Abstract
The aim of this study was to analyze the relationship between serum pro-hepcidin concentration and the anemia profiles of rheumatoid arthritis (RA) and to estimate the pro-hepcidin could reflect the disease activity of RA. RA disease activities were measured using Disease Activity Score 28 (DAS28), tender/swollen joint counts, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). Anemia profiles such as hemoglobin, iron, total iron binding capacity (TIBC), ferritin, and transferrin levels were measured. Serum concentration of pro-hepcidin, the prohormone of hepcidin, was measured using enzyme-linked immunosorbent assay (ELISA). Mean concentration of serum pro-hepcidin was 237.6±67.9 ng/mL in 40 RA patients. The pro-hepcidin concentration was correlated with rheumatoid factor, CRP, ESR, and DAS28. There was a significant correlation between pro-hepcidin with tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6. The pro-hepcidin concentration was significantly higher in the patients with active RA (DAS28>5.1) than those with inactive to moderate RA (DAS28≤5.1). However, the pro-hepcidin concentration did not correlate with the anemia profiles except hemoglobin level. There was no difference of pro-hepcidin concentration between the patients with anemia of chronic disease and those without. In conclusion, serum concentration of pro-hepcidin reflects the disease activity, regardless of the anemia states in RA patients, thus it may be another potential marker for disease activity of RA.
Keywords: Arthritis, Rheumatoid; Anemia; Hepcidin; Prohepcidin
INTRODUCTION
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease mainly affected the peripheral joints, but extra-articular systems are often affected. Anemia is one of the common extra-articular manifestations in RA. The prevalence of anemia is 26.9%-77.6% in patients with RA (1). The leading cause of the anemia in RA is chronic inflammation, with the pattern of a normochromic and normocytic anemia. It is associated with decreased serum iron and total iron-binding capacity (TIBC), but iron store is increased or normal. Iron deficiency anemia due to long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) and folate deficiency due to methotrexate therapy also result in the anemia in RA (2). Hemolytic anemia is a rare cause of anemia in RA.
There are possible mechanisms in the anemia of chronic inflammatory diseases in RA. First, inflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and interferon-γ, induce inadequate erythropoiesis in bone marrow, inhibiting erythroid progenitor differentiation and driving the apoptosis of erythroid progenitors. Second, the inflammatory cytokines mediate the inhibition of peripheral iron utilization. Third, the erythropoietin production in response to anemia is relatively impaired. Forth, red cell survival is slightly reduced in RA patients (2, 3). In general, a degree of anemia correlates with RA disease activity and serum TNF-α concentration (4). The patients with anemia are likely to have more severe joint diseases than those without anemia in RA.
Hepcidin, a 25 amino acid peptide hormone, regulates intestinal iron absorption, recycling, and tissue storage (5). Beyond the iron regulation, it is closely linked with inflammation and infection (5, 6). The inflammatory signal, chiefly IL-6, induces hepcidin synthesis, then the upregulated hepcidin decreases duodenal iron absorption and increases sequestration of iron by macrophage, resulting in hypoferremia (7). This is another possible mechanism of anemia of chronic inflammatory disease such as RA and systemic lupus erythematosus (SLE). This is supported by following two evidences. First, hepcidin mRNA level in adipose tissue correlates with IL-6 and C-reactive protein (CRP) (8). Second, when IL-6 is infused to healthy individuals, the hepcidin level increases, and serum iron and transferrin saturation decrease (7).
The aim of this study is to analyze the relationship between serum pro-hepcidin, a prohormone of hepcidin, concentration and the anemia profiles of RA and to determine whether the pro-hepcidin could reflect RA disease activity.
MATERIALS AND METHODS
Patients
Forty patients with RA were enrolled in this study and the patients all met the American College of Rheumatology 1987 revised criteria for the classification of RA (9). Informed consents were obtained from the patients before the study, and this study was approved by the Ethical Committees in Konkuk Medical Center (KUH1010061).
Disease activity evaluation in RA patients
When the sera were obtained, clinical assessments, such as tender joint counts, swollen joint counts, and 100 mm-visual analogue scale (VAS) were performed by one rheumatologist. Erythrocyte sedimentation rate (ESR), CRP, the titer of IgM rheumatoid factors (RF) and the titer of antibodies to cyclic citrullinated peptide (anti-CCP Ab) were measured. From these clinical and laboratory data, Disease Activity Score 28 (DAS28) was calculated.
Laboratory study for anemia
Laboratory analysis for anemia, such as complete blood count (CBC), hemoglobin (Hb), hematocrit, serum iron, TIBC, ferritin, and transferrin levels, are measured using standard laboratory methods. Anemia was defined as Hb <13 g/dL in male and <12 g/dL in female. Anemia of chronic disease was defined as normo- to microcytic anemia with decreased serum iron concentrations, normal to decreased transferrin levels and normal or increased serum ferritin levels (10). Patients whose anemia did not result from anemia of chronic disease were excluded in this study.
Serum concentration of pro-hepcidin and proinflammatory cytokines (TNF-α, IL-1β, and IL-6)
Serum pro-hepcidin concentration was measured using the DRG® Hepcidin Prohormone Enzyme Immunoassay Kit (DRG Instruments, Marburg, Germany), according to the manufacturer's instructions. Pro-hepcidin is the 84 amino acid precursor of the active hepcidin peptide.
TNF-α, IL-6, and IL-1β in serum were measured by sandwich enzyme-linked immunosorbent assay (ELISA), as previously described (11). Briefly, 4 µg/mL of monoclonal antibodies to human TNF-α, IL-6, and IL-1β (R&D Systems, Minneapolis, MN, USA) was added to a 96-well plate (Nunc, Denmark) and incubated overnight at 4℃. After incubating the plate with a blocking solution consisting of phosphate buffered saline (PBS) containing 1% bovine serum albumin and 0.05% Tween 20 for 2 hr at room temperature, test samples and the standard recombinant cytokines (R&D Systems) were added to the 96-well plate and the plate incubated at room temperature for 2 hr. After washing four times with PBS containing Tween 20, 200 ng/mL of biotinylated monoclonal antibodies to human cytokines (R&D Systems) were added and the reaction was allowed to proceed for 2 hr at room temperature. After washing, 2000-fold diluted streptavidin-alkaline-phosphatase (Sigma Bioscience, St. Louis, MO, USA) was added, and the reaction was allowed to proceed for a further 2 hr. After the plates were washed four times, 1 mg/mL of p-nitrophenylphosphate (Sigma) dissolved in diethanolamine (Sigma) was added to induce the color reaction, which was stopped by adding 1N NaOH. An automated microplate reader (Vmax, Molecular Devices, Palo Alto, CA, USA) set at 405 nm was used to measure the optical density. The sensitivity limit was 15.6 pg/mL for TNF-α, IL-6, and IL-1β. Recombinant human cytokines diluted in culture medium over a concentration range of 10-2,000 pg/mL were used as calibration standards. A standard curve was drawn by plotting optical density versus the log of the concentration of recombinant cytokines.
Statistical analysis
Data are expressed as the mean±standard deviation (SD). Statistical analysis was performed using Student's t test for independent samples. Correlation coefficients were determined by Pearson's correlation test. P values less than 0.05 were considered significant.
RESULTS
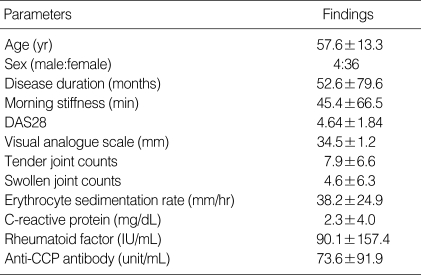
The clinical characteristics of the 40 RA patients were as follows: age 57.6±13.3 yr, disease duration 52.6±79.6 months, duration of morning stiffness 45.4±66.5 min, and DAS28 4.64±18.4. RF was positive (>18 IU/mL) in 67.5% of the patients. 75% of the patients had positive anti-CCP Ab (>20 Unit/mL). Table 1 shows the additional clinical data of the RA patients included in the study.
Table 1.
Clinical characteristics of the 40 patients with rheumatoid arthritis
Data were expressed by mean±SD.
DAS28, Disease Activity Score 28; Anti-CCP antibody, anti-cyclic citrullinated peptide antibody.
The laboratory profiles relative to anemia were as follows; Hb 11.9±1.5 g/dL, serum iron 63.9±35.9 µg/dL, TIBC 291.6±43.9 µg/dL, ferritin 116.4±168.6 ng/mL, and transferrin 235.3±37.9 ng/mL. When the patients were divided into anemic and non-anemic groups, the patients with anemia of chronic disease had lower serum iron (78.9±39.9 vs. 47.4±20.7 µg/dL, P<0.001), higher ESR (32.2±23.9 vs. 44.8±24.3 mm/hr, P=0.02), and higher CRP (1.3±1.8 vs. 3.4±5.3 mg/dL, P=0.03), than the patients without anemia. Other clinical and laboratory parameters (disease duration, morning stiffness, 100 mm VAS, DAS28, tender/swollen joint counts), RF and anti-CCP Ab titers were not different between anemic and non-anemic groups.
In 40 RA patients, the mean concentration of serum prohepcidin was 237.6±67.9 ng/mL. Serum pro-hepcidin concentration negatively correlated with serum iron (r=-0.23, P=0.04), but it did not correlate with TIBC, ferritin and transferrin. There was no difference of pro-hepcidin concentration between the patients with anemic and non-anemic groups.
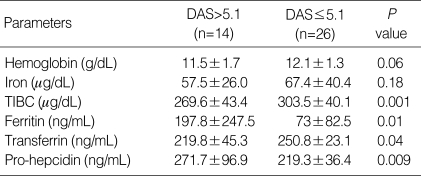
Next, we determined the relationship between serum prohepcidin concentration and RA disease activity parameters. The pro-hepcidin concentration correlated with DAS28 (r=0.4, P<0.001), tender joint count (r=0.3, P=0.003), RF titer (r=0.51, P<0.001), ESR (r=0.53, P<0.001), and CRP (r=0.22, P=0.05). When the patients were divided into two groups using DAS28 score: patients with active RA (DAS28 >5.1, n=14) and those with inactive to moderate RA (DAS28 ≤5.1, n=26), the serum pro-hepcidin concentration was higher in patients with active disease than in those inactive to moderate disease (271.7±95.7 ng/mL vs. 219.3±37.6 ng/mL; P=0.01, Table 2). There is no difference of pro-hepcidin concentration in the groups with inactive and moderate diseases. However, the pro-hepcidin concentration was not dependent of current medication including steroid, disease modifying anti-rheumatic drugs (DMARDs), and biologics.
Table 2.
Laboratory parameters relative to anemia and the serum concentrations of pro-hepcidin in the 14 patients with active RA (DAS>5.1) and the 26 patients with inactive to moderate RA (DAS≤5.1)
Data were expressed by mean±SD.
TIBC, total iron binding capacity; DAS, Disease Activity Score.
Finally, we analyzed the relationship between pro-hepcidin with proinflammatory cytokines such as TNF-α, IL-1β, and IL-6. Mean concentrations of serum TNF-α, IL-1β, and IL-6 were 197.8±22.6 pg/mL, 252.5±5.8 pg/mL, and 262.3±17.3 pg/mL, respectively. There was significant correlation between the serum concentration of pro-hepcidin with the serum concentrations of TNF-α (r=0.58, P<0.001), IL-1β (r=0.59, P<0.001), and IL-6 (r=0.4, P=0.01).
DISCUSSION
Hepcidin synthesis is regulated by extrinsic or intrinsic iron loading, anemia, and hypoxemia. Moreover, the hepcidin production is induced by a particular cytokine, IL-6, during inflammation (7). In a previous study, IL-6 is induced within 3 hr after injection of lipopolysaccharide (LPS) and urinary hepcidin peaks within 6 hr, followed by decrease in serum iron (12). IL-6 is one of the proinflammatory cytokines, which has central role in the pathogenesis of RA, a representative inflammatory disease. IL-6 induces acute phase response, and differentiates B and T cells, and promotes joint destruction in RA (13-15). Serum levels of IL-6 are correlated with RA disease activity and clinical symptoms. Because anemia is a common extra-articular manifestation of RA, we hypothesized IL-6-hepcidin could be the possible link in RA to the anemia.
A recent study showed that RA patients have higher serum concentration of pro-hepcidin than patients with SLE and healthy volunteers, but the pro-hepcidin concentration doesn't correlate with RA disease activity scores, TNF-α, or IL-6 (14). However, in our study, serum pro-hepcidin concentration correlated with RA disease activity parameters such as DAS28, tender joint count, ESR and CRP. The serum concentration of pro-hepcidin also correlated with proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6. In the previous study, serum concentrations of TNF-α, IL-1β, and IL-6 are correlated with RA disease activity indexes, such as DAS28, ESR, and CRP (16-18). The roles of these proinflammatory cytokines in the RA pathogenesis are well known and the inhibitors of each cytokines are used universally as the therapy for active RA. Correlation of pro-hepcidin with proinflammatory cytokines suggests that pro-hepcidin was induced by the proinflammatory cytokines, especially IL-6. Moreover, when the patients were divided two groups by disease activities, the RA patients with active disease had higher concentration of serum pro-hepcidin than those with inactive to moderate disease. This result suggested that serum pro-hepcidin concentration could be another useful marker for RA disease activity as acute phase reactants, active joint counts or proinflammatory cytokines. Our results showed different results from the previous study in RA (14), it is plausible that the patients in our study were older Asian with more active RA than the patients who were enrolled in the previous study (mean age: 57.6 vs. 46.4 yr, and mean DAS28: 4.64 vs. 3.59). These differences of characteristics of patients could be the cause of different results. Further study should be warranted to elucidate this contradictory result.
On the other hand, pro-hepcidin did not correlate anemiazzzz profiles, except for negative correlation with serum iron concentration. It was postulated that serum pro-hepcidin could not represent the bioactive hepcidin (12), thus serum pro-hepcidin concentration did not reflect actual anemic parameters, as supported by a previous studies (19, 20). After injection of LPS, serum pro-hepcidin levels don't change significantly, although pro-inflammatory cytokine induction, urinary hepcidin excretion, and serum iron are decreased (12). No difference of serum pro-hepcidin between the RA patients with or without anemia may be explained that serum pro-hepcidin could not reflect the iron state exactly in human disease setting. Another explanation is that hepcidin is overproduced by the inflammatory response in RA, and the over-expressed hepcidin might disturb the normal physiologic response in the iron metabolism. Many arguments on the correlation between pro-hepidin and anemia indicate that measurement of serum pro-hepcidin could not substitute bioactive hepcidin. Therefore, available method for measurement of hepdicin is needed to reveal accurate relationship of inflammatory diseases and anemia. We postulate that there is different clinical significance between hepcidin and pro-hepcidin, the former might regulate iron metabolism and the latter might regulate inflammatory process. More research is needed to serial measurement of serum pro-hepcidin in same patients as the disease activity changes.
In conclusion, proinflammatory cytokines play a major role in the development of anemia of RA, as they inhibit erythropoiesis, and hepcidin might involve in minor mechanism of anemia in the inflammatory disease, as it regulates iron metabolism inappropriately. Hepcidin or pro-hepcidin might play an important role in RA, as the mediator linking anemia and the inflammatory cytokines. Pro-hepcidin could be another useful marker for RA disease activity.
Footnotes
This work was supported by Konkuk University in 2006.
References
- 1.Wilson A, Yu HT, Goodnough LT, Nissenson AR. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):50S–57S. doi: 10.1016/j.amjmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Bowman SJ. Hematological manifestations of rheumatoid arthritis. Scand J Rheumatol. 2002;31:251–259. doi: 10.1080/030097402760375124. [DOI] [PubMed] [Google Scholar]
- 3.Bertero MT, Caligaris-Cappio F. Anemia of chronic disorders in systemic autoimmune diseases. Haematologica. 1997;82:375–381. [PubMed] [Google Scholar]
- 4.Vreugdenhil G, Lowenberg B, Van Eijk HG, Swaak AJ. Tumor necrosis factor alpha is associated with disease activity and the degree of anemia in patients with rheumatoid arthritis. Eur J Clin Invest. 1992;22:488–493. doi: 10.1111/j.1365-2362.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 5.Ganz T. Hepcidin--a regulator of intestinal iron absorption and iron recycling by macrophages. Best Pract Res Clin Haematol. 2005;18:171–182. doi: 10.1016/j.beha.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–788. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 7.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bekri S, Gual P, Anty R, Luciani N, Dahman M, Ramesh B, Iannelli A, Staccini-Myx A, Casanova D, Ben Amor I, Saint-Paul MC, Huet PM, Sadoul JL, Gugenheim J, Srai SK, Tran A, Le Marchand-Brustel Y. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131:788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 10.Weiss G. Pathogenesis and treatment of anaemia of chronic disease. Blood Rev. 2002;16:87–96. doi: 10.1054/blre.2002.0193. [DOI] [PubMed] [Google Scholar]
- 11.Asturias JA, Arilla MC, Aguirre M, Gomez-Bayon N, Martinez A, Palacios R, Sanchez-Gascon F, Martinez J. Quantification of profilins by a monoclonal antibody-based sandwich ELISA. J Immunol Methods. 1999;229:61–71. doi: 10.1016/s0022-1759(99)00115-5. [DOI] [PubMed] [Google Scholar]
- 12.Kemna E, Pickkers P, Nemeth E, van der Hoeven H, Swinkels D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood. 2005;106:1864–1866. doi: 10.1182/blood-2005-03-1159. [DOI] [PubMed] [Google Scholar]
- 13.Cronstein BN. Interleukin-6--a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- 14.Koca SS, Isik A, Ustundag B, Metin K, Aksoy K. Serum pro-hepcidin levels in rheumatoid arthritis and systemic lupus erythematosus. Inflammation. 2008;31:146–153. doi: 10.1007/s10753-008-9060-8. [DOI] [PubMed] [Google Scholar]
- 15.Raj DS. Role of interleukin-6 in the anemia of chronic disease. Semin Arthritis Rheum. 2009;38:382–388. doi: 10.1016/j.semarthrit.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Petrovic-Rackov L, Pejnovic N. Clinical significance of IL-18, IL-15, IL-12 and TNF-alpha measurement in rheumatoid arthritis. Clin Rheumatol. 2006;25:448–452. doi: 10.1007/s10067-005-0106-0. [DOI] [PubMed] [Google Scholar]
- 17.Senturk T, Kinikli G, Turgay M, Tutkak H, Duman M, Tokgoz G. Evaluation of interleukin-6 in rheumatoid arthritis as an activity criterion. Rheumatol Int. 1996;16:141–144. doi: 10.1007/BF01419726. [DOI] [PubMed] [Google Scholar]
- 18.Altomonte L, Zoli A, Mirone L, Scolieri P, Magaro M. Serum levels of interleukin-1b, tumour necrosis factor-a and interleukin-2 in rheumatoid arthritis. Correlation with disease activity. Clin Rheumatol. 1992;11:202–205. doi: 10.1007/BF02207957. [DOI] [PubMed] [Google Scholar]
- 19.Kulaksiz H, Gehrke SG, Janetzko A, Rost D, Bruckner T, Kallinowski B, Stremmel W. Pro-hepcidin: expression and cell specific localisation in the liver and its regulation in hereditary haemochromatosis, chronic renal insufficiency, and renal anaemia. Gut. 2004;53:735–743. doi: 10.1136/gut.2003.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadley KB, Johnson LK, Hunt JR. Iron absorption by healthy women is not associated with either serum or urinary prohepcidin. Am J Clin Nutr. 2006;84:150–155. doi: 10.1093/ajcn/84.1.150. [DOI] [PubMed] [Google Scholar]