Abstract
We examined whether the presence and severity of preoperative hydronephrosis have prognostic significance in patients who underwent radical cystectomy for transitional cell carcinoma of the bladder. The medical records of 457 patients who underwent radical cystectomy for bladder cancer between 1986 and 2005 were retrospectively reviewed. Following the Society for Fetal Urology grading system, patients were divided into low-, and high-grade hydronephrosis groups. Clinicopathologic factors associated with preoperative hydronephrosis and survival were evaluated. Of a total of 406 patients, unilateral hydronephrosis was found in 74 (18.2%), bilateral hydronephrosis in 11 (2.7%), and no hydronephoris in 321 (79.1%). Low-grade hydronephrosis was found in 57 (12.2%) patients and high-grade hydronephrosis in 28 (6%). Preoperative hydronephrosis was related to higher pT stage and lymph node invasion. In univariate analysis, the presence of hydronephrosis, hydronephrosis grade, age, pT and pN stage, tumor grade, surgical margin, number of retrieved nodes, carcinoma in situ, and lymphovascular invasion were significant prognostic factors for cancer-specific survival. In multivariate analysis, bilateral hydronephrosis and high-grade hydronephrosis remained significant predictors for decreased survival. The presence of preoperative hydronephrosis, and high-grade hydronephrosis are significant prognostic factors in patients with bladder cancer after radical cystectomy.
Keywords: Hydronephrosis, Prognosis, Urinary Bladder Neoplasms, Cystectomy
INTRODUCTION
The incidence of preoperative hydronephrosis in patients with bladder cancer ranges from 7.2 to 54.1% (1, 2). Although pathologic stage and lymph node (LN) status are known to be the most important prognostic factors, the prognostic significance of preoperative hydronephrosis after radical cystectomy has been investigated in other studies with conflicting results (3-7). Some researchers have shown that preoperative hydronephrosis is related to poor survival while others have suggested that it is not a prognostic factor in multivariate analysis. To our knowledge, the relationship between the severity of preoperative hydronephrosis and prognosis after radical cystectomy has not yet been evaluated. Therefore, in this study, we investigated whether the presence and severity of preoperative hydronephrosis have prognostic significance in patients who underwent radical cystectomy for transitional cell carcinoma (TCC) of the bladder.
MATERIALS AND METHODS
We performed a retrospective review of 457 patients who underwent radical cystectomy and urinary diversion for bladder cancer at our institution between 1986 and 2005. Fifth-one patients with non-TCC pathology, upper urinary tract carcinoma, obstructing stones, neoadjuvant chemotherapy or radiotherapy, incomplete medical records were excluded.
Preoperative hydronephrosis was defined as dilation of the ureter and renal pelvis with or without secondary changes in the renal parenchyma, diagnosed by computed tomography (CT) or renal ultrasound. All CT and ultrasound images were reviewed by a uroradiologist. Based on the Society for Fetal Urology (SFU) grading system (8), the patients without calyx or pelvic dilation were classified as grade 0, pelvic dilation only as grade 1, and the tumors accompanying mild calyx dilation as grade 2. Patients with severe calyx dilation were classified as grade 3, and tumors with calyx dilation accompanied by renal parenchymal atrophy as grade 4. Then, cases were divided into low- and high-grade hydronephrosis groups. The low-grade hydronephrosis group included patients with SFU grade 1 or 2 hydronephrosis, and the high-grade hydronephrosis group included those with SFU grade 3 or 4 hydronephrosis.
All patients had a complete preoperative evaluation including physical examination, complete blood count, serum creatinine, electrolyte analysis, chest radiography, abdominal ultrasound, CT and bone scan. All patients underwent a bilateral pelvic lymphadenectomy to the bifurcation of the common iliac vessels.
Pathologic T and N stage, margin status, tumor grade, carcinoma in situ (CIS), lymphovascular invasion (LVI), and squamous differentiation were recorded. Pathologic staging was done according to the 2002 tumor node metastasis (TNM) classification. During the median follow-up duration of 66.3 months (range, 3-232 months), patients were examined every 3 months for the first 2 yr, and 6-months intervals for 5 yr, and annually thereafter. Clinicopathologic factors associated with preoperative hydronephrosis were evaluated by chi-square test. Cancer-specific survival curves were calculated by Kaplan-Meier method. Univariate and multivariate analysis were performed using log rank test and Cox proportional hazards model. Statistical analysis was performed using SPSS version 12.0 and significance was defined as P<0.05.
RESULTS
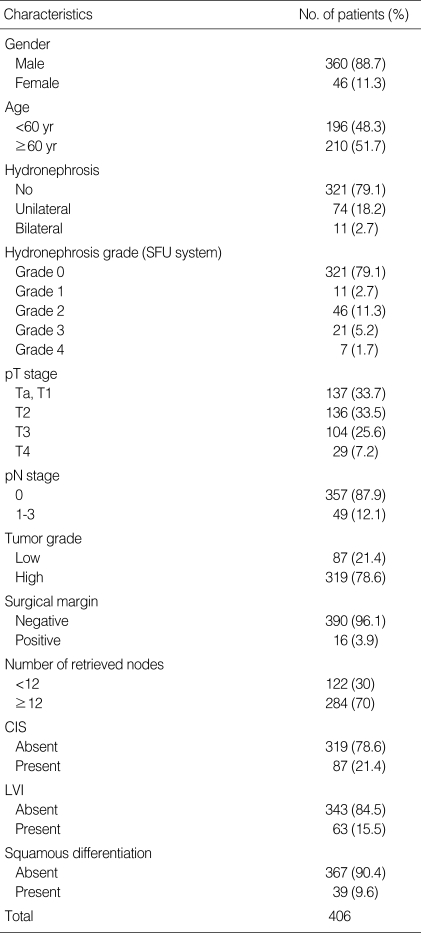
The study group was composed of 360 men and 46 women and the median age was 60.8 yr (range, 27-79 yr). Of a total of 406 patients, unilateral hydronephrosis was found in 74 (18.2%), bilateral hydronephrosis in 11 (2.7%), and no hydronephrosis in 321 (79.1%). Low-grade hydronephrosis was found in 57 (12.2%) patients and high-grade hydronephrosis in 28 (6%). Clinicopathologic characteristics of all patients are shown in Table 1.
Table 1.
Characteristics of 406 patients
SFU, Society of Fetal Urology; pT, pathologic T; pN, pathologic N; CIS, carcinoma in situ; LVI, lymphovascular invasion.
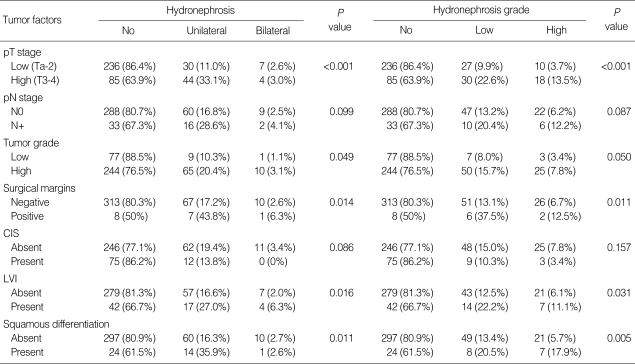
Chi-square test was performed to determine whether there was a correlation between the presence and severity of preoperative hydronephrosis and clinicopathologic factors. The presence of preoperative hydronephrosis was related to higher pT stage (≥T3), LN invasion, positive surgical margin, higher tumor grade, presence of LVI, and squamous differentiation. The severity of preoperative hydronephrosis was related to higher pT stage (≥T3), LN invasion, positive surgical margin, presence of LVI, and squamous differentiation (Table 2).
Table 2.
Correlation between clinicopathologic factors and preoperative hydronephrosis
pT, pathologic T; pN, pathologic N; CIS, carcinoma in situ; LVI, lymphovascular invasion.
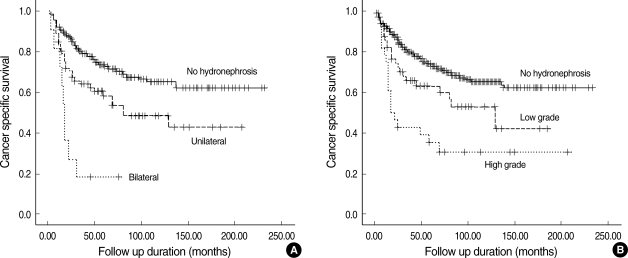
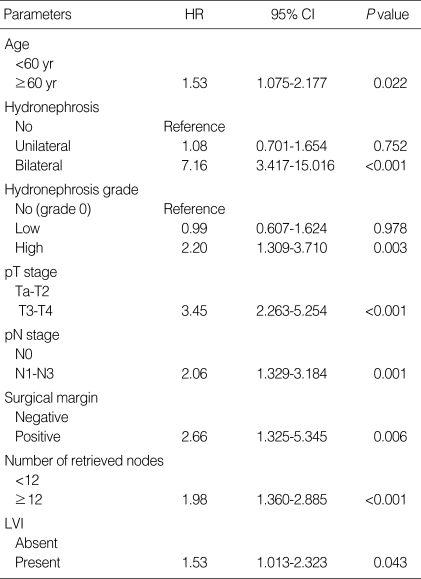
The mean cancer-specific survival of all patients was 49.5 months (range, 3-232 months). The 3- and 5-yr cancer-specific survival rates, estimated by the Kaplan-Meier method, were 75.5% and 69.3%, respectively. When the study population was divided into 3 groups based on the hydronephrosis status, patients without preoperative hydronephrosis showed a better median cancer-specific survival (52 months; range, 3-232 months) compared to those with unilateral hydroneprhosis (42 months; range, 3-207 months; P=0.002) or bilateral hydronephrosis (17 months; range, 3-76 months; P<0.001) by log rank test (Fig. 1A). When the patients were placed into 3 groups based on hydronephrosis grade, patients without preoperative hydronephrosis showed a better median cancer-specific survival (52 months; range, 3-232 months) compared to those with low-grade hydronephrosis (41 months; range, 3-184 months; P=0.021) or high-grade hydronephrosis (20 months; range, 3-232 months; P<0.001) by log rank test (Fig. 1B). In univariate analysis of other clinicopathologic variables, patient age (<60 yr vs. ≥60 yr; P=0.048), T stage (Ta-2 vs. T3-4; P<0.001), N stage (N0 vs. N1-3; P<0.001), tumor grade (low vs. high, P=0.002), surgical margin positivity (P=0.001), number of retrieved nodes (<12 vs. ≥12; P<0.001), CIS (P=0.025), and LVI (P<0.001) were significant prognostic factors for cancer-specific survival after radical cystectomy. In multivariate analysis performed using a Cox proportional hazards regression model, bilateral hydronephrosis (hazard ratio [HR] 7.16; P<0.001) as well as high grade hydronephrosis (HR 2.20; P=0.003) remained significant predictors for decreased survival when controlled for age, pathologic T (pT) and pathologic N (pN) stage, surgical margin positivity, number of retrieved nodes, and LVI (Table 3).
Fig. 1.
Cancer-specific survival curves (A) for patients without hydronephrosis, with unilateral hydronephrosis, and with bilateral hydronephrosis and (B) for patients without hydronephrosis, with low-grade hydronephrosis, and with high-grade hydronephrosis.
Table 3.
Multivariate analysis of cancer-specific survival
HR, hazard ratio; CI, confidence interval; pT, pathologic T; pN, pathologic N; LVI, lymphovascular invasion.
We also analyzed 269 patients with muscle invasive bladder cancer excluding patients with non-muscle invasive bladder cancer (pTa-1), and compared between organ confined disease (T2) and non-organ confined disease (≥T3). The mean cancer-specific survival of 269 patients with muscle invasive bladder cancer was 55.03 months (range, 3-227 months). The presence and severity of preoperative hydronephrosis was associated with non-organ confined disease. When the study population was divided into 3 groups based on the hydronephrosis status, patients with bilateral hydronephrosis showed a poorer median cancer-specific survival (16 months) compared to those with no hydronephrosis (41 months; P<0.001) or unilateral hydronephrosis (37.5 months; P=0.007) by log rank test. But there was no difference in cancer-specific survival between no hydronephrosis and unilateral hydronephrosis groups (P=0.322). When the patients were splitted into 3 groups based on hydronephrosis grade, patients with high-grade hydronephrosis showed a poorer median cancer-specific survival (18 months) compared to those with no hydronephrosis (41 months; P=0.003) or low-grade hydronephrosis (37.5 months; P=0.048) by log rank test. There was no difference in cancer-specific survival between no hydronephrosis and high-grade hydronephrosis groups (P=0.625). In multivariate analysis, bilateral hydronephrosis (HR 4.84; P<0.001) as well as high grade hydronephrosis (HR 1.78; P=0.030) were significant predictors for decreased survival when controlled for pT and pN stage, surgical margin positivity, number of retrieved nodes, and LVI.
DISCUSSION
In addition to TNM stage, known as the most important prognostic factor in patients who underwent radical cystectomy for bladder TCC, clinicopathologic factors such as patient age, LVI, and CIS have been reported as significant predictors (9, 10). However, many studies have presented different results regarding the significance of preoperative hydronephrosis. Haleblian et al. (3) analyzed 415 patients and found that bilateral preoperative hydronephrosis has a significant relation to increased stage and overall survival. Leibovitch et al. (1) studied 122 cases and reported that patients without preoperative hydronephrosis had better cancer-specific survival compared to those with preoperative hydronephrosis. They suggested that hydronephrotic patients with ureteral orifice involvement of bladder cancer on cystoscopic finding had a tendency to show low-stage disease compared to hydronephrotic patients without ureter orifice involvement. Bartsch et al. (4) reported the similar findings, showing that hydronephrotic patients without tumors at the orifice on cystoscopy have a 70% risk of having non-organ confined disease and a 40% risk of having LN metastasis compared to those with tumors at the ureteral orifice, who have significantly lower risks at 41.7% and 19.4%, respectively. They also reported that preoperative hydronephrosis was an independent prognostic factor for recurrence-free survival. Yang et al. (11) analyzed 310 patients and identified preoperative hydronephrosis as an independent predictor for cancer-specific survival in univariate analysis, but significance was not found in multivariate analysis. Canter et al. (12) showed that bilateral hydronephrosis was found to have a HR of 3.87 and 2.75 for disease-specific survival and overall survival, respectively on multivariate analysis.
This study included 406 patients with urothelial carcinoma of the urinary bladder and focused on the presence and grade of preoperative hydronephrosis as prognostic factors for cancer-specific survival. To our knowledge, this is the first investigation to stratify hydronephrotic patients according to the hydronephrosis grade and analyze the prognostic significance of the hydronephrosis grade. The SFU grading system, introduced in 1993, is a subjective, 5-point numerical grading system that assigns a grade of 0 to 4 based on the appearance of the calyces and renal pelvis and renal parenchymal thickness (13). To clarify the differences between hydronephrosis grades, patients with SFU grade 1 and 2 were assigned to the low-grade group and those with SFU grade 3 and 4 to the high-grade group. The number of patients with low-grade and high-grade hydronephrosis was 57 (14.0%) and 28 (6.9%), respectively.
Bladder cancer that causes gradual ureteral orifice obstruction might result in the development of hydronephrosis. Consequently, the degree of hydronephrosis and T stage might be correlated. Although we found that high-grade or bilateral hydronephrosis were related to the higher pT stage on chi-square analysis, some patients with high-grade or bilateral hydronephrosis showed non-muscle invasive bladder cancer. Among 28 patients with high-grade hydronephrosis and 11 patients with bilateral hydronephrosis, Ta-1 stage was found in 3 and 2 patients, respectively.
The grade and presence of preoperative hydronephrosis were significant predictors for cancer-specific survival on log-rank test. In multivariate analysis, high-grade hydronephrosis and bilateral hydronephrosis had an HR of 2.20 and 7.16 for cancer-specific survival, respectively. In addition, analysis of patients with muscle invasive bladder cancer (pT2-4) excluding pTa-1, showed that high-grade hydronephrosis and bilateral hydronephrosis were significant predictors with HR of 1.78 and 4.84 for cancer-specific survival.
This study has 2 limitations. First, it is a retrospective review, which limits the power of its results. Second, cystoscopic evaluation of the bladder cancer was not involved in the analysis. Tumor location related to the ureteral orifice could affect the prognosis after radical cystectomy as shown by Leibovitch et al. (1) and Bartsch et al. (4). Despite these limitations, our data supports that preoperative hydronephrosis, especially the grade of preoperative hydronephrosis is an independent predictor for cancer-specific survival in patients with bladder cancer after cystectomy.
In conclusion, the presence and high grade of preoperative hydronephrosis are related to the higher pT stage and are significant prognostic factors for cancer-specific survival in patients who underwent radical cystectomy for bladder cancer. This could be helpful in deciding the therapeutic plans and assessing the prognosis of patients before the operation.
References
- 1.Leibovitch I, Ben-Chaim J, Ramon J, Madjar I, Engelberg IS, Goldwasser B. The significance of ureteral obstruction in invasive transitional cell carcinoma of the urinary bladder. J Surg Oncol. 1993;52:31–35. doi: 10.1002/jso.2930520109. [DOI] [PubMed] [Google Scholar]
- 2.Bowles WT, Silber I. Carcinoma of the bladder: a computer analysis of 516 patients. J Urol. 1972;107:245–247. doi: 10.1016/s0022-5347(17)60993-5. [DOI] [PubMed] [Google Scholar]
- 3.Haleblian GE, Skinner EC, Dickinson MG, Lieskovsky G, Boyd SD, Skinner DG. Hydronephrosis as a prognostic indicator in bladder cancer patients. J Urol. 1998;160:2011–2014. doi: 10.1097/00005392-199812010-00018. [DOI] [PubMed] [Google Scholar]
- 4.Bartsch GC, Kuefer R, Gschwend JE, de Petriconi R, Hautmann RE, Volkmer BG. Hydronephrosis as a prognostic marker in bladder cancer in a cystectomy-only series. Eur Urol. 2007;51:690–698. doi: 10.1016/j.eururo.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Scrimger RA, Murtha AD, Parliament MB, Venner PM, Hanson J, Houle G, Chetner M. Muscle-invasive transitional cell carcinoma of the urinary bladder: a population-based study of patterns of care and prognostic factors. Int J Radiat Oncol Biol Phys. 2001;51:23–30. doi: 10.1016/s0360-3016(01)01591-7. [DOI] [PubMed] [Google Scholar]
- 6.Bassi P, Ferrante GD, Piazza N, Spinadin R, Carando R, Pappagallo G, Pagano F. Prognostic factors of outcome after radical cystectomy for bladder cancer: a retrospective study of a homogeneous patient cohort. J Urol. 1999;161:1494–1497. [PubMed] [Google Scholar]
- 7.Thrasher JB, Frazier HA, Robertson JE, Dodge RK, Paulson DF. Clinical variables which serve as predictors of cancer-specific survival among patients treated with radical cystectomy for transitional cell carcinoma of the bladder and prostate. Cancer. 1994;73:1708–1715. doi: 10.1002/1097-0142(19940315)73:6<1708::aid-cncr2820730626>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 8.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23:478–480. doi: 10.1007/BF02012459. [DOI] [PubMed] [Google Scholar]
- 9.Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M, Raghavan D, Skinner DG. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 10.Karakiewicz PI, Shariat SF, Palapattu GS, Gilad AE, Lotan Y, Rogers CG, Vazina A, Gupta A, Bastian PJ, Perrotte P, Sagalowsky AI, Schoenberg M, Lerner SP. Nomogram for predicting disease recurrence after radical cystectomy for transitional cell carcinoma of the bladder. J Urol. 2006;176:1354–1362. doi: 10.1016/j.juro.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 11.Yang MH, Yen CC, Chen PM, Wang WS, Chang YH, Huang WJ, Fan FS, Chiou TJ, Liu JH, Chen KK. Prognostic-factors-based risk-stratification model for invasive urothelial carcinoma of the urinary bladder in Taiwan. Urology. 2002;59:232–239. doi: 10.1016/s0090-4295(01)01590-4. [DOI] [PubMed] [Google Scholar]
- 12.Canter D, Guzzo TJ, Resnick MJ, Brucker B, Vira M, Chen Z. Hydronephrosis is an independent predictor of poor clinical outcome in patients treated for muscle-invasive transitional cell carcinoma with radical cystectomy. Urology. 2008;72:379–383. doi: 10.1016/j.urology.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 13.Keays MA, Guerra LA, Mihill J, Raju G, Al-Asheeri N, Geier P, Gaboury I, Matzinger M, Pike J, Leonard MP. Reliability assessment of society for fetal urology ultrasound grading system for hydronephrosis. J Urol. 2008;180:1680–1683. doi: 10.1016/j.juro.2008.03.107. [DOI] [PubMed] [Google Scholar]