Abstract
This study was conducted to investigate the etiology, the clinical characteristics and prognosis of acute necrotizing encephalopathy (ANE) in Korean children. Six children (1 yr to 7 yr) patients with ANE were enrolled. They were diagnosed by clinical and radiological characteristics and their clinical data were retrospectively analyzed. In a search of clinically plausible causes, brain MRI in all patients, mitochondrial DNA studies for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes (MELAS) and myoclonus epilepsy and ragged red fibers (MERRF) in four patients, and genomic typing on HLA DRB/HLA DQB genes in three patients were performed. All had precedent illnesses and the main initial symptoms included mental change (83%), seizures (50%), and focal deficits (50%). MRI revealed increased T2 signal density in the bilateral thalami and/or the brainstem in all patients. Mitochodrial DNA studies for MELAS and MERRF were negative in those children and HLA-DRB1*1401, HLA-DRB3*0202, and HLA-DQB1*0502 seemed to be significant. A high dose steroid was given to all patients, which seemed to be partly effective except for 2 patients. In conclusion, ANE is relatively rare, but can result in serious neurological complication in children. Early detection and appropriate treatment may lead to a better neurological outcome.
Keywords: Acute Necrotizing Encephalopathy, Mitochodrial, MELAS Syndrome, MERRF Syndrome, HLA-DR Antigens, HLA-DQ Antigens
INTRODUCTION
Acute necrotizing encephalopathy (ANE) is a unique acute encephalopathy that predominantly affects infants and young children (1). It is characterized by the features of acute encephalopthy such as seizures and rapid alteration of consciousness after a nonspecific viral illness. Since Mizuguchi et al. proposed this condition as a new entity in 1995 (2), it has occasionally been reported in both Asian and Western countries. An increased level of serum aminotransferase activiy and cerebrospinal fluid protein, are the most common abnormalities (1-4). Diagnosis is made mainly by the characteristic findings of computed tomography or magnetic resonance imaging (MRI), which typically show symmetric lesions in the thalami, with variable involvement of the white matter, basal ganglia, brainstem, and cerebellum (1, 4, 5). Regardless of the treatment, the prognosis of ANE varies widely from complete recovery to death (1-6). With the widespread use of MRI, this unique condition is becoming more familiar. However, the etiology, pathogenesis, guidelines of treatments, or prognostic factors still remain unclear. In this report, we described 6 children with ANE to elucidate the genetic propensity, the clinical/neuroradiological characteristics, the response to medical treatment and prognostic factors.
MATERIALS AND METHODS
A total of 6 children with ANE (aged 1 yr to 7 yr at onset, 3 males and 3 females) was evaluated. The patients were admitted to Kyungpook National University Hospital, Daegu, Korea, from 1999 through 2007. Diagnosis was made mainly by clinical and radiological characteristics. Serological tests or polymerase chain reaction for certain viruses such as Herpes simplex virus, cytomegalovirus, Epstein-Barr virus, influenza virus, and mycoplasma were done in 5 out of them. Brain MRIs were taken in all patients at the time of initial presentation. In a search of other clinically plausible causes, mitochodrial DNA studies for mitochondrial encephalomyopathy and lacticacidosis and strokelike episode (MELAS) and myoclonus epilepsy and ragged red fibers (MERRF), particularly point mutation at nt 3243, nt 3271, nt 3252, nt 8344, nt 8356, etc. in the tRNA were screened in four patients. In addition, genomic typing was performed on HLA DRB/HLA DQB genes using polymerase chain reaction (PCR)-SSOP/ SSP techniques with Gel immunoelectrophoresis in three patients. This clinical research was approved by Internal Review Board (74005-1540).
RESULTS
A total of six children was enrolled in the study. Past medical histories were uneventful in all patients and they had normal developmental milestones. The family histories were also unremarkable. No patients were exposed to any drugs or chemical substances known to cause toxic encephalopathies.
Clinical features of the subjects
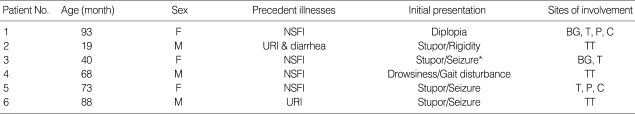
Clinical characteristics of the subjects are summarized in Table 1. All had precedent illnesses and five out of them (83%) had fever. The initial neurological symptoms include mental change in five patients (83%), seizures (50%) and focal neurological signs (50%).
Table 1.
Clinical features of the subjects with ANE
*Status epilepticus.
ANE, acute necrotizing encephalopathy; NSFI, non specific febrile illness; URI, upper respiratory tract infection; BG, Basal ganglia; T, Thalamus; TT, Thalamotegmantum; P, Pons; C, Cerebellum.
Laboratory findings
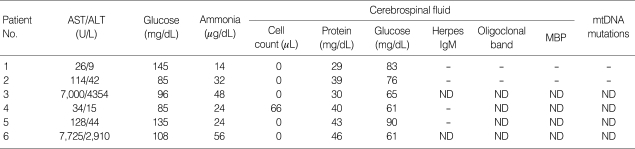
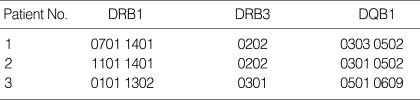
Table 2 shows laboratory findings of the subjects. Increased level of serum aminotransferase and lactic dehydrogenase activities may indicate hepatic dysfunction, but their levels varied highly from case to case. Four patients (67%) showed elevation of serum aminotransferase, which increased sharply to 7,000 U/L in 2 out of them. Severe cases revealed laboratory findings compatible with disseminated intravascular coagulation but the clinical symptoms were almost absent. In addition, serum ammonia levels were normal. Serological tests or polymerase chain reaction for certain viruses such as Herpes simplex virus, cytomegalovirus, Epstein-Barr virus, influenza virus, and mycoplasma were unremarkable. Mitochodrial DNA studies for MELAS and MERRF were done in 4 patients, and none of them showed any mutations. Genomic typing of HLA DRB/HLA DQB genes in three patients (patient 3, 4, and 5) using PCR-SSOP/SSP techniques with gel immunoelectrophoresis showed HLA-DRB1*1401, HLA-DRB3*0202, and HLA-DQB1*0502 which were possibly significant (Table 3).
Table 2.
Laboratory findings of the subjects with ANE
ANE, acute necrotizing encephalopathy; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ND, not done; MBP, myelin basic protein; mtDNA, mitochondrial DNA.
Table 3.
HLA-DRB and DQB alleles of 3 patients with ANE
ANE, acute necrotizing encephalopathy.
Cerebrospinal fluid study revealed normal protein levels in all patients and mild pleocytosis in one patient (patient 4). Herpes IgM antibody, oligoclonal band and myelin basic protein were unremarkable in all subjects.
Radiological findings
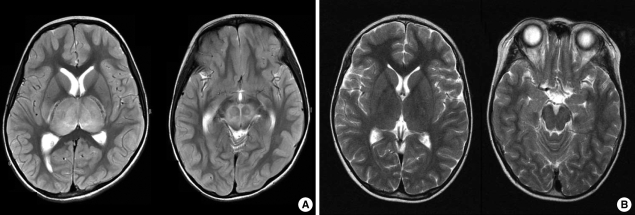
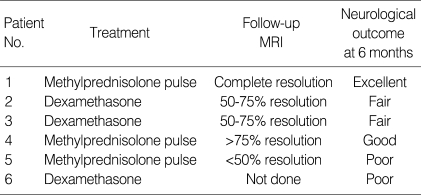
As shown in Fig. 1, brain MRI revealed increased signal density on T2-weighted imaging in the bilateral thalami and brain stem in almost all patients. Two out of them had lesions in cerebellum (Table 1). The findings were consistent with a unique pattern of ANE. Follow-up MRI was done in 2 weeks to 7 months. In one patient, brain findings had completely resolved, However, in 4 patients of 5 followed-up, a certain degree of sequelae were found (Table 4).
Fig. 1.
Radiological findings in a patient with ANE. (A) MRI shows symmetric, multifocal brain lesions involving bilateral thalami and upper brain stem tegmentum without involvement of other CNS regions. (B) Follow-up MRI shows complete resolution of previous lesions.
Table 4.
Treatment and its outcome in the subjects with ANE
*Definition of outcome.
Excellant, complete resolution; Good, almost complete resolution or minimal degree of neurological sequelae (mobile, almost no cognitive/social/emotional impairment); Fair, moderate degree of neurological sequelae (mobile with difficulty, mild to moderate cognitive/social/emotional impairment); Poor, severe degree of neurological sequelae (immobile, severe cognitive/social/emotional impairment).
Treatment and outcome
With respect to the treatment, steroids have been used in two regimens: intravenous dexamethasone (patient 2, 3, 6) or methylprednisolone pulse therapy (patient 1, 4, 5). In the dexamethasone group, 1 mg/kg/day of dexamethasone was administered in 4 divided doses for at least 5 days. In the methylprednisolone group, 30 mg/kg/day of methylprednisolone was administrered for 5 days. As shown in Table 4, a patient (patient 1) showed excellent outcomes without any neurological sequelae at 6 months after the illness. The other three patients (patient 2, 3, 4) showed a relatively good to fair outcome. Even though they had initial weakness, spasticity on extremities or memory disturbance, their symptoms improved remarkably. Two patients developed epilepsy later on and required prophylactic antiepileptic drugs. Even so, they remained seizure free for more than years, and eventually were taken off antiepileptic drugs without any sequelae. A patient (patient 6) died of cardiorespiratory compromise.
DISCUSSION
ANE was proposed as a novel disease entity by Mizuguchi et al. in 1995 (2). Patients with ANE manifest fulminating neurologic deterioration with preceding non-specific febrile illness and frequently undergo intractable convulsions. Serious neurological signs such as decorticate, decerebrate posturing or long tract signs may appear. Its mortality is considered to reach as high as 30% (1, 2). Mizuguchi et al. proposed the following diagnostic criteria for acute necrotizing encephalopathy: 1) acute encephalopathy following a viral febrile disease and rapid deterioration in the level of consciousness, convulsion; 2) increased cerebrospinal fluid (CSF) protein without CSF pleocytosis; 3) CT or MRI findings for symmetric, multifocal brain lesions involving bilateral thalami, cerebral periventricular white matter, internal capsule, putamen, upper brain stem tegmentum and cerebellar medulla without involvement of other CNS regions; 4) elevation of serum aminotrasferase of variable degrees without hyperammonemia; 5) exclusion of other resembling diseases such as overwhelming bacterial and viral infections, fulminant hepatitis, Reye syndrome, Leigh encephalopathy and related mitochondrial cytopathies, acute disseminated encephalomyelitis or other types of encephalitis, vasculitis, cerebral infarction, and so on (1). Despite the fact that Mizuguchi et al. proposed the diagnostic criteria of ANE, atypical or milder cases have been reported. Yoshikawa et al. suggested clinical diversity in ANE. They experienced 2 atypical cases that had selective reversible thalamic involvement and milder clinical manifestations, and that ended up in complete recovery without any sequelae (4). Patients with normal cerebrospinal fluid protein and low levels of trasnsaminase, asymmetric thalamic involvement and no lesion in the brain stem tend to recover well (6). Considering the fact that ANE is a rare neurological condition, we experienced a variety of different cases. They met the diagnostic criteria proposed by Mizuguchi et al. (2).
ANE is known to be one of the neurological complications of viral infections, such as influenza A and B, human herpesvirus 6, varicella zoster or mycoplasmal infection (7-14). In addition, it is reportedly associated with the measles virus in an animal study (15). Serological tests or polymerase chain reaction for certain viruses, and mycoplasma were unremarkable in our study. As shown in Table 2, the subjects showed various results in laboratory findings. Interestingly CSF protein was within the normal range in all cases, but all had typical thalamic involvement on the images. In an attempt to exclude other resembling encephalopathies, Herpes IgM, oligoclonal band and myelin basic proteins in CSF were examined and shown unremarkable findings in most cases. Mutations of the mitochondrial DNA were screened to rule out mitochondrial encephalopathies in four out of subjects, which all came back negative (16, 17). Considering the fact of their similarity to mitochondrial disorders on brain MRI, we believe that mitochondrial dysfunction is partly related in this condition, whether primary or not.
As is already known, ANE is a relatively uncommon form of acute encephalopathy especially in East Asia, although European and American cases have been reported. It is thought to have some racial or geographic predilection (1, 6). In this study the HLA typing of 3 patients with ANE were evaluated to see genetic propensity. Genomic typing was performed on their HLA DRB/HLA DQB genes and HLA-DRB1*1401, HLA-DRB3*0202, and HLA-DQB1*0502 were found to be significant as compared with known data of HLA alleles in Korean (Table 3) (18). This is the first study using genomic typing of HLA DRB/HLA DQB genes that has never been done so far and the results may provide the immunogenetic background of ANE. However, further studies are needed to elucidate the condition.
A postmortem examination of the central nervous system could not be conducted, but bilateral thalamic necrosis is the histological hallmark of acute necrotizing encephalopathy. Selective vulnerability of the thalami seems to be a determining factor in acute necrotizing encephalopathy, because the affected areas are generally supplied by the terminal branches of the intracerebral arteries (3). It showed fresh necrosis, brain edema in the margin of the lesion, and petechiae and congestion (5, 19). A local breakdown of the blood-brain barrier and an immunogenetic mechanism were suggested as the pathogenesis of acute necrotizing encephalopathy (3). It has also been suggested that the ischemic and necrotic process from vasculitis or hypoxic injuries are related to the rapid deterioration in ANE (10, 20). Some cytokine-mediated processes may play a role in ANE (1, 2, 4, 10, 21). Patients with ANE often have signs of systemic inflammatory response such as shock, multiple organ failure, and disseminated intravascular coagulation. This indicates that macrophage activation and hypercytokinemia may be involved in the pathogenesis of ANE (22).
As illustrated in Fig. 1, almost all subjects showed radiologically pathognomonic findings of ANE. Based on previously limited experience, the MRI of brain seems to be a very important diagnostic tool in ANE. With respect to the treatment for ANE, anti-inflammatory treatment may be effective (22-24). As shown in Table 4, the result of this study indicates early methylprednisolone pulse therapy seems to be partly effective for the children with ANE. However, well designed, comparative, multi-center studies are still required to evaluate the effectiveness of the regimen.
Regarding the outcome in patients, younger than 2 yr of age, those with high serum aminotransferase level, high protein levels of cerebrospinal fluid, and those with brain stem lesions are thought to be poor prognostic factors (3-7, 10). In other reports, reversible or asymmetric brain involvement, focal neurologic signs showed a relatively fair prognosis (4, 8, 25). In this study, we experienced 6 patients with different manifestation of ANE. They ended up having relatively fair to good results except for two bad outcomes (patient 5, 6). As mentioned earlier, we believe that high dose steroid is fairly effective and can be a prognostic determinant when used in earlier stages of treatment. Findings on follow-up MRI seem to allow us to predict the outcome as shown in Table 4. In addition, it is confirmed that normal serum aminotransferase level, normal protein level of cerebrospinal fluid, and few neurological signs may be good prognostic factors for ANE.
In conclusion, ANE is an uncommon neurological complication of acute infection in children and is to be further elucidated in many aspects. However, early detection and appropriate treatment may lead to better neurological outcomes.
References
- 1.Mizuguchi M. Acute necrotizing encephalopathy in childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997;19:81–92. doi: 10.1016/s0387-7604(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 2.Mizuguchi M, Abe J, Mikkaichi K, Noma S, Yoshida K, Yamanaka T, Kamoshita S. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995;58:555–561. doi: 10.1136/jnnp.58.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Millan B, Teijeira S, Penin C, Garcia JL, Navarro C. Acute necrotizing encephalopathy of childhood: report of a spanish case. Pediatr Neurol. 2007;37:438–441. doi: 10.1016/j.pediatrneurol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Yoshikawa H, Watanabe T, Abe T, Oda Y. Clinical diversity in acute necrotizing encephalopathy. J Child Neurol. 1999;14:249–255. doi: 10.1177/088307389901400407. [DOI] [PubMed] [Google Scholar]
- 5.Yagishita A, Nakano I, Ushioda T, Otsuki N, Hasegawa A. Acute encephalopathy with bilateral thalamotegmental involvement in infants and children: imaging and pathology findings. Am J Neuroradiol. 1995;16:439–447. [PMC free article] [PubMed] [Google Scholar]
- 6.Mastroyianni S, Gionnis D, Voudris K, Skardoutsou A, Mizuguchi M. Acute necrotizing encephalopathy of childhood in non-Asian patients: report of three cases and literature review. J Child Neurol. 2006;21:872–879. doi: 10.1177/08830738060210101401. [DOI] [PubMed] [Google Scholar]
- 7.Olgar S, Ertugrul T, Nisli K, Aydin K, Caliskan M. Influenza A associated acute necrotizing encephalopathy. Neuropediatrics. 2006;37:166–168. doi: 10.1055/s-2006-924164. [DOI] [PubMed] [Google Scholar]
- 8.Okumura A, Kidokoro H, Mizuguchi M, Kurahashi H, Hirabayashi Y, Morishima T, Watanabe K. The mildest form of acute necrotizing encephalopathy associated with influenza A. Neuropediatrics. 2006;37:261–263. doi: 10.1055/s-2006-924431. [DOI] [PubMed] [Google Scholar]
- 9.Grose C. The puzzling picture of acute necrotizing encephalopathy after influenza A and B virus infection in young children. Pediatr Infect Dis J. 2004;23:253–254. doi: 10.1097/01.inf.0000114901.70040.33. [DOI] [PubMed] [Google Scholar]
- 10.Ohasaka M, Houkin K, Takigami M, Koyanagi I. Acute necrotizing encephalopathy associated with human herpesvirus-6 infection. Pediatr Neurol. 2006;34:160–163. doi: 10.1016/j.pediatrneurol.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Skelton BW, Hollingshead MC, Sledd AT, Phillips CD, Castillo M. Acute necrotizing encephalopathy of childhood: typical findings in an atypical disease. Pediatr Radiol. 2008;38:810–813. doi: 10.1007/s00247-008-0823-z. [DOI] [PubMed] [Google Scholar]
- 12.Tran TD, Kubota M, Takeshita K, Yanagisawa M, Sakakihara Y. Varicella-associated acute necrotizing encephalopathy with a good prognosis. Brain Dev. 2001;23:54–57. doi: 10.1016/s0387-7604(00)00199-6. [DOI] [PubMed] [Google Scholar]
- 13.Kirton A, Busche K, Ross C, Wirrell E. Acute necrotizing encephalopathy in Caucasian children: two cases and review of the literature. J Child Neurol. 2005;20:527–532. doi: 10.1177/088307380502000612. [DOI] [PubMed] [Google Scholar]
- 14.Ashtekar CS, Jaspan T, Thomas D, Weston V, Gayatri NA, Whitehouse WP. Acute bilateral thalamic necrosis in a child with Mycoplasma pneumonia. Dev Med Child Neurol. 2003;45:634–637. doi: 10.1017/s0012162203001154. [DOI] [PubMed] [Google Scholar]
- 15.Libert UG, Schneider-Schaulies S, Baczko K, ter Meulen V. Antibody-induced restriction of viral gene expression in measles encephalitis in rat. J Virol. 1990;64:706–713. doi: 10.1128/jvi.64.2.706-713.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chou HF, Liang WC, Zhang Q, Goto Y, Jong YJ. Clinical and genetic features in a MELAS child with a 3271T>C mutation. Pediatr Neurol. 2008;38:143–146. doi: 10.1016/j.pediatrneurol.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 17.Qi Y, Zhang Y, Wang Z, Yang Y, Yuan Y, Niu S, Pei P, Wang S, Ma Y, Bu D, Zou L, Fang F, Xiao J, Sun F, Zhang Y, Wu Y, Wang S, Xiong H, Wu X. Screening of common mitochondrial mutations in Chinese patients with mitochondrial encephalomyopathies. Mitochondrion. 2007;7:147–150. doi: 10.1016/j.mito.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Oh HH, Kwon SH, Kim CW, Choe BH, Ko CW, Jung HD, Suh JS, Lee JH. Moelcular anaysis of HLA Class II-associated susceptibility to neuroinflammatory disease in Korean children. J Korean Med Sci. 2004;19:426–430. doi: 10.3346/jkms.2004.19.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Kim IO, Lim MK, Park MS, Choi CG, Kim HW, Kim JE, Choi SJ, Koh YH, Yang DM, Choo SW, Chung MJ, Yoon HK, Goo HW, Lee M. Acute necrotizing encephalopathy in Korean infants and children: imaging findings and diverse clinical outcome. Korean J Radiol. 2004;5:171–177. doi: 10.3348/kjr.2004.5.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goo HW, Choi CG, Yoon CH, Ko TS. Acute necrotizing encephalopathy: diffusion MR imaging and localized proton MR spectroscopic findings in two infants. Korean J Radiol. 2003;4:61–65. doi: 10.3348/kjr.2003.4.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ichiyama T, Endo S, Kaneko M, Isumi H, Matsubara T, Furukawa S. Serum cytokine concentration of influenza-associated acute necrotizing encephalopathy. Pediatr Int. 2003;45:734–736. doi: 10.1111/j.1442-200x.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 22.Okumura A, Mizuguchi M, Kidokoro H, Tanaka M, Abe S, Hosoya M, Aiba H, Maegaki Y, Yamamoto H, Tanabe T, Noda E, Imataka G, Kurahashi H. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009;31:221–227. doi: 10.1016/j.braindev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Akiyoshi K, Hamada Y, Yamada H, Kojo M, Izumi T. Acute necrotizing encephalopathy associated with hemophagocytic syndrome. Pediatr Neurol. 2006;34:315–318. doi: 10.1016/j.pediatrneurol.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Manara R, Franzoi M, Cogo P, Battistella PA. Acute necrotizing encephalopathy: combined therapy and favorable outcome in a new case. Childs Nerv Syst. 2006;22:1231–1236. doi: 10.1007/s00381-006-0076-9. [DOI] [PubMed] [Google Scholar]
- 25.Wong AM, Simon EM, Zimmerman RA, Wang HS, Toh CH, Ng SH. Acute necrotizing encephalopathy of childhood: correlation of MR findings and clinical outcome. AJNR Am J Neuroradiol. 2006;27:1919–1923. [PMC free article] [PubMed] [Google Scholar]