Measures
For the subjects with schizotypal personality disorder, there was a statistically significant negative correlation between the degree of impairment due to odd speech assessed by the interviewer (one of the criteria for diagnosis of schizotypal personality disorder) and volume of the left superior temporal gyrus (rs=−0.46, p<0.04). There was no correlation between Thought Disorder Index scores and superior temporal gyrus volumes, although the Thought Disorder Index score was elevated (mean=26, SD=23.1).
Discussion
This report examining a group of female schizotypal personality disorder and comparison subjects found that, contrary to predictions, the schizotypal personality disorder group did not show volume abnormalities in the superior temporal gyrus. This is not due to insufficient study group size, given the small effect size. To our knowledge, this is the first MRI study that has focused specifically on female schizotypal subjects.
Within the schizotypal personality disorder group, there was left-right difference in superior temporal gyrus volumes in those subjects with a positive family history of mental illness and a tendency for the left superior temporal gyrus gray matter volume to be smaller that was not present in those subjects who did not have a family history of mental illness. Note that the number of subjects in these two groups are small, so the data must be interpreted in that light. Moreover, most of the subjects reported a first-degree relative with affective disease, not schizophrenia, but that might be expected to dilute the finding. However no independent confirmation was available. It is possible that some of these relatives may have had comorbid schizotypal personality disorder, given the high rates of comorbidity (9). Nonetheless, it may be that female subjects with schizotypal personality disorder and a positive family history of major mental illness may have anatomical features similar to male subjects with schizotypal personality disorder.
Of note, however, subjects with schizotypal personality disorder with more odd speech had more reduced left superior temporal gyrus volumes. The qualities of the odd speech by definition include speech that is vague or over-inclusive. In addition, pilot data suggested that female subjects with schizotypal personality disorder demonstrate formal thought disorder.
These results point to a neuroanatomical difference between male and female subjects with schizotypal personality disorder, since male subjects demonstrated frank superior temporal gyrus abnormalities (4, 5) whereas female subjects did not. This finding suggests that the interaction between gender and disease may play a pivotal role in the generation of abnormal brain morphometry and clinical symptoms in the schizophrenia spectrum. Similar to our data, Gur et al. (10) demonstrated gender differences in the superior temporal gyrus in schizophrenic subjects, with female subjects exhibiting normal volumes. Bryant et al. (11) showed a gender effect for the whole temporal lobe for male schizophrenia subjects who had reduced volumes compared with male comparison subjects, but no difference between female schizophrenia subjects and their gender-matched comparison subjects. No gender effect on superior temporal gyrus was noted, however, in that publication, although as the authors noted themselves, gray and white matter were not analyzed separately, which many consider key for the evaluation of this brain region (1). Some of the differences in findings also may be due to the overlap of volumes between subject groups and inherent measuring difficulties due to greater variability in the surface morphometry of the superior temporal gyrus in schizophrenic subjects relative to comparison subjects (12). Moreover, the effect of gender on the volume of superior temporal gyrus is not clear. Even within healthy nonpsychiatric populations, some investigators report a main effect of gender on the superior temporal gyrus (13) while others do not, when using surface-based modeling approaches (14), or when using semiautomatic volume measures (15). Nonetheless, the current data and clinical data suggest that there is heterogeneity within the schizophrenia spectrum and that gender appears to be a critical factor in producing divergence.
Although the superior temporal gyrus was of normal volume in this group of female subjects with schizotypal personality disorder, it may not be functioning normally, as evinced by the correlation with odd speech. Functional MRI studies of this population may be an intriguing way of testing this hypothesis.
Acknowledgments
Supported in part by NIMH grants to Dr. McCarley (MH-52807, MH-40799) and Dr. Shenton (MH-01110, MH-50740); VA Merit Awards to Drs. McCarley and Shenton; a VA Career Development Award (Dr. Dickey); and a VA Psychiatry Research/Neuroscience Fellowship (Dr. Frumin).
The authors thank Marie Fairbanks for her administrative support for this project.
Footnotes
Presented in part at the 57th annual meeting of the Society of Biological Psychiatry, Philadelphia, May 16–18, 2002.
References
- 1.Shenton M, Dickey C, Frumin M, McCarley R. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW. Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res. 1998;32:161–167. doi: 10.1016/s0022-3956(97)00038-1. [DOI] [PubMed] [Google Scholar]
- 3.Dickey CC, McCarley RW, Shenton ME. The brain in schizotypal personality disorder: a review of the structural MRI and CT findings. Harv Rev Psychiatry. 2002;10:1–15. doi: 10.1080/10673220216201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Downhill J, Buchsbaum M, Hazlett E, Barth S, Roitman S, Nunn M, Lekarev O, Wei T, Shihabuddin L, Mitropoulou V, Silverman J, Siever L. Temporal lobe volume determined by magnetic resonance imaging in schizotypal personality disorder and schizophrenia. Schizophr Res. 2001;48:187–199. doi: 10.1016/s0920-9964(00)00131-6. [DOI] [PubMed] [Google Scholar]
- 5.Dickey CC, McCarley RW, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Hirayasu Y, Fischer I, Teh EK, Van Rhoads R, Jakab M, Kikinis R, Jolesz FA, Shenton ME. Schizotypal personality disorder and MRI abnormalities of temporal lobe gray matter. Biol Psychiatry. 1999;45:1393–1402. doi: 10.1016/s0006-3223(99)00030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickey C, Shenton M, Fraone S, Niznikiewicz M, Voglmaier M, Seidman L, Hirayasu Y, Kwon J, Fischer I, Anderson J, Frumin M, McCarley R. Reduced left Heschl’s gyrus volume in schizotypal personality disorder (abstract) Biol Psychiatry. 2000;47:13S. [Google Scholar]
- 7.Johnston M, Holzman P. Assessing Schizophrenic Thinking: A Clinical and Research Instrument for Measuring Thought Disorder. San Francisco: Jossey-Bass; 1979. [Google Scholar]
- 8.Shenton ME, Kikinis R, Jolesz FA, Pollack SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, McCarley RW. Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. doi: 10.1056/NEJM199208273270905. [DOI] [PubMed] [Google Scholar]
- 9.Dickey CC, Shenton ME, Hirayasu Y, Fischer I, Voglmaier MM, Niznikiewicz MA, Seidman LJ, Fraone S, McCarley RW. Large CSF volume not attributable to ventricular volume in schizotypal personality disorder. Am J Psychiatry. 2000;157:48–54. doi: 10.1176/ajp.157.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gur R, Turetsky B, Cowell P, Finkelman C, Maany V, Grossman R, Arnold S, Bilker W, Gur R. Temporolimbic volume reductions in schizophrenia. Arch Gen Psychiatry. 2000;57:769–775. doi: 10.1001/archpsyc.57.8.769. [DOI] [PubMed] [Google Scholar]
- 11.Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M. Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry. 1999;156:603–609. doi: 10.1176/ajp.156.4.603. [DOI] [PubMed] [Google Scholar]
- 12.Narr K, Thompson P, Sharma T, Moussai J, Blanton R, Anvar B, Edris A, Krupp R, Rayman J, Khaledy M, Toga A. Three-dimensional mapping of temporo-limbic regions and the lateral ventricles in schizophrenia: gender effects. Biol Psychiatry. 2001;50:84–97. doi: 10.1016/s0006-3223(00)01120-3. [DOI] [PubMed] [Google Scholar]
- 13.Flaum M, Swayze VW, II, O’Leary DS, Yuh WTC, Ehrhardt JC, Arndt SV, Andreasen NC. Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry. 1995;152:704–714. doi: 10.1176/ajp.152.5.704. [DOI] [PubMed] [Google Scholar]
- 14.Narr KL, Thompson PM, Sharma T, Moussai J, Zoumalan C, Rayman J, Toga AW. Three-dimensional mapping of gyral shape and cortical surface asymmetries in schizophrenia: gender effects. Am J Psychiatry. 2001;158:244–255. doi: 10.1176/appi.ajp.158.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein J, Seidman L, Horton N, Makris N, Kennedy D, Caviness V, Faraone S, Tsuang M. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]