Abstract
Myocardial regeneration using stem and progenitor cell transplantation in the injured heart has recently become a major goal in the treatment of cardiac disease. Experimental studies and clinical applications have generally been encouraging, although the functional benefits that have been attained clinically are modest and inconsistent. Low cell retention and engraftment after myocardial delivery is a key factor limiting the successful application of cell therapy, irrespective of the type of cell or the delivery method. In order to improve engraftment, accurate methods for tracking cell fate and quantifying cell survival need to be applied. Several laboratory techniques (histological methods, real time quantitative polymerase chain reaction, radiolabeling) have provided invaluable information about cell engraftment. In vivo imaging (nuclear medicine modalities, bioluminescence and magnetic resonance imaging) has the potential to provide quantitative information non-invasively, enabling longitudinal assessment of cell fate. In the present review, we present several available methods for assessing cell engraftment, and we critically discuss their strengths and limitations. In addition to providing insights about the mechanisms mediating cell loss after transplantation, these methods can evaluate techniques for augmenting engraftment, such as tissue engineering approaches, preconditioning and genetic modification, allowing optimization of cell therapies.
Keywords: stem cells, engraftment, imaging, reporter genes
Stem cell transplantation has emerged as a new therapeutic option for ischemic cardiomyopathy1 2, 3, although not yet at the level of routine clinical utility. Numerous studies in recent years have shown that cell therapy administered after myocardial infarction can improve cardiac function and limit infarct size 4, 5. Despite the encouraging initial results, the beneficial effects are modest in clinical applications to date, and much remains to be optimized. The best cell type, time and route of delivery are still under investigation. However, irrespective of the specific cell type used for transplantation, low retention and engraftment after cardiac cell delivery are persistent obstacles to successful myocardial regeneration.
Many different approaches have been used to augment cell survival. In order to accurately assess their efficacy, reliable methods for quantification of engraftment need to be developed. Ideally, these methods should be sensitive enough to detect even low numbers of cells, non-invasive, allow longitudinal tracking of cell fate and be free from artifacts. At present, no technique satisfies all these requirements. Here, we review the various methods available for engraftment quantification, present their relative strengths and limitations, and briefly discuss methods that have been used effectively to improve the survival of the transplanted cells.
Assessment of engraftment
a) Histological methods
i. Applications
Techniques based on histology offer the possibility not only to track injected cells in the tissue but also to determine their fate 6, 7. The majority of experimental studies that attempt to explore cell survival and function after intracardiac delivery apply a labeling method to allow detection by histological methods or immunohistochemistry. Cell labeling can be achieved by several, widely available techniques. Membrane (DiI, PKH26) or nuclear (Hoechst 33342, DAPI) stains can be used, with simple and non-toxic protocols 8–10. Since direct labeling techniques are subject to dilution by cell proliferation, the use of genetic labeling and reporter genes is usually preferred. Plasmid transfection or viral transduction can be used to express reporter genes in the therapeutic cell before delivery in the heart. These genes encode proteins that allow subsequent detection of the cells by specific staining techniques (X-gal stain for β-galactosidase) or fluorescent microscopy 10–12. GFP and eGFP are by far the most widely-used fluorescent probes for this purpose. However, a wide array of proteins of different colors, excitation and emission wavelengths exist (RFP, YFP, mCherry, etc) and allow researchers to perform simultaneously detection of any desired combination of target molecules 13. Fig. 1 shows a typical application, from our own work, of lentiviral transduction of cardiosphere-derived cells (CDCs) for eGFP overexpression and subsequent cell detection, by immunocytochemistry, after intramyocardial injection into infarcted mouse or rat hearts.
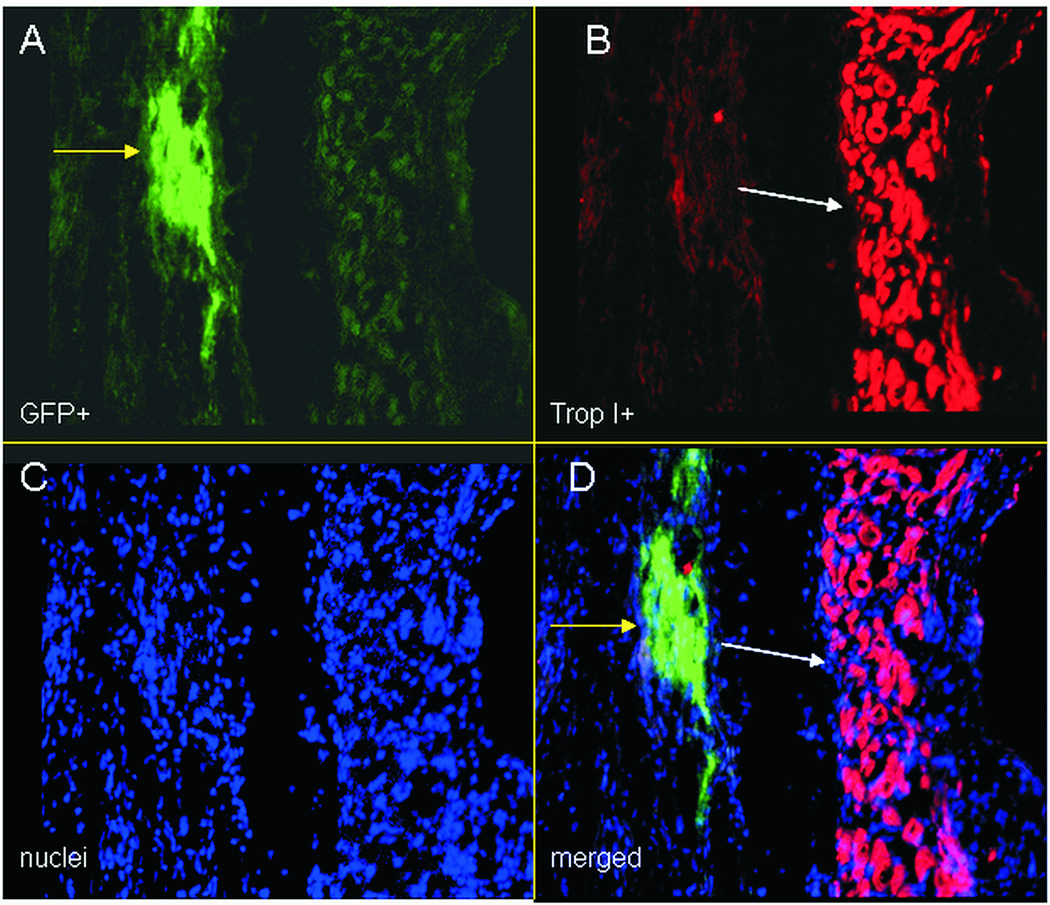
Figure 1.
Immunocytochemistry images of eGFP overexpressing cardiac derived stem cells transplanted in the hearts of infarcted rats, at 21 days post cell injection. A: eGFP+ cells (green-yellow arrow), B: cardiac Troponin I+ cells (cardiomyocytes-red-white arrow), at the infarct border zone, C: nuclei (stained with Hoechst 33342-blue), d: merged image. eGFP does not co-localize with cardiac TropI, indicating that cells have not differentiated yet.
In order to overcome the limitations of cell labeling, specific antigens existing exclusively in the injected cells and not in the recipient can be targeted by immunofluorescence. This is possible in certain experimental settings, such as xenotransplantation (detection of species-specific antigens) or sex-mismatched (sex-specific antigens) transplantation 6. Differentiation of the cells can be addressed simultaneously, by using antibodies against lineage-specific markers. Fig. 2 illustrates the use of this method to detect human CDCs (identified by human nuclear antigen expression) injected into immunodeficient (SCID) mice, demonstrating their differentiation into cardiomyocytes by expression of troponin I.
Figure 2.
Immunocytochemistry images of human cardiac derived stem cells transplanted in the hearts of infarcted immunocompromised (SCID) mice, at 6 weeks post cell injection. A: white light image, B: nuclei (blue) and human nuclear specific antigen (green), C: merged image of cardiac troponin I (red) and human specific nuclear antigen (green), indicating in vivo differentiation of the CDCs into cardiomyocytes.
Finally, transgenic animals expressing the reporter gene, either constitutively or under the control of a tissue-specific promoter, can be used as cell donors, with wild type animals of the same strain or immunodeficient animals serving as recipients 7, 14, 15. Given that the animals carry the gene in the germline, no somatic gene transfer vectors are required. In one particularly trenchant transgenic application, genetic fate mapping can be used to determine the origin of potential cardiac progenitors or their fate after delivery in the myocardium 16, 17. A bi-transgenic MerCreMer-Z/EG mouse model was generated by crossbreeding cardiomyocyte-specific MerCreMer andZ/EG mice 18. The Z/EG reporter mouse has a cytomegalovirus (CMV) enhhancer/chicken beta-actin promoter driving floxed beta-galactosidase, followed by multiple stop codons and subsequently eGFP. Double heterozygous bi-transgenic MerCreMer-Z/EG mice express eGFP exclusively in cardiomyocytes after induction of Cre recombination by 4-OH-Tamoxifen treatment. Using this model, we demonstrated that c-kit+ cells in the one-week outgrowth of myocardial biopsies are not of cardiomyocyte origin16.
Quantum dots offer an alternative approach for cell labeling and detection by microscopy. These are nanoparticles that can be easily taken up by the cells, even without the need for transfection agents. They are very bright (require short exposure times), exert higher photostability than the more commonly used dyes, and have emission wavelengths in the red and near infra-red range, where autofluorescence of the myocardium is less of a problem 19. These attributes of quantum dots lead to increased sensitivity for cell detection by fluorescence microscopy. Rosen and coworkers developed an efficient protocol for labeling human mesenchymal stem cells (MSCs) with quantum dots 20. They demonstrated the lack of any detrimental effect of the labeling on cell properties (viability and differentiation), and were able to identify injected cells in the myocardium of rats or dogs, even at 8 weeks post transplantation.
ii. Strengths and limitations
Histologic-microscopic approaches for cell tracking have several strengths. Technologies, reagents and expertise are widely available. Information about cell viability, location and fate as well as quantitative results can be obtained 7. However, there are equally important limitations that hamper the applicability of these approaches for cell tracking:
Animals have to be biopsied or sacrificed for the collection of specimens, therefore longitudinal tracking of cell fate is difficult or impossible in the same animal.
A large number of experiments is required in order to obtain information about long-term cell fate, since several animals need to be sacrificed at each time point.
Quantitation is subject to variability and sampling errors. Only a few sections (and usually only a limited number of optical fields per section), sampled from the whole heart, are typically examined.
Importantly, results are susceptible to artifacts, particularly when immunofluorescence is used to track labeled cells. The myocardium is notorious for high levels of autofluorescence, therefore researchers must be cautious with the interpretation of their results and always examine appropriate controls 6. Confocal microscopy should also be used for these types of experiments, particularly when more than one target is examined and information about cell fate is being sought; otherwise, superimposed cells can lead to erroneous interpretations 21. Even more subtle errors might confound histological assessments. Laflamme and Murry elegantly demonstrated that macrophages can phagocytose labeled cells including the organelles labeled by fluorescent dyes (membranes or nuclei)21. Immunocytochemistry can subsequently detect the label in a viable, healthy cell and lead to the assumption that this is the injected stem or progenitor cell. Fusion events can confound histological interpretation and more complicated labeling techniques (dual reporter systems or recombination systems) need to be applied in order to rule out this possibility7, 22, 23.
Limitations of labeling techniques should also be taken into account. Intercalating dyes such as DAPI can be toxic for the cells. Nuclear stains, such as Hoechst 33342, released by dead cells, can label neighboring host cells and yield false-positive results 22. The same theoretical concern also exists for quantum dots, since nanoparticles released by dead, labeled cells can be readily taken up by macrophages and generate spurious results.
Genetic labeling is superior from the particle persistence/reuptake point of view; however, limitations also exist with this approach. Simple transfection and adenoviral vectors do not confer stable transgene expression, therefore the absence of reporter gene detection does not necessarily mean cell loss. Adeno-associated viruses (AAV) and lentiviruses are more appropriate vectors for engraftment studies; however, the possibility of transgene silencing, particularly when non-mammalian promoters are used, should always be taken into account 24, 25. Even cells from transgenic animals are not immune to transgene silencing 26–28. Therefore, careful validation experiments should accompany these types of studies, in order to confirm stable reporter gene expression.
b) Fluorescent In Situ Hybridization (FISH)
FISH is an invaluable technique for cell detection by microscopy. FISH allows, with the use of an appropriate probe, the detection of a target cell-specific sequence. In the same section, several other targets can be explored by plain immunofluorescence simultaneously, to determine cell identity and fate. The usual applications of these techniques are in xenotransplantation or sex-mismatched cell transplantation models. FISH can detect male-specific sequences in the Y chromosome or human-specific sequences, when human cells are injected in immunocompromised animals 6, 14, 21, 29, 30. Fig. 3 shows the application of this technique to detect human CDCs in the myocardium of SCID mice, using a human-specific pan-centromeric sequence as the target. The advantage of this approach lies in the fact that it does not require cell labeling for detection of the injected cell. The targets are stably present in the cells (species- or sex-specific genomic sequences) and in that way they are not subject to silencing. However, the technique is labor-intensive, susceptible to artifacts (false positives or negatives), requires careful optimization of the staining protocol for optimal sensitivity and specificity, and appropriate controls. It also has the same generic limitations as other histology-microscopy techniques, namely the requirement for animal sacrifice for tissue harvest and limited quantitation potential.
Figure 3.
Fluorescent in situ hybridization (FISH) image of human cardiac derived stem cells injected into infarcted SCID mice, at 4 days post injection. Human specific sequences (A:red dots and B: white dots) as well mouse specific sequences (A:green dots and C:white dots) were used as targets in the nuclei (A:blue). Red arrows in panel A point to the nuclei containing human sequences (human cardiac derived cells). In panel B, red arrows point to the area corresponding to the human nuclei in A. Green arrows in panel A point to the nuclei containing mouse sequences (mouse cardiomyocytes). In panel C, green arrows point to the area corresponding to the mouse nuclei.
c) Ex vivo quantitation of particles
In vitro labeling with particles has also been used to quantify cell survival after cell delivery. In a swine model, Freyman and co-workers injected iridium-labeled mesenchymal stem cells and evaluated cell survival at 14 days, by ex vivo quantitation of the amount of iridium in the heart, using a standard curve and atomic absorption spectrometry 31. The technique requires animal sacrifice and does not allow longitudinal tracking of cell fate, but more importantly, like all particle approaches, is susceptible to overestimation of engraftment. Signal from particles that persist in the heart despite cell death (in macrophages or in the interstitium) will be misinterpreted as indicating cell survival.
d) Ex vivo quantitation of reporter gene products
Enzymatic activity of reporter gene products, such as firefly luciferase or β-galactosidase, can also be quantified ex vivo and provide information about cell engraftment. In order to correlate enzyme activity to cell numbers, standard curves are constructed from serial dilutions of known number of cells mixed with myocardial tissue, in vitro. Suzuki and co-workers used an ex vivo assay for β-galactosidase to quantify engraftment of skeletal myoblasts (derived from transgenic animals stably expressing β-galactosidase) in rat hearts, after retrograde coronary vein infusion, up to 14 days after delivery 32. Robey and co-workers injected myoblasts retrovirally-transduced with β-galactosidase into infarcted mice and used a chemiluminescent assay for ex vivo β-galactosidase activity measurement 33. They even applied correction for the activity derived from enzyme released by dead cells, based on the half-life of free β-galactosidase in the myocardium.
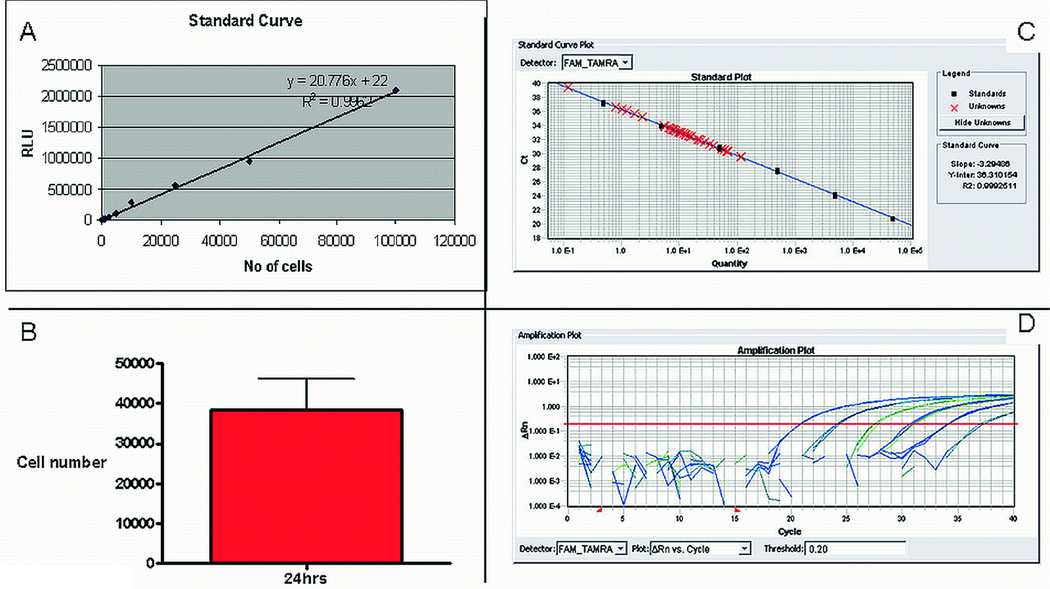
Luciferase is a very popular reporter gene, since it can be used for both in vivo imaging and ex vivo luciferase activity assessment. Luciferase assays have the advantage of very high sensitivity, and background is essentially zero. We have successfully used ex vivo luciferase assay to quantify engraftment in rats and pigs 34 , 24 hrs after intramyocardial or intracoronary injection of lentivirally-transduced CDCs expressing firefly luciferase. A standard curve is created with known numbers of cells mixed with rat or pig tissue and used for absolute quantification. The standard is used to convert luciferase activity (relative light units-RLU) measured in tissue lysates into cell numbers. It is essential to use a standard curve derived from the same cell preparation that was injected, since transduction efficiency and transgene copy number per cell may vary by experiment, even if the same multiplicity of infection is used every time. An example of quantification of cell engraftment by this method, in 3 rats 24 hrs after direct intramyocardial CDC delivery is shown in Figure 4. Figure 4A shows the standard curve used in the experiment, Figure 4B the absolute number of cells per whole rat heart. Since one million cells were injected in each rat, an average of 3.8% was retained in the heart 24hrs post-injection.
Figure 4.
A: Standard curve correlating ex vivo luciferase activity in rat heart homogenates with known cell numbers of luciferase-expressing cardiac derived stem cells, indicating the excellent sensitivity and linearity of the assay. B: Absolute quantification of CDC number at 24hrs after direct intramyocardial injection, in 3 rats, using ex vivo luciferase assay. C: A quantitative real time PCR standard curve used for ex vivo quantification of male cell numbers injected in female hearts. A genomic sequence of the SRY gene is used as target. The curve shows excellent sensitivity of the assay, high efficiency of the reaction and linearity of the relationship between SRY gene copies and cell numbers. D: amplification plots of the reactions corresponding to the points of the standard curve. Data were analyzed with the SDS 2.1 software, by Applied Biosystems.
Ex vivo assays can provide reliable quantitative results; however, they require animal sacrifice and destruction of the tissue samples. They provide information about surviving cell number but not about cell fate or differentiation. In addition, gene silencing or unstable transfection may confound the results of these assays, particularly with long-term applications (weeks to months).
e) Direct radiolabeling for ex vivo quantitation of radioactivity
Direct radiolabeling of stem cells with radiopharmaceuticals is also used for quantitation of engraftment. The method is not difficult to use in laboratories that are experienced with handling radioactivity. Tracers with long half lives have been attached to molecules that can then accumulate in a cell. Fluoro-deoxyglucose, a glucose analogue that is taken up by the cell through glucose transporters, can be labeled with 3H. Thymidine, labeled with 3H or 14C, can be incorporated in the DNA as thymine. After cell labeling, baseline radioactivity contained within the cells is measured. At several time points after cell transplantation, animals are sacrificed, tissue is lysed and radioactivity is measured in appropriate scintillation counters. Suzuki et al described a protocol for labeling myoblasts with 14C-thymidine before intramyocardial injection in infarcted mice 35. At several time points after cell transplantation, animals were sacrificed, whole hearts were lysed, radioactivity was measured in the lysates and the percent of engrafted cells was reported.
The method has the important advantage of accurate quantitation. However it also has significant disadvantages:
Handling of radioactivity requires significant caution, since these assays frequently involve the use of tracers with long half lives.
Animal sacrifice precludes longitudinal testing.
Importantly, interpretation of results is not straightforward. There can be ‘contamination’ of the signal from radioactivity released by dead cells that have not been washed out rapidly enough.
Cell proliferation cannot be assessed, since there is only a fixed initial amount of radioactivity that can be distributed in daughter cells. Results at any time point can indicate the loss of the injected cell population but, if proliferation has occurred, there is underestimation of the actual size of the engrafted cell population.
f) Real time polymerase chain reaction (PCR)
Another approach that bypasses the drawbacks of gene transfer and genetic modification is quantitative, real-time PCR, with cell-specific sequences as targets for the reaction. It is used in xenotransplantation models (human-specific sequences such as the Alu can serve as targets) or in sex-mismatched transplantation, where male cells are injected into female animals and the Y-chromosome specific SRY gene is the target 33, 36. A standard curve derived from dilutions of genomic DNA (human or male, depending on the experimental setting) is used for quantification (Figure 4C and 4D). Numerous experimental studies have been published where engraftment was quantified by real time PCR.
Real time PCR can provide absolute quantification and, since the whole heart of a rodent can be homogenized and assessed, the results are not susceptible to sampling errors. No genetic modification of cells is needed, and limitations of cell labeling techniques are avoided. More importantly, cell proliferation can be assessed, since the genetic marker is stably present in the cells’ genome. Indeed, Suzuki et al. elegantly demonstrated the superiority of real time qPCR over direct radiolabeling in measuring the true size of the engrafted cell population, in experiments involving the delivery of muscle precursor cells in mice 35. The radioactivity-based assay showed a decrease of the cell population at 3 days post cell injection, while qPCR demonstrated an increase due to cell proliferation. On the other hand, real time PCR has the disadvantages of all the ex vivo assays. Tissue is destroyed, results can be obtained only at single time points, and no information about cell differentiation and localization is available.
g) Noninvasive imaging methods
It is obvious from the abovementioned assays that no laboratory method is ideal for assessing cell engraftment. Even the ones that allow accurate quantification require animal sacrifice and preclude longitudinal cell tracking. There is a clear need for noninvasive quantitative methods; imaging modalities have the potential to play a major role in this setting.
i) Bioluminescence imaging
Optical bioluminescence imaging (BLI) is used frequently for cell tracking in cell transplantation studies. Genetically-engineered cells overexpressing firefly luciferase are transplanted into the recipient animals. Subsequently, the animals are injected with the substrate of luciferase, D-luciferin. In the presence of ATP, O2 and Mg2+, luciferin is oxidized and photons of visible light are emitted. Animals undergo imaging in a dark chamber and light is detected using highly-sensitive cameras. Numerous studies involving BLI for cell tracking in the heart have been published 37–41. We have used BLI to study CDC engraftment in a syngeneic rat model of direct intramyocardial injections in the infarcted heart. A third-generation lentivirus was used to stably transduce the cells with firefly luciferase, driven by the constitutively active CAG promoter. Imaging results were validated by qPCR, and both techniques provided similar results (Figure 5 and Figure 6).
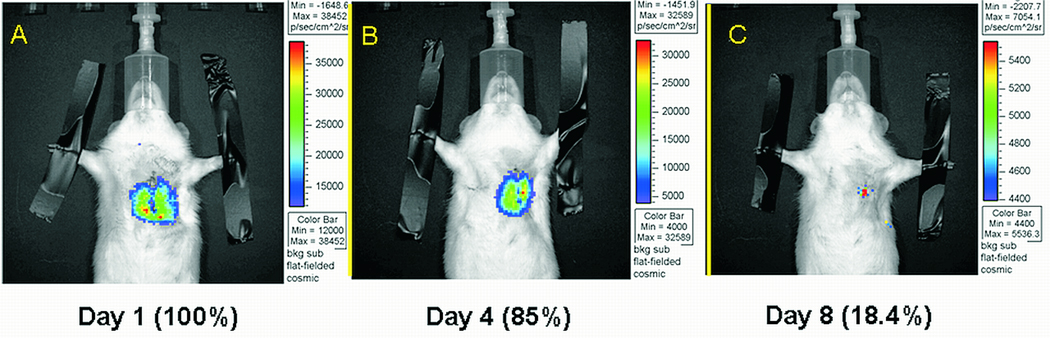
Figure 5.
Engraftment of cardiac derived stem cells in a syngeneic rat, myocardial infarction model, assessed by in vivo Bioluminescence Imaging (A: Day 1, B: Day 2, C: Day 8 post cell injection).
Figure 6.
Validation of engraftment results obtained by Bioluminescence Imaging (A), using quantitative real time PCR as the gold standard (B). Both techniques yield similar cell survival patterns, although BLI results have greater variability.
BLI has many significant advantages. It is an easy technique, provides quantitative results, it is sensitive (approximately 1000 cells can be visualized in a rat, even smaller numbers in a mouse), non toxic, and allows serial imaging at consecutive time points. Importantly, only viable cells can generate signal, since the reaction requires transcription, translation and ATP. However, there are also several limitations:
Light in vivo is significantly scattered, the spatial resolution is low and cells cannot be located accurately.
Only surface images are obtained, therefore the results are sensitive to the depth of signal origin 42. In the case of cardiac imaging, the same number of cells will yield a much smaller signal if the cells are injected in the posterior wall of the left ventricle, instead of the anterior.
A major drawback is the difficulty in obtaining reliable baseline signal intensity. It has been reported that bioluminescence signal in vivo reaches its peak value approximately 24 hrs after cell injection 43. This was also the case in our experience with the method. The reason is not clear, but this limitation essentially does not allow any information about cell viability during the first 24 hrs to be retrieved, although a very significant amount of cell loss occurs during this period 44. Because the results are sensitive to the timing of acquisition, significant errors can be made if normalization is performed according to day 1 post-injection signal intensities that do not really represent the peak values. These inherent limitations result in significant variability in the quantitative results obtained by bioluminescence imaging. Figure 6 shows the large standard deviations of engraftment results obtained by BLI, in comparison to qPCR.
As with all transcriptionally-dependent reporters, gene silencing may lead to a falsely low estimate of engraftment.
ii) Direct radiolabeling for SPECT or PET
Direct radiolabeling of stem cells with radiotracers for in vivo imaging is a widely available and frequently used method for quantitation of cell engraftment. Since there is significant experience in nuclear medicine with white blood cell labeling for detection of clinically occult sites of inflammation, it is not surprising that radiolabeling of cells has already been used for cell tracking in many experimental and several clinical applications 45. 111Indium oxine and 99mTc HMPAO have been used in conjunction with Single Photon Emission Tomography (SPECT) imaging, and 18FDG with Positron Emission Tomography (PET) for tracking bone marrow derived or endothelial progenitor cells delivered to the myocardium intravenously or via the coronary arteries 10, 31, 46–52. Many interesting observations have been made using these imaging approaches. They have made clear that only a small percentage of the injected cells is retained in the heart, even at relatively short periods of time after infusion 48–51. In one clinical study, CD34+ selected cells showed significantly higher retention rates in comparison to the unselected mononuclear bone marrow population 53 . In an experimental study, the intracoronary delivery of a single, large intracoronary bolus of cells resulted in higher retention rates than the standard technique of intermittent cell infusions during balloon occlusion of antegrade flow, calling into question the efficacy of the method applied in most clinical studies until now 47. However, a major safety concern not addressed in this experimental study was the possibility that infusing a concentrated cell population in the myocardial microcirculation may lead to cell plugging in the capillaries and creation of microinfarcts. Indeed, the increased cell retention could be secondary to such microvascular sludging, a phenomenon previously reported with intracoronary administration of mesenchymal stem cells54 (but which, for CDCs at least, can be avoided by systematic optimization of dosage and delivery34). Imaging studies, both experimental and clinical, have also demonstrated that, with certain stem and progenitor cell populations such as MSCs and circulating progenitor cells (CPCs), homing to the infarcted myocardium is possible even after intravenous cell administration, although the percentage of cells accumulating in the heart is again very low(<3%). We have used radiolabeling of cardiac stem cells with 18FDG and assessed cardiac retention of CDCs by in vivo PET imaging, 1hr post direct intramyocardial delivery, in a rat myocardial infarction model (Figure 7)55. Even with this direct approach, retention was on average <20%. Importantly, we validated the results obtained using real time qPCR, as described above, and found almost identical retention rates, proving the accuracy of this imaging approach.
Figure 7.
Positron Emission Tomography (PET) images of directly radiolabeled with 18FDG cardiac derived stem cells (red) injected in the infarct area of rats, at 1hr post cell injection. 13NH3 (green) was used as perfusion tracer to delineate normally perfused myocardium. In vivo PET images are co-registered with CT images, for better anatomic detail and attenuation correction. (A: transverse image orientation, B: coronal, C: sagittal).
Both SPECT and PET can be used for tracking radiolabeled stem cells. SPECT is widely available, simple and requires tracers that are applied in everyday clinical practice. However, there are problems with acquiring accurate quantitative data, mainly due to errors derived from photon scattering. PET is more sensitive, has higher spatial resolution and quantitation is more straightforward and clinically applicable. Unfortunately, PET scanners are more expensive, not widely available and usually require an on-site or nearby cyclotron (for production of the necessary tracers).
Nuclear imaging techniques have several important advantages for studying cell engraftment. They provide information about cell localization, homing and migration. Newer hybrid scanners, combining CT with PET or SPECT, are even more appropriate for these studies. Anatomic CT images have higher resolution and quantitative data are more accurate, since the co-registered CT facilitates attenuation correction 56. In addition, in vivo imaging can allow quantitation of cell persistence at several time points in the same animal. This of course is only feasible when tracers with sufficiently long half lives are used, such as 111In for SPECT (4.2 days). Most PET tracers, such as 18FDG (half life of 110 min), have very short half lives and can only be used to assess acute retention. Importantly, the successful use of the positron emitter 64Cu-PTSM has also been reported for cell tracking 57. This compound has a half life of 12 hours and therefore can allow the use of PET imaging for cell tracking for up to three days.
With direct radiolabeling of cells for in vivo imaging, there is no need for systemic radiotracer administration. This is an important advantage, because there is no blood pool activity and image background is minimal. Cells can be visualized even when they are labeled with very low amounts of radioactivity, therefore systemic radiotoxicity can be avoided. This is particularly true for PET imaging that has higher sensitivity than SPECT. Using 18FDG for cell labeling and a dedicated small animal PET scanner, we were able to visualize cells and reliably quantify intramyocardial retention, even when injected activities were consistently less than 1µCi.
A word of caution is also necessary in relation to potential radiotoxicity to the cells themselves, due to labeling. Several reports have found measurable toxicity related to indium labeling of blood cells, hematopoietic or mesenchymal stem cells 58–60. In our experience, cardiac-derived stem cells were also very sensitive to exposure to 18FDG; very small doses had to be used in order to avoid adverse effects of the labeling procedure on cell viability and proliferation. We believe that simply checking acute viability after radiolabeling with Trypan Blue (standard method in most published studies) is not adequate to rule out radiotoxicity to the cells. Radioactivity could damage more subtle functions of the cells. As a minimal precaution for dividing cells, the absence of any effect on cell proliferation for a few days after labeling should be confirmed before a radiolabeling method is considered safe. This is even more important if clinical applications are being considered.
Finally, the limitations of radiolabeling for ex vivo radioactivity measurements also apply to the imaging studies. Tracer released from dead cells can contaminate the signal for a certain period of time until it is washed out from the myocardium. For tracers with sufficient half lives to allow longitudinal imaging, it should always be kept in mind that cell proliferation cannot be assessed and engraftment may be underestimated. In addition, efflux of the radiolabel from viable cells can also confound the quantitative results, since it is indistinguishable from cell loss.
iii) Reporter genes for SPECT or PET
Reporter genes offer the possibility to circumvent the aforementioned limitations of direct radiolabeling 61–63. Reporter genes for in vivo imaging encode a membrane receptor, transporter or enzyme that is not normally expressed in the target cell. This protein allows the uptake and accumulation of a systemically-injected radiotracer exclusively in the genetically-modified cells. In that way, the signal is specific, and since tracer uptake requires protein synthesis and metabolic activity of the cells, only viable cells can generate signal. When the transgene is stably integrated in the host genome (which is the case when lentiviruses and retroviruses are used as vectors), daughter cells also express the reporter gene. In that way, proliferating cells can also be visualized, allowing longitudinal and reliable cell tracking.
Three reporter genes have been used successfully for cardiac nuclear imaging . A) The herpes virus thymidine kinase, either as the wild type (HSV1-tk), which phosphorylates and traps intracellularly the pyrimidine derivative 2-deoxy-2-fluoro-5-iodo-1-b-D-arabinofuranosyluracil (FIAU), labeled with 124I or 18F for PET imaging, or its truncated mutant form (HSV1-sr39tk), which uses as substrate the acycloguanosine analogue 9-[4-18F-fluoro-3-(hydroxymethyl) butyl]guanine (18F-FHBG) 39, 41, 45, 64. B) the sodium-iodide symporter, a protein normally expressed in the thyroid, the stomach and to a lesser extent in the salivary glands and choroid plexus, which allows cellular uptake of iodide at the expense of the transmembrane sodium gradient. NIS can be ectopically expressed in cells and used for PET imaging with 124I as tracer or for SPECT imaging with 99mTc (pertechnetate). C) the D2 dopaminergic receptor, which can be used for PET imaging with the 18F labeled tracers 2-18F-(fluoroethyl)spiperone, 18F-fluoroclebopride, and 18F-4-fluorobenzyltrozamicol]64. Only thymidine kinase and NIS have been used for stem cell tracking studies until now. Wu et al injected rat cardiomyoblasts expressing the mutant thymidine kinase after adenoviral transduction into the myocardium of nude athymic rats. They were able to detect the cells by in vivo PET on day 2 after injection 41. Imaging results were confirmed by autoradiography. Our group injected lentivirally-transduced CDCs overexpressing NIS directly in the myocardium of infarcted rats, in a syngeneic, sex-mismatched transplantation model 65. Using SPECT (with 99mTc pertechnetate) and PET (124I as tracer), we were able to detect the injected cells up to day 6 post injection (Figure 8). In vivo imaging results were validated by ex vivo imaging and by qPCR. SPECT was more sensitive than PET in this experimental setting, due to limitations of 124I as a tracer (mainly because of the high-energy photons that this compound emits in addition to positrons, resulting in an increase in the background and reduction of contrast). Using SPECT and 99mTc, approximately 105 NIS+ cells could be detected in vivo, in this rat model.
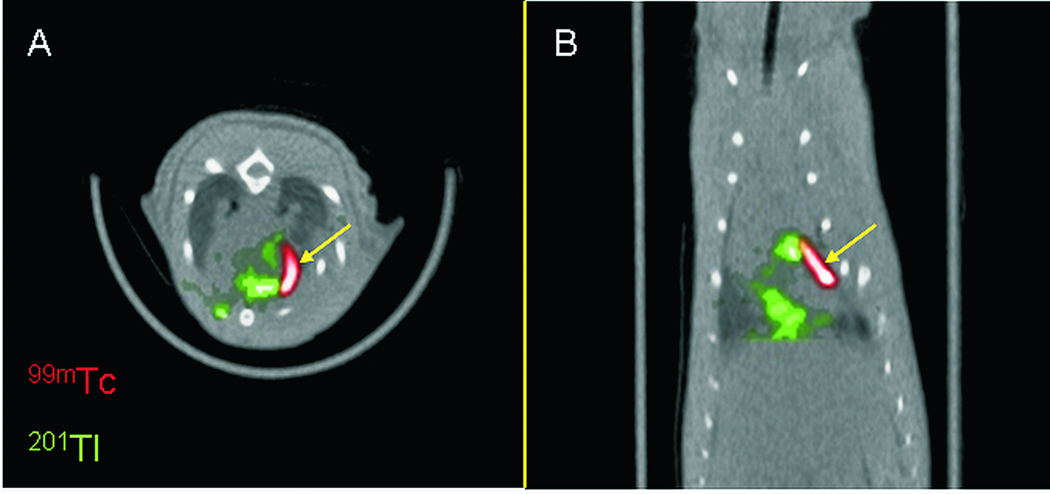
Figure 8.
Single Photon Emission Computed Tomography (SPECT) images of human sodium iodide symporter (hNIS) overexpressing cardiac derived stem cells (red) injected in the infarct area of rats, at one day post cell injection. 99mTc-pertechnetate (99mTc-red) was used as tracer for the hNIS+ cells. 201 Thalium (201Tl-green) was used as perfusion tracer to delineate normally perfused myocardium. Images were obtained in a hybrid SPECT/CT scanner. (A: transverse image orientation, B: coronal).
Reporter gene approaches also have important limitations. Genetic modification may alter cell properties and compromise the functional benefit from cell transplantation 63. Transgene expression in the cell progeny is not guaranteed, even when viral vectors are used 66. Adenoviruses are cleared by the immune system. The recombinant adeno-associated viruses currently used for gene transfer rarely integrate in the host genome, but remain as episomes in the host nucleus 66. Since they are non-replicating, the episomes become diluted with cell proliferation 67. Even when viruses that integrate in the host genome are used, transgene expression can be silenced by DNA methylation, particularly when strong viral promoters such as CMV drive the expression of the reporter gene 25. In addition, there are safety concerns about mutagenesis and oncogenicity of this type of viral vector, since they integrate preferentially adjacent to oncogenes, that may be activated by this process 68.
Reporter genes have certain drawbacks from the imaging point of view as well:
Quantification is not as straightforward, since uptake (percent of injected dose-%ID) in the areas of interest depends not only on the number of labeled cells, but also on the dose injected, arterial concentration of the tracer, regional blood flow and tracer uptake kinetics 69. Therefore, complex kinetic models need to be constructed and validated, if absolute quantification is required.
In general, reporter gene approaches are less sensitive than direct radiolabeling, which means that more viable cells are needed for detection.
Tracers have to be injected systemically to reach the genetically-modified cells. Therefore there is a substantial amount of radioactivity circulating in the blood pool that increases the background and decreases the contrast. This is particularly important for cardiac studies, where blood in the atrial and ventricular cavities can be a significant source of signal. In this type of study, an optimal time window should be defined where accumulation of radiotracer in the cells is adequate while blood pool activity has sufficiently decreased, in order to image with the highest possible contrast. This also explains why reporter genes that encode for transporters or intracellular enzymes (NIS or thymidine kinase) are potentially superior to the ones encoding membrane receptors (dopamine receptors), since they allow accumulation of tracer in the labeled cell, in contrast to reversible extracellular binding 63. Indeed, in a study where thymidine kinase and D2 receptor were compared as reporter genes for PET, after adenoviral transduction of the rat myocardium, signal to background was higher with thymidine kinase 64. In addition, even with thymidine kinase and NIS, a degree of tracer leak from the cell (resulting in signal loss) is inevitable, stressing the importance of defining the optical timing for image acquisition 70.
Theoretically thymidine kinase should have an advantage as a reporter gene over NIS, since phosphorylating the substrate leads to its intracellular entrapment, whereas iodide or pertechnetate are free to diffuse out of the cell. However, in a study where the efficiency as PET reporter genes of both wild-type and mutant thymidine kinase was compared to that of NIS, after adenoviral transduction of the rat myocardium, signal to background was higher with NIS 70. In addition, thymidine kinase is a viral enzyme which can elicit immunogenic reactions that may not only limit its long-term efficiency but even harm the labeled cells. For this purpose, a human mitochondrial thymidine kinase was developed and used recently as a reporter gene for tracking genetically-engineered cytolytic T cells in the brain, in a small clinical study 71. This novel reporter gene has not yet been used to track cells in the myocardium. NIS, as a human protein, on the other hand, has great potential for clinical translation. It can be used for SPECT imaging with widely-available tracers, eliminating the need for the complex radiosynthesis required for production of the thymidine kinase substrates. It can also be used for PET imaging, although 124I has a long half life and potential toxicity to the thyroid, therefore its clinical applicability is problematic. However, the positron emitter 94mTc pertechnetate can be an alternative PET tracer to track NIS-labeled cells in future clinical applications.
iv) Magnetic Resonance Imaging (MRI)
Cell labeling with iron particles for visualization by MRI is one of the most frequently applied methods for cell tracking. Several iron formulations have been used for this purpose, including dextran-coated iron oxide nanoparticles (ferumoxides), cross-linked iron particles and micron-sized iron particles. This approach is attractive, because MRI provides anatomic information of the highest quality for cell localization and does not require ionizing radiation. In addition, most of the protocols that have been used for cell labeling have repeatedly been found to be non-toxic for cells. However, there are important pitfalls that limit the usefulness of these techniques. We and others have demonstrated that iron nanoparticles released by dead cells are taken up by macrophages and persist in the myocardium for up to 5 weeks, generating signals that could readily be misinterpreted as representing robust cell survival72–74. We used a model of xenogeneic transplantation, where human CDCs or MSCs, labeled with ferumoxides and genetically-engineered to overexpress β-galactosidase, were injected in the myocardium of immunocompetent rats. At 3 weeks post cell injection, MRI could clearly identify black spots in myocardium (areas of signal loss) corresponding to the presence of iron. The iron-specific Prussian Blue stain showed many iron-containing cells in myocardial tissue sections. However X-gal stain could not identify any surviving β-galactosidase positive cells, and immunostaining revealed that all the iron-containing cells were macrophages. In addition to iron labeling and 1H-MRI, cells have been labeled with perfluorocarbon particles and detected by 19F-MRI 75. The positive signals (cells) appear as ‘hot’ spots and can be localized anatomically after superimposition of the conventional proton MRI picture to the 19F one. This approach has the advantage of low background and high sensitivity, however requires specialized technical expertise and is susceptible to the same kind of artifacts with any other particle approach, namely persistence of the label after cell death resulting in false-positive signals. Such methods may prove to be particularly useful for quantification of acute cell retention and biodistribution, rather than long-term engraftment.
Optimization of engraftment
a) Factors influencing acute retention and long-term engraftment
Irrespective of the method used to assess engraftment, the cell type and transplantation model, it is widely recognized that only a small fraction of the delivered cells are acutely retained (within the first hour); cell loss is compounded by further attrition over the first 1–2 days and again in weeks. During intracoronary or direct intramyocardial injection, cells can be washed away by blood flow 47, 50, 76. In the case of direct intramyocardial injection, a significant portion of the cells are also lost due to leak from the injection site, pushed by the contracting myocardium and washed by local bleeding. We have shown that both myocardial contraction, by accentuating leakage, and coronary blood flow, by washing away the cells, have a profound effect on acute cell retention. When cells are injected in the normally-contracting and perfused myocardium, less than 20% are retained 1 hr post injection (measured by in vivo PET). When cells are injected after induction of cardiac arrest, with the heart still and no coronary flow, retention was increased to more than 70% 77, 78.
A number of fundamental questions need to be addressed and resolved, in order to improve acute retention. The optimal delivery route (intracoronary, retrograde transvenous, intramyocardial) has not been determined yet. This of course depends on the clinical setting (acute myocardial infarction, chronic ischemic cardiomyopathy, transcatheter or open-chest approaches). In an experimental swine model where the intramyocardial, intracoronary and retrograde transvenous routes for cell delivery were compared using 111Indium labeled peripheral mononuclear cells, direct intramyocardial cell delivery resulted in the higher engraftment, whereas the retrograde transvenous approach was proven feasible and superior to the intracoroanary route 46.
The optimal cell dose for any given delivery route remains an open question. It may appear reasonable that a higher dose would result in better long-term engraftment; however, delivering a large number of cells is not always feasible and safe. In a dose-finding study conducted in a swine model, Johnston and coworkers have shown that increasing the number of intracoronary-administered CDCs to more than 2.5×107 indeed augments engraftment, but at the same time results in myocardial damage (as evidenced by increases in the serum ischemic biomarker TnI)34. Bone marrow mononuclear cells can be safely administered in higher doses via the intracoronary route, due to their smaller size 79. For direct intramyocardial cell injections in rodents, delivery of larger number of cells has resulted in better engraftment80. For clinical applications, the optimal cell concentration, volume and number of injections remain to be determined. Interestingly, in one study conducted in sheep, the functional benefit of directly administered MSCs was higher in the subgroups that received the lower cell numbers81. Unfortunately, cell retention was not directly measured in this study.
Myocardial substrate is another factor affecting cell retention and engraftment. In the acute myocardial infarction setting, retention after direct intramyocardial cell delivery was lower when reperfusion was established76. In a rat infarct model, MSCs were directly injected at 1hr, 1week and 2 weeks post myocardial infarction. Functional benefit and engraftment were better at one week, suggesting that, at later time points, lack of homing signals in the injured myocardium may have adversely affected cell retention, whereas cell administration too early after an acute myocardial infarction may lead to suboptimal engraftment, due to the intensity of the inflammatory response in the acute stage 82.
Of the cells that are retained in the heart 24 hrs after delivery, a small percentage (from <1 to 20% depending on the cell type) will stably engraft as indexed at 3 weeks. A combination of factors have been implicated in this process, with lack of connection to the host extracellular matrix (anoikis), ischemic death and apoptosis apparently playing the most important roles 83. It is therefore reasonable to expect that a combination of approaches will be required in order to achieve meaningful increases in cell retention and engraftment.
b) Tissue-engineered matrices
Tissue-engineered three-dimensional matrices have been used as vehicles for cell delivery, in order to reduce washout from the injection site. Matrices can also provide a scaffold for the cells to attach and prevent apoptosis triggering due to anoikis. In addition, 3D matrices can serve as carriers for growth factors that can increase cell survival and proliferation after transplantation. Kutchka and coworkers seeded rat cardiomyoblasts into a collagen matrix, mixed with matrigel, with or without VEGF or FGF and implanted them into infarcted hearts, in an heterotopic transplantation rat model. Engraftment was assessed by optical bioluminescence imaging 40. They were able to show that engraftment was increased, both at early (day 5 after cell injection) and late (day 14) time points, when cells were transplanted embedded in the 3D collagen matrices. The addition of FGF or VEGF did not further increase engraftment.
In another tissue engineering approach, Davis and coworkers used biotinylated oligopeptides, able to self-assemble into nanofibers, as a delivery vehicle of IGF1 to the myocardium 84. In a rat model of acute myocardial infarction, nenonatal cardiomyocytes delivered together with these nanofibers exerted higher functional benefit in comparison to cells or nanofibers alone. Engraftment was not quantified in this study; therefore, it is not possible to attribute the favorable effect to increased cell survival, although such a mechanism is certainly plausible.
Memon et al compared the efficacy of delivering myoblasts either by direct injection or by implantation of monolayer cell sheets epicardially, in a rat acute myocardial infarction model85. They demonstrated more significant attenuation of remodeling in the animals of the cell sheet group, in terms of ejection fraction, left ventricular dimensions, fibrosis and capillary density. Recently, Matsuura and coworkers used the same technique to administer clonally-expanded cardiac-derived stem cell sheets in mice, and reported similar favorable effects on left ventricular function after myocardial infarction 86.
Christman and co-workers injected skeletal myoblasts embedded in fibrin glue in the hearts of rats, 7 days after the induction of myocardial infarction 87. They demonstrated increased myoblast survival at 5 weeks by histology and less infarct expansion, in the cells plus fibrin glue group. Although fibrin glue is an injectable material, there are concerns that intravascular injections of the activated thrombin may promote thrombosis, and lead to increases in infarct size. Several clinical cases of thrombosis have been reported after inadvertent intravascular application of fibrin glue88, 89. Therefore, we developed a modified protocol, where, after intramyocardial injection of cardiac-derived stem cells, the glue was applied only epicardially, as a plug to the injection site, in order to prevent leak of the injected cells after removal of the needle. We were able to demonstrate an almost doubling of the acute retention of the cells, by in vivo PET and real time PCR. Increased retention rate resulted in a significant increase in engraftment at 3 weeks and a larger functional benefit by CDC therapy, in comparison to intramyocardial injections of cells suspended in plain vehicle (PBS).
c) Preconditioning of cells
Several pre-treatments with cytokines, growth factors, prosurvival factors and physical stimuli have been used in order to increase cell survival; after transplantation 90. Suzuki et al subjected skeletal myoblasts to heat shock prior to ischemic-reoxygenation injury 91. They observed less ischemic and apoptotic death of heat-shocked cells in comparison to non-treated cells. Subsequently, in an in vivo experiment, they compared survival of heat-shocked myoblasts to untreated cells, after intracoronary delivery in rat hearts, using a heterotopic cardiac transplantation model. They found a significant increase in cell survival at 8 weeks, assessed by a β-galactosidase assay. Similarly, Laflamme et al demonstrated that heat shock was effective in promoting human embryonic stem cell engraftment in the hearts of athymic rats 92. At one week post cell injection, they observed that the grafts formed by heat-shock-treated cells were nearly three times larger than the respective ones formed by untreated cells.
Ischemic preconditioning is a powerful way to significantly reduce ischemic cell death in the setting of myocardial infarction. Another possibility to enhance cell survival takes advantage of drugs that mimic the effects of ischemic preconditioning, by opening mitochondrial potassium channels. Niagara and co-workers showed that pretreatment of skeletal myoblasts with diazoxide, a mitochondrial potassium channel opener, increased their survival almost two-fold after direct intramyocardial injection in the infarcted rat heart 93. Importantly, the functional benefit in the animals that received diazoxide-treated cells was also greater, indicating that increased cell survival is closely related to the effectiveness of cell transplantation.
A variety of growth and angiogenic factors have also been used in order to enhance cell engraftment. Kofidis et al injected eGFP-labeled mouse ESCs with or without IGF-1 and observed better functional improvement and enhanced cardiomyocyte differentiation of ESCs in the animals treated with cells and IGF-1 94. Engraftment was not quantified in this study. Pasha et al pretreated mesenchymal stem cells with SDF-1 before intramyocardial injection in the infarcted rat heart 95. They were able to demonstrate a significant increase in cell survival (by histology) compared to transplantation of non-pretreated cells. In addition, fibrosis in the infarct area was less, and improvement in cardiac function was greater, when pretreated cells were used. These favorable effects of SDF-1 were abolished by a selective antagonist of CXCR4, the receptor of SDF-1. VEGF has also been used effectively in several studies to enhance survival and function of various cell types. Pons and co-workers injected MSCs and VEGF in infarcted mouse hearts and demonstrated increased cell engraftment and better cardiac function in comparison to animals injected with MSCs or VEGF alone 96. Recently, Higuchi et al used Positron Emission Tomography to track endothelial progenitor cells genetically engineered to overexpress the human NIS, in infarcted rat hearts 97. Using 124Iodine as tracer, they showed increased cell engraftment after pretreatment of cells with VEGF and atrorvastatin.
Despite these encouraging results, it should be remembered that multiple mechanisms mediate cell loss after transplantation; thus, pretreatment with single factors may not suffice to achieve a clinically meaningful increase in engraftment. With this rationale, Laflamme and coworkers used a combination of growth factors and drugs to enhance engraftment of human embryonic stem cell derived cardiomyocytes in the infarcted hearts of immunodeficient rats 98. Their cocktail consisted of Matrigel as a cell attachment material to prevent anoikis, two components for suppression of mitochondrial death pathways (a cell permeant Bcl-XL derived peptide and cyclosporine A), pinacidil as a preconditioning agent (mitochondrial ATP-dependent potassium channel opener), IGF-1 as a pro-survival factor (activation of Akt pathways) and a broad-spectrum caspase inhibitor (ZVAD-fmk), for apoptosis inhibition. This “kitchen sink” approach yielded a significant increase in histologically-assessed graft formation, in comparison to animals that received non-pretreated cells (control), cells subjected to heat shock (very poor engraftment) or heat-shock-treated cells in Matrigel (better engraftment than control but less than the cocktail-treated cells). Robey et al, from the same group, demonstrated the efficacy of a combination approach using carbamylated-erythropoietin (as a pro-survival factor) and heat-shock for pretreatment of human embryonic stem cell derived cardiomyocytes to enhance engraftment (by real time PCR) in the infarcted mouse heart 33.
d) Genetic engineering of cells
Despite the success in experimental models of cell preconditioning, the effect of the pretreatment is only transient and therefore the magnitude of the increase in cell engraftment may (or may not) be adequate for a clinically-meaningful result. Another approach in this direction is the genetic modification of the cells, in order to stably express pro-survival or angiogenic factors. Mangi et al transduced mesenchymal stem cells with a retrovirus to overexpress Akt, an important pro-survival signal, and injected them intramyocardially in infarcted rats 99. They showed a significantly larger functional benefit and smaller infarct size in the animals treated with Akt-overexpressing cells in comparison to the ones that received lacZ expressing cells. In this proof-of-concept study, cell engraftment was not quantified directly. However, in a similar experiment, Jiang et al transduced MSC with adenovirus to overexpress angiopoietin-1 and Akt and injected them in the myocardium of infarcted rats 8, 100. At 3 months after transplantation, they were able to demonstrate, by real time PCR, increased cell survival in the animals that received genetically-engineered cells in comparison to the ones transplanted with non-transduced cells. In addition, functional improvement and vascular density in the infarct area were increased in the animals treated with Akt and angiopoietin-1 cells. Kutschka et al injected ESC derived cardiomyoblasts, adenovirally transduced to overexpress the anti-apoptotic gene Bcl-2, in infarcted rat hearts, using a heterotopic cardiac transplantation model 101. Bcl-2 overexpressing cells survived better than control non-transduced ones at 4 weeks (assessed by bioluminescence), although cardiac function was similarly preserved in both groups. A similar increase in cell survival was observed with Bcl-2-overexpressing MSCs, in a rat myocardial infarction model 102. Tang and co-workers transiently transfected mouse MSCs with a hypoxia inducible vector overexpressing human heme oxygenase-1. Heme oxygenase-1 (HO-1) has been shown to confer protection in the myocardium during ischemia, through antioxidant and antiapoptotic activity. HO-1 and lacZ-overexpressing MSCs were injected intramyocardially into the myocardium of infarcted mice, 1hr after MI induction 103. At 7 days, MSC engraftment was 5 times higher in the HO-1 group (assessed by real time PCR), indicating a pro-survival effect of the hypoxia-induced expression of this enzyme. Left ventricular function was also more effectively preserved by HO-1 overexpressing MSCs.
Genetic modifications have several limitations that need to be considered, particularly if clinical translatability is concerned:
Any intervention in the cell’s gene expression profile should not have detrimental effects to the cell’s desired function.
Pro-survival gene overexpression carries the risk of oncogenesis, at least theoretically. This concern has been raised specifically for Akt overexpression strategies 104.
Vector safety is also an issue, particularly with lentiviruses and retroviruses that stably integrate in the cell genome. If only transient transgene expression is required, plain transfection may be preferable 105.
The regulatory hurdles to clinical application of genetically-modified cells will be substantial, as safety criteria for both cell therapy and gene therapy will have to be satisfied.
Conclusions
Suboptimal cell engraftment appears as one of the major hurdles for successful myocardial regeneration with stem cell therapy. Many experimental studies have connected improved engraftment with superior functional benefits of cell therapy. The development of reliable, accurate quantitative tools for tracking cell fate and assessing cell viability after transplantation is crucial, not only to understand the mechanisms of cell attrition but also to enhance cell survival. Within this setting, in vivo imaging will undoubtedly play major role in the future, since reporter gene technologies, combined with recent improvements in multimodality imaging, will be able to provide the required information non-invasively. Improvement of engraftment demands interventions in many different technical and biological aspects of cell transplantation. Delivery methods should be optimized in order to increase acute cell retention. Resistance of cells to ischemia by preconditioning, inhibition of apoptotic death, amelioration of acute inflammatory reactions, stimulation of cell proliferation by growth factors and gene therapy are all potential targets for augmenting engraftment. In this regard, pre-clinical studies in clinically-relevant large-animal models (acute myocardial infarction or chronic ischemic cardiomyopathy) should be carefully designed and conducted, before these interventions can be translated into clinical trials. The risks of these approaches to cell function and to the recipient’s health should be carefully evaluated, particularly if they involve genetic modifications or the use of viral vectors. The encouraging, albeit modest, functional benefits observed in stem cell clinical trials have occurred despite the universally-acknowledged low rates of cell survival. These support the notion that improvement of long-term cell engraftment is a goal that should be pursued aggressively, in order to take full advantage of the potential of myocardial regeneration therapy.
Acknowledgments
Sources of Funding. Supported by NIH and the Donald W. Reynolds Foundation.
Non-standard Abbreviations and Acronyms
- CDC
cardiosphere derived cells
- DiI
1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate
- DAPI
4',6-diamidino-2-phenylindole
- NIS
sodium iodide symporter
- Tc-HMPAO
technetium hexamethylpropylenamine oxime
Footnotes
Subject codes: [130] Animal models of human disease, [147] Growth factors/cytokines, [150] Imaging
Disclosures. Dr Eduardo Marbán holds equity in a private company (Capricor Inc.) that licenses techniques used to manufacture cardiac stem cells. Dr Rachel Ruckdeschel Smith is employed by Capricor Inc. Dr John Terrovitis has a consultant relationship with Capricor Inc. Capricor provided no funding for the present work.
References
- 1.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 2.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 3.Mazhari R, Hare JM. Advances in cell-based therapy for structural heart disease. Prog Cardiovasc Dis. 2007;49:387–395. doi: 10.1016/j.pcad.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circ.Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 6.Anversa P, Leri A, Rota M, Hosoda T, Bearzi C, Urbanek K, Kajstura J, Bolli R. Concise review: stem cells, myocardial regeneration, and methodological artifacts. Stem Cells. 2007;25:589–601. doi: 10.1634/stemcells.2006-0623. [DOI] [PubMed] [Google Scholar]
- 7.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annu Rev Physiol. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 8.Jiang S, Haider H, Idris NM, Salim A, Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 9.Torre-Perez N, Montero JA, Zuzarte-Luis V, Garcia-Porrero JA, Rubio N, Blanco J, Nistal JF, Hurle JM. Migration and Differentiation of Human Umbilical Cord Stem Cells After Heart Injury in Chicken Embryos. Stem Cells Dev. 2008 doi: 10.1089/scd.2007.0239. [DOI] [PubMed] [Google Scholar]
- 10.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 11.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 12.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schar M, Gerstenblith G, Weiss RG, Marban E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 13.Encinas JM, Enikolopov G. Identifying and quantitating neural stem and progenitor cells in the adult brain. Methods Cell Biol. 2008;85:243–272. doi: 10.1016/S0091-679X(08)85011-X. [DOI] [PubMed] [Google Scholar]
- 14.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res. 2005;96:127–137. doi: 10.1161/01.RES.0000151843.79801.60. [DOI] [PubMed] [Google Scholar]
- 15.Scherschel JA, Soonpaa MH, Srour EF, Field LJ, Rubart M. Adult bone marrow-derived cells do not acquire functional attributes of cardiomyocytes when transplanted into peri-infarct myocardium. Mol Ther. 2008;16:1129–1137. doi: 10.1038/mt.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis DR, Zhang Y, Smith RR, Cheng K, Terrovitis J, Malliaras K, Li TS, White A, Makkar R, Marban E. Validation of the cardiosphere method to culture cardiac progenitor cells from myocardial tissue. PLoS One. 2009;4:e7195. doi: 10.1371/journal.pone.0007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970–974. doi: 10.1038/nm1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McVeigh ER. Emerging imaging techniques. Circ Res. 2006;98:879–886. doi: 10.1161/01.RES.0000216870.73358.d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosen AB, Kelly DJ, Schuldt AJ, Lu J, Potapova IA, Doronin SV, Robichaud KJ, Robinson RB, Rosen MR, Brink PR, Gaudette GR, Cohen IS. Finding fluorescent needles in the cardiac haystack: tracking human mesenchymal stem cells labeled with quantum dots for quantitative in vivo three-dimensional fluorescence analysis. Stem Cells. 2007;25:2128–2138. doi: 10.1634/stemcells.2006-0722. [DOI] [PubMed] [Google Scholar]
- 21.Laflamme MA, Murry CE. Regenerating the heart. Nat.Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 22.Reinecke H, Minami E, Zhu WZ, Laflamme MA. Cardiogenic differentiation and transdifferentiation of progenitor cells. Circ Res. 2008;103:1058–1071. doi: 10.1161/CIRCRESAHA.108.180588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noiseux N, Gnecchi M, Lopez-Ilasaca M, Zhang L, Solomon SD, Deb A, Dzau VJ, Pratt RE. Mesenchymal stem cells overexpressing Akt dramatically repair infarcted myocardium and improve cardiac function despite infrequent cellular fusion or differentiation. Mol Ther. 2006;14:840–850. doi: 10.1016/j.ymthe.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Mutskov V, Felsenfeld G. Silencing of transgene transcription precedes methylation of promoter DNA and histone H3 lysine 9. Embo J. 2004;23:138–149. doi: 10.1038/sj.emboj.7600013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krishnan M, Park JM, Cao F, Wang D, Paulmurugan R, Tseng JR, Gonzalgo ML, Gambhir SS, Wu JC. Effects of epigenetic modulation on reporter gene expression: implications for stem cell imaging. Faseb J. 2006;20:106–108. doi: 10.1096/fj.05-4551fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier-Mariette C, Henry I, Montfort L, Capgras S, Forlani S, Muschler J, Nicolas JF. CpG content affects gene silencing in mice: evidence from novel transgenes. Genome Biol. 2003;4:R53. doi: 10.1186/gb-2003-4-9-r53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta AK, Majumdar SS, Alam P, Gulati N, Brahmachari V. Epigenetic regulation of cytomegalovirus major immediate-early promoter activity in transgenic mice. Gene. 2009;428:20–24. doi: 10.1016/j.gene.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Scrable H, Stambrook PJ. A genetic program for deletion of foreign DNA from the mammalian genome. Mutat Res. 1999;429:225–237. doi: 10.1016/s0027-5107(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 29.Ghodsizad A, Niehaus M, Kogler G, Martin U, Wernet P, Bara C, Khaladj N, Loos A, Makoui M, Thiele J, Mengel M, Karck M, Klein HM, Haverich A, Ruhparwar A. Transplanted human cord blood-derived unrestricted somatic stem cells improve left-ventricular function and prevent left-ventricular dilation and scar formation after acute myocardial infarction. Heart. 2009;95:27–35. doi: 10.1136/hrt.2007.139329. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Murtuza B, Fukushima S, Smolenski RT, Varela-Carver A, Coppen SR, Yacoub MH. Targeted cell delivery into infarcted rat hearts by retrograde intracoronary infusion: distribution, dynamics, and influence on cardiac function. Circulation. 2004;110:II225–II230. doi: 10.1161/01.CIR.0000138191.11580.e3. [DOI] [PubMed] [Google Scholar]
- 33.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 1077 p following 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki K, Murtuza B, Beauchamp JR, Brand NJ, Barton PJ, Varela-Carver A, Fukushima S, Coppen SR, Partridge TA, Yacoub MH. Role of interleukin-1beta in acute inflammation and graft death after cell transplantation to the heart. Circulation. 2004;110:II219–II224. doi: 10.1161/01.CIR.0000138388.55416.06. [DOI] [PubMed] [Google Scholar]
- 36.Muller-Ehmsen J, Whittaker P, Kloner RA, Dow JS, Sakoda T, Long TI, Laird PW, Kedes L. Survival and development of neonatal rat cardiomyocytes transplanted into adult myocardium. J Mol Cell Cardiol. 2002;34:107–116. doi: 10.1006/jmcc.2001.1491. [DOI] [PubMed] [Google Scholar]
- 37.Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kutschka I, Chen IY, Kofidis T, von Degenfeld G, Sheikh AY, Hendry SL, Hoyt G, Pearl J, Blau HM, Gambhir SS, Robbins RC. In vivo optical bioluminescence imaging of collagen-supported cardiac cell grafts. J Heart Lung Transplant. 2007;26:273–280. doi: 10.1016/j.healun.2006.11.604. [DOI] [PubMed] [Google Scholar]
- 39.Cao F, Lin S, Xie X, Ray P, Patel M, Zhang X, Drukker M, Dylla SJ, Connolly AJ, Chen X, Weissman IL, Gambhir SS, Wu JC. In vivo visualization of embryonic stem cell survival, proliferation, and migration after cardiac delivery. Circulation. 2006;113:1005–1014. doi: 10.1161/CIRCULATIONAHA.105.588954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kutschka I, Chen IY, Kofidis T, Arai T, von Degenfeld G, Sheikh AY, Hendry SL, Pearl J, Hoyt G, Sista R, Yang PC, Blau HM, Gambhir SS, Robbins RC. Collagen matrices enhance survival of transplanted cardiomyoblasts and contribute to functional improvement of ischemic rat hearts. Circulation. 2006;114:I167–I173. doi: 10.1161/CIRCULATIONAHA.105.001297. [DOI] [PubMed] [Google Scholar]
- 41.Wu JC, Chen IY, Sundaresan G, Min JJ, De A, Qiao JH, Fishbein MC, Gambhir SS. Molecular imaging of cardiac cell transplantation in living animals using optical bioluminescence and positron emission tomography. Circulation. 2003;108:1302–1305. doi: 10.1161/01.CIR.0000091252.20010.6E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virostko J, Chen Z, Fowler M, Poffenberger G, Powers AC, Jansen ED. Factors influencing quantification of in vivo bioluminescence imaging: application to assessment of pancreatic islet transplants. Mol Imaging. 2004;3:333–342. doi: 10.1162/15353500200404133. [DOI] [PubMed] [Google Scholar]
- 43.Gheysens O, Lin S, Cao F, Wang D, Chen IY, Rodriguez-Porcel M, Min JJ, Gambhir SS, Wu JC. Noninvasive evaluation of immunosuppressive drug efficacy on acute donor cell survival. Mol Imaging Biol. 2006;8:163–170. doi: 10.1007/s11307-006-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qiao H, Surti S, Choi SR, Raju K, Zhang H, Ponde DE, Kung HF, Karp J, Zhou R. Death and Proliferation Time Course of Stem Cells Transplanted in the Myocardium. Mol Imaging Biol. 2009 doi: 10.1007/s11307-009-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akins EJ, Dubey P. Noninvasive imaging of cell-mediated therapy for treatment of cancer. J Nucl Med. 2008;49 Suppl 2:180S–195S. doi: 10.2967/jnumed.107.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 47.Doyle B, Kemp BJ, Chareonthaitawee P, Reed C, Schmeckpeper J, Sorajja P, Russell S, Araoz P, Riederer SJ, Caplice NM. Dynamic tracking during intracoronary injection of 18F-FDG-labeled progenitor cell therapy for acute myocardial infarction. J Nucl Med. 2007;48:1708–1714. doi: 10.2967/jnumed.107.042838. [DOI] [PubMed] [Google Scholar]
- 48.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 49.Kang WJ, Kang HJ, Kim HS, Chung JK, Lee MC, Lee DS. Tissue distribution of 18F-FDG-labeled peripheral hematopoietic stem cells after intracoronary administration in patients with myocardial infarction. J Nucl Med. 2006;47:1295–1301. [PubMed] [Google Scholar]
- 50.Blocklet D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E, Egrise D, Degaute JP, Goldman M, Goldman S. Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells. 2006;24:333–336. doi: 10.1634/stemcells.2005-0201. [DOI] [PubMed] [Google Scholar]
- 51.Schachinger V, Aicher A, Dobert N, Rover R, Diener J, Fichtlscherer S, Assmus B, Seeger FH, Menzel C, Brenner W, Dimmeler S, Zeiher AM. Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation. 2008;118:1425–1432. doi: 10.1161/CIRCULATIONAHA.108.777102. [DOI] [PubMed] [Google Scholar]
- 52.Goussetis E, Manginas A, Koutelou M, Peristeri I, Theodosaki M, Kollaros N, Leontiadis E, Theodorakos A, Paterakis G, Karatasakis G, Cokkinos DV, Graphakos S. Intracoronary infusion of CD133+ and CD133−CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006;24:2279–2283. doi: 10.1634/stemcells.2005-0589. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 54.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intracoronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 55.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow PL, Rannou FR, Chatziioannou AF. Attenuation correction for small animal PET tomographs. Phys Med Biol. 2005;50:1837–1850. doi: 10.1088/0031-9155/50/8/014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adonai N, Nguyen KN, Walsh J, Iyer M, Toyokuni T, Phelps ME, McCarthy T, McCarthy DW, Gambhir SS. Ex vivo cell labeling with 64Cu-pyruvaldehydebis(N4-methylthiosemicarbazone) for imaging cell trafficking in mice with positron-emission tomography. Proc Natl Acad Sci U S A. 2002;99:3030–3035. doi: 10.1073/pnas.052709599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kraitchman DL, Tatsumi M, Gilson WD, Ishimori T, Kedziorek D, Walczak P, Segars WP, Chen HH, Fritzges D, Izbudak I, Young RG, Marcelino M, Pittenger MF, Solaiyappan M, Boston RC, Tsui BM, Wahl RL, Bulte JW. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451–1461. doi: 10.1161/CIRCULATIONAHA.105.537480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balaban EP, Simon TR, Frenkel EP. Toxicity of indium-111 on the radiolabeled lymphocyte. J Nucl Med. 1987;28:229–233. [PubMed] [Google Scholar]
- 60.Brenner W, Aicher A, Eckey T, Massoudi S, Zuhayra M, Koehl U, Heeschen C, Kampen WU, Zeiher AM, Dimmeler S, Henze E. 111In-labeled CD34+ hematopoietic progenitor cells in a rat myocardial infarction model. J Nucl Med. 2004;45:512–518. [PubMed] [Google Scholar]
- 61.Acton PD, Zhou R. Imaging reporter genes for cell tracking with PET and SPECT. Q J Nucl Med Mol Imaging. 2005;49:349–360. [PubMed] [Google Scholar]
- 62.Beeres SL, Bengel FM, Bartunek J, Atsma DE, Hill JM, Vanderheyden M, Penicka M, Schalij MJ, Wijns W, Bax JJ. Role of imaging in cardiac stem cell therapy. J Am Coll Cardiol. 2007;49:1137–1148. doi: 10.1016/j.jacc.2006.10.072. [DOI] [PubMed] [Google Scholar]
- 63.Serganova I, Ponomarev V, Blasberg R. Human reporter genes: potential use in clinical studies. Nucl Med Biol. 2007;34:791–807. doi: 10.1016/j.nucmedbio.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Chen IY, Wu JC, Min JJ, Sundaresan G, Lewis X, Liang Q, Herschman HR, Gambhir SS. Micro-positron emission tomography imaging of cardiac gene expression in rats using bicistronic adenoviral vector-mediated gene delivery. Circulation. 2004;109:1415–1420. doi: 10.1161/01.CIR.0000121727.59564.5B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terrovitis J, Kwok KF, Lautamaki R, Engles JM, Barth AS, Kizana E, Miake J, Leppo MK, Fox J, Seidel J, Pomper M, Wahl RL, Tsui B, Bengel F, Marban E, Abraham MR. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. J Am Coll Cardiol. 2008;52:1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gray SJ, Samulski RJ. Optimizing gene delivery vectors for the treatment of heart disease. Expert Opin Biol Ther. 2008;8:911–922. doi: 10.1517/14712598.8.7.911. [DOI] [PubMed] [Google Scholar]
- 67.Stiehler M, Duch M, Mygind T, Li H, Ulrich-Vinther M, Modin C, Baatrup A, Lind M, Pedersen FS, Bunger CE. Optimizing viral and non-viral gene transfer methods for genetic modification of porcine mesenchymal stem cells. Adv Exp Med Biol. 2006;585:31–48. doi: 10.1007/978-0-387-34133-0_3. [DOI] [PubMed] [Google Scholar]
- 68.Mikkers H, Berns A. Retroviral insertional mutagenesis: tagging cancer pathways. Adv Cancer Res. 2003;88:53–99. doi: 10.1016/s0065-230x(03)88304-5. [DOI] [PubMed] [Google Scholar]
- 69.Su H, Forbes A, Gambhir SS, Braun J. Quantitation of cell number by a positron emission tomography reporter gene strategy. Mol Imaging Biol. 2004;6:139–148. doi: 10.1016/j.mibio.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Miyagawa M, Anton M, Wagner B, Haubner R, Souvatzoglou M, Gansbacher B, Schwaiger M, Bengel FM. Non-invasive imaging of cardiac transgene expression with PET: comparison of the human sodium/iodide symporter gene and HSV1-tk as the reporter gene. Eur J Nucl Med Mol Imaging. 2005;32:1108–1114. doi: 10.1007/s00259-005-1854-4. [DOI] [PubMed] [Google Scholar]
- 71.Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, Gambhir SS. Noninvasive detection of therapeutic cytolytic T cells with 18F-FHBG PET in a patient with glioma. Nat Clin Pract Oncol. 2009;6:53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash IM, Leor J. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116:I38–I45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 73.Terrovitis J, Stuber M, Weiss RG, Leppo M, Youssef A, Gerstenblith G, Marban E, Abraham MR. Iron-labeled Stem Cells Seen by Magnetic Resonance Imaging: Dead or Alive? AHA sessions. 2006 doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 74.Chen IY, Greve JM, Gheysens O, Willmann JK, Rodriguez-Porcel M, Chu P, Sheikh AY, Faranesh AZ, Paulmurugan R, Yang PC, Wu JC, Gambhir SS. Comparison of optical bioluminescence reporter gene and superparamagnetic iron oxide MR contrast agent as cell markers for noninvasive imaging of cardiac cell transplantation. Mol Imaging Biol. 2009;11:178–187. doi: 10.1007/s11307-008-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Partlow KC, Chen J, Brant JA, Neubauer AM, Meyerrose TE, Creer MH, Nolta JA, Caruthers SD, Lanza GM, Wickline SA. 19F magnetic resonance imaging for stem/progenitor cell tracking with multiple unique perfluorocarbon nanobeacons. Faseb J. 2007;21:1647–1654. doi: 10.1096/fj.06-6505com. [DOI] [PubMed] [Google Scholar]
- 76.Dow J, Simkhovich BZ, Kedes L, Kloner RA. Washout of transplanted cells from the heart: a potential new hurdle for cell transplantation therapy. Cardiovasc Res. 2005;67:301–307. doi: 10.1016/j.cardiores.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 77.Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132:628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 78.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009 doi: 10.1016/j.jacc.2009.04.097. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 80.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, Fleischmann BK, Hescheler J, Schwinger RH. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol. 2006;41:876–884. doi: 10.1016/j.yjmcc.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 81.Hamamoto H, Gorman JH, 3rd, Ryan LP, Hinmon R, Martens TP, Schuster MD, Plappert T, Kiupel M, St John-Sutton MG, Itescu S, Gorman RC. Allogeneic mesenchymal precursor cell therapy to limit remodeling after myocardial infarction: the effect of cell dosage. Ann Thorac Surg. 2009;87:794–801. doi: 10.1016/j.athoracsur.2008.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu X, Wang J, Chen J, Luo R, He A, Xie X, Li J. Optimal temporal delivery of bone marrow mesenchymal stem cells in rats with myocardial infarction. Eur J Cardiothorac Surg. 2007;31:438–443. doi: 10.1016/j.ejcts.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 83.Yau TM, Kim C, Ng D, Li G, Zhang Y, Weisel RD, Li RK. Increasing transplanted cell survival with cell-based angiogenic gene therapy. Ann Thorac Surg. 2005;80:1779–1786. doi: 10.1016/j.athoracsur.2005.04.079. [DOI] [PubMed] [Google Scholar]
- 84.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Memon IA, Sawa Y, Fukushima N, Matsumiya G, Miyagawa S, Taketani S, Sakakida SK, Kondoh H, Aleshin AN, Shimizu T, Okano T, Matsuda H. Repair of impaired myocardium by means of implantation of engineered autologous myoblast sheets. J Thorac Cardiovasc Surg. 2005;130:1333–1341. doi: 10.1016/j.jtcvs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 86.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest. 2009;119:2204–2217. doi: 10.1172/JCI37456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 88.Goerler H, Oppelt P, Abel U, Haverich A. Safety of the use of Tissucol Duo S in cardiovascular surgery: retrospective analysis of 2149 patients after coronary artery bypass grafting. Eur J Cardiothorac Surg. 2007;32:560–566. doi: 10.1016/j.ejcts.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 89.Lamm P, Adelhard K, Juchem G, Weitkunat R, Milz S, Kilger E, Gotz A, Reichart B. Fibrin glue in coronary artery bypass grafting operations: casting out the Devil with Beelzebub? Eur J Cardiothorac Surg. 2007;32:567–572. doi: 10.1016/j.ejcts.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 90.Haider H, Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki K, Smolenski RT, Jayakumar J, Murtuza B, Brand NJ, Yacoub MH. Heat shock treatment enhances graft cell survival in skeletal myoblast transplantation to the heart. Circulation. 2000;102:III216–III221. doi: 10.1161/01.cir.102.suppl_3.iii-216. [DOI] [PubMed] [Google Scholar]
- 92.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, Muskheli V, Murry CE. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–671. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niagara MI, Haider H, Jiang S, Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 94.Kofidis T, de Bruin JL, Yamane T, Balsam LB, Lebl DR, Swijnenburg RJ, Tanaka M, Weissman IL, Robbins RC. Insulin-like growth factor promotes engraftment, differentiation, and functional improvement after transfer of embryonic stem cells for myocardial restoration. Stem Cells. 2004;22:1239–1245. doi: 10.1634/stemcells.2004-0127. [DOI] [PubMed] [Google Scholar]
- 95.Pasha Z, Wang Y, Sheikh R, Zhang D, Zhao T, Ashraf M. Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res. 2008;77:134–142. doi: 10.1093/cvr/cvm025. [DOI] [PubMed] [Google Scholar]
- 96.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Su H. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Higuchi T, Anton M, Saraste A, Dumler K, Pelisek J, Nekolla SG, Bengel FM, Schwaiger M. Reporter Gene PET for Monitoring Survival of Transplanted Endothelial Progenitor Cells in the Rat Heart After Pretreatment with VEGF and Atorvastatin. J Nucl Med. 2009 doi: 10.2967/jnumed.109.067801. [DOI] [PubMed] [Google Scholar]
- 98.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O'Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 99.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 100.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 101.Kutschka I, Kofidis T, Chen IY, von Degenfeld G, Zwierzchoniewska M, Hoyt G, Arai T, Lebl DR, Hendry SL, Sheikh AY, Cooke DT, Connolly A, Blau HM, Gambhir SS, Robbins RC. Adenoviral human BCL-2 transgene expression attenuates early donor cell death after cardiomyoblast transplantation into ischemic rat hearts. Circulation. 2006;114:I174–I180. doi: 10.1161/CIRCULATIONAHA.105.001370. [DOI] [PubMed] [Google Scholar]
- 102.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 103.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 104.Rosen N, She QB. AKT and cancer--is it all mTOR? Cancer Cell. 2006;10:254–256. doi: 10.1016/j.ccr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 105.Haider HK, Elmadbouh I, Jean-Baptiste M, Ashraf M. Nonviral vector gene modification of stem cells for myocardial repair. Mol Med. 2008;14:79–86. doi: 10.2119/2007-00092.Haider. [DOI] [PMC free article] [PubMed] [Google Scholar]