Abstract
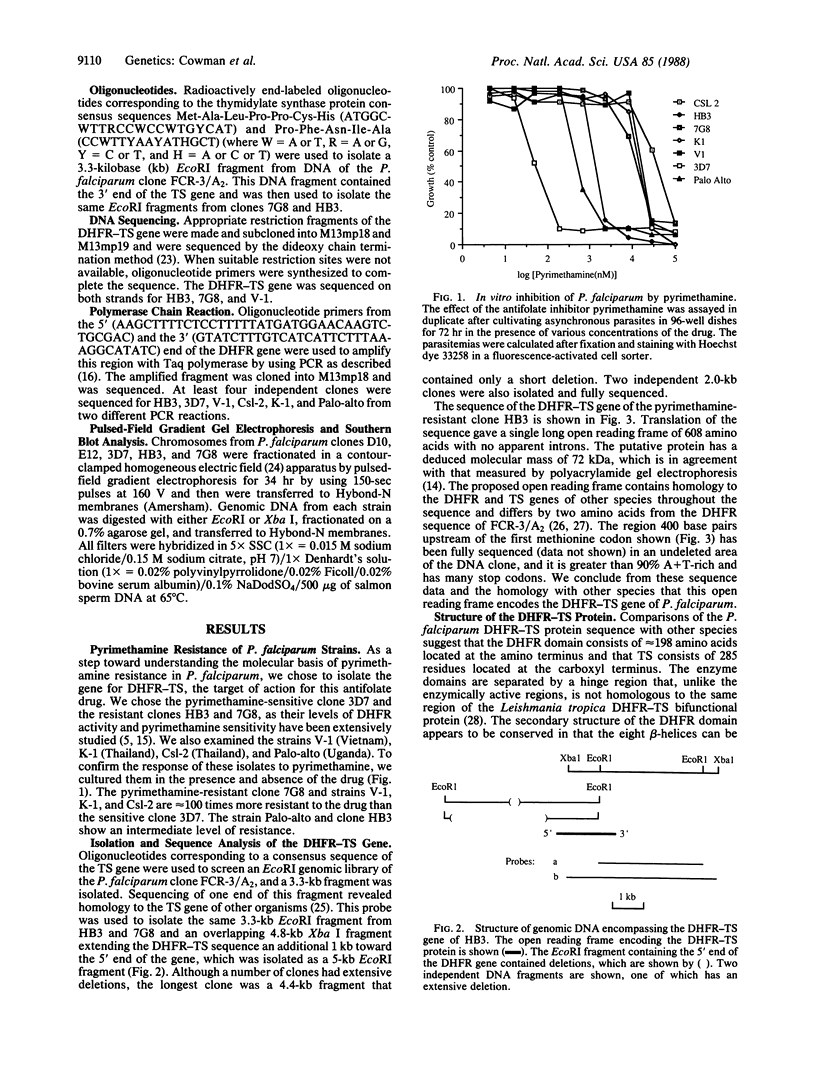
We describe the isolation and the sequence of the gene for the bifunctional enzyme dihydrofolate reductase-thymidylate synthase (DHFR-TS; EC 1.5.1.3 and EC 2.1.1.45, respectively) from two pyrimethamine-resistant clones of Plasmodium falciparum, HB3 and 7G8. We have also derived the sequence of the DHFR portion of the gene, by amplification using polymerase chain reaction, for the pyrimethamine-sensitive clone 3D7 and the pyrimethamine-resistant strains V-1, K-1, Csl-2, and Palo-alto. The deduced protein sequence of the resistant DHFR portion of the enzyme from HB3 contained a single amino acid difference from the pyrimethamine-sensitive clone 3D7. It is highly likely that this difference is involved in the mechanism of drug resistance in HB3. The sequence of the DHFR gene from other pyrimethamine-resistant strains contains the same amino acid difference from the sensitive clone 3D7. However, they all differ at one other site that may influence pyrimethamine resistance. The DHFR-TS gene is present as a single copy on chromosome 4 in all pyrimethamine-sensitive and pyrimethamine-resistant isolates tested. Therefore, the molecular basis of pyrimethamine resistance in the parasites tested is not amplification of the DHFR-TS gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anders R. F., Brown G. V., Edwards A. Characterization of an S antigen synthesized by several isolates of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6652–6656. doi: 10.1073/pnas.80.21.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyal H. S., Inselburg J. Plasmodium falciparum: induction, selection, and characterization of pyrimethamine-resistant mutants. Exp Parasitol. 1986 Aug;62(1):61–70. doi: 10.1016/0014-4894(86)90008-1. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Ellenberger T. E., Cordingley J. S. Primary structure of the gene encoding the bifunctional dihydrofolate reductase-thymidylate synthase of Leishmania major. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2584–2588. doi: 10.1073/pnas.83.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco A. E., Battye F. L., Brown G. V. Plasmodium falciparum: rapid quantification of parasitemia in fixed malaria cultures by flow cytometry. Exp Parasitol. 1986 Oct;62(2):275–282. doi: 10.1016/0014-4894(86)90032-9. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. X., Mueller C., Wendlinger M., Zolg J. W. Kinetic and molecular properties of the dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant clones of the human malaria parasite Plasmodium falciparum. Mol Pharmacol. 1987 Apr;31(4):430–437. [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppel R. L., Bianco A. E., Culvenor J. G., Crewther P. E., Brown G. V., Anders R. F., Kemp D. J. A cDNA clone expressing a rhoptry protein of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Aug;25(1):73–81. doi: 10.1016/0166-6851(87)90020-x. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Diggens S. M., Gutteridge W. E., Trigg P. I. Altered dihydrofolate reductase associated with a pyrimethamine-resistant Plasmodium berghei berghei produced in a single step. Nature. 1970 Nov 7;228(5271):579–580. doi: 10.1038/228579a0. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Reductions in methotrexate and folate influx in methotrexate-resistant lines of Leishmania major are independent of R or H region amplification. J Biol Chem. 1987 Oct 5;262(28):13501–13506. [PubMed] [Google Scholar]
- Ellis J., Irving D. O., Wellems T. E., Howard R. J., Cross G. A. Structure and expression of the knob-associated histidine-rich protein of Plasmodium falciparum. Mol Biochem Parasitol. 1987 Nov;26(1-2):203–214. doi: 10.1016/0166-6851(87)90144-7. [DOI] [PubMed] [Google Scholar]
- Ferone R., Burchall J. J., Hitchings G. H. Plasmodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol. 1969 Jan;5(1):49–59. [PubMed] [Google Scholar]
- Ferone R. Dihydrofolate reductase from pyrimethamine-resistant Plasmodium berghei. J Biol Chem. 1970 Feb 25;245(4):850–854. [PubMed] [Google Scholar]
- Ferone R., Roland S. Dihydrofolate reductase: thymidylate synthase, a bifunctional polypeptide from Crithidia fasciculata. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5802–5806. doi: 10.1073/pnas.77.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett C. E., Coderre J. A., Meek T. D., Garvey E. P., Claman D. M., Beverley S. M., Santi D. V. A bifunctional thymidylate synthetase-dihydrofolate reductase in protozoa. Mol Biochem Parasitol. 1984 Apr;11:257–265. doi: 10.1016/0166-6851(84)90070-7. [DOI] [PubMed] [Google Scholar]
- Green T. J., Gadsden G., Seed T., Jacobs R., Morhardt M., Brackett R. Cloning and characterization of Plasmodium falciparum FCR-3/FMG strain. Am J Trop Med Hyg. 1985 Jan;34(1):24–30. doi: 10.4269/ajtmh.1985.34.24. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Bzik D. J., Horii T. Pyrimethamine resistant Plasmodium falciparum: overproduction of dihydrofolate reductase by a gene duplication. Mol Biochem Parasitol. 1987 Nov;26(1-2):121–134. doi: 10.1016/0166-6851(87)90136-8. [DOI] [PubMed] [Google Scholar]
- Kan S. C., Siddiqui W. A. Comparative studies on dihydrofolate reductases from Plasmodium falciparum and Aotus trivirgatus. J Protozool. 1979 Nov;26(4):660–664. doi: 10.1111/j.1550-7408.1979.tb04216.x. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Thompson J. K., Walliker D., Corcoran L. M. Molecular karyotype of Plasmodium falciparum: conserved linkage groups and expendable histidine-rich protein genes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7672–7676. doi: 10.1073/pnas.84.21.7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Studies on identifying the folylpolyglutamate binding sites of Lactobacillus casei thymidylate synthetase. Arch Biochem Biophys. 1982 Jul;216(2):551–558. doi: 10.1016/0003-9861(82)90244-2. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Welsh J. A., Dame J. B., Quakyi I. A., Graves P. M., Drake J. C., Allegra C. J. Mechanism of pyrimethamine resistance in recent isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 1984 Nov;26(5):656–659. doi: 10.1128/aac.26.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Walliker D., Wellems T. E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirawaraporn W., Yuthavong Y. Kinetic and molecular properties of dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant Plasmodium chabaudi. Mol Biochem Parasitol. 1984 Mar;10(3):355–367. doi: 10.1016/0166-6851(84)90033-1. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Kaneda S., Ayusawa D., Shimizu K., Gotoh O., Seno T. Nucleotide sequence of a functional cDNA for human thymidylate synthase. Nucleic Acids Res. 1985 Mar 25;13(6):2035–2043. doi: 10.1093/nar/13.6.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Walter R. D. Altered dihydrofolate reductase in pyrimethamine-resistant Plasmodium falciparum. Mol Biochem Parasitol. 1986 Apr;19(1):61–66. doi: 10.1016/0166-6851(86)90066-6. [DOI] [PubMed] [Google Scholar]