Abstract
Idiopathic pulmonary alveolar proteinosis (PAP) is a rare disorder in which lipoproteinaceous material accumulates within alveoli. There were few reports on Asian populations with idiopathic PAP. We retrospectively reviewed 38 patients with idiopathic PAP in Korea. We assessed clinical features, therapeutic efficacy and outcomes of whole lung lavage in patients with idiopathic PAP. The mean age at diagnosis was 52 yr. Eighty six percent of patients were symptomatic at diagnosis. Dyspnea and cough were the most common symptoms. Crackles were the most common physical examination finding. On pulmonary function test, a mild restrictive ventilatory defect was common, with a predicted mean forced vital capacity (FVC) of 77% and forced expiratory volume in one second (FEV1) of 84.6%. Diffusing capacity was disproportionately reduced at 67.7%. Arterial blood gas analysis revealed hypoxemia with a decreased PaO2 of 69.0 mmHg and an increased D(A-a)O2 of 34.2 mmHg. After whole lung lavage, PaO2, D(A-a)O2 and DLCO were significantly improved, but FVC and total lung capacity (TLC) were not different. This is the first multicenter study to analyze 38 Korean patients with idiopathic PAP. The clinical features and pulmonary parameters of Korean patients with idiopathic PAP are consistent with reports in other published studies. Whole lung lavage appears to be the most effective form of treatment.
Keywords: Pulmonary Alveolar Proteinosis, Irrigation, Treatment Outcome, Koreans
INTRODUCTION
Pulmonary alveolar proteinosis (PAP) was first described by Rosen et al. in 1958 (1). It is a rare disorder in which lipoproteinaceous material accumulates within alveoli. The clinical course of the disease is variable, ranging from spontaneous resolution to death due to pneumonia or respiratory failure. Pulmonary alveolar proteinosis occurs in three clinically distinct forms: congenital, secondary, and idiopathic (acquired). Congenital PAP is a heterogeneous group of disorders (2) caused by mutations in the genes encoding surfactant protein B, C or βc chain of the receptor for granulocyte-macrophage colony stimulating factor (GM-CSF) (3, 4). Secondary PAP occurs as a consequence of any one of a heterogeneous group of underlying clinical conditions (hematologic cancers, pharmacologic immunosuppression, inhalation of inorganic dust or toxic fumes, and certain infections) that impair alveolar macrophage function, resulting in surfactant accumulation (5). Idiopathic (acquired) PAP is a disorder of unknown etiology that is thought to represent approximately 90% of PAP cases. The prevalence of idiopathic pulmonary alveolar proteinosis has been estimated to be 0.37 per 100,000 persons (6-9). The median age at the time of diagnosis is 39 yr; most patients are men, and 72% have a history of smoking (10). Idiopathic pulmonary alveolar proteinosis is an enigmatic and fascinating disorder (1). Recent observations in transgenic mice and humans, however, have provided important clues about its pathogenesis. The first real clue was provided by the discovery that mice deficient in GM-CSF develop a lung phenotype that is biochemically, histologically, physiologically, and ultrastructurally indistinguishable from idiopathic PAP (11, 12).
The observation of PAP in GM-CSF-deficient mice was quickly followed by the evaluation of GM-CSF therapy, first in a single patient and then in several small series of patients (13-16). GM-CSF deficiency has not been reported in humans (17, 18). However, a second vital pathogenic clue was the observation that primary PAP is specifically and strongly associated with very high levels of GM-CSF autoantibodies (19).
So far, most reports have focused on PAP patients from western countries, but reports on Asian populations are extremely rare, with only one report based on a Japanese population (20). The first case in Korea was reported in 1987 (21) and Kim and colleagues reviewed twelve cases in 1999 (22).
We reviewed the clinical features, treatment outcomes, and prognosis of patients with idiopathic PAP in Korea.
MATERIALS AND METHODS
We retrospectively reviewed patients with idiopathic PAP that were diagnosed between 1993 and 2007. The data were collected from 10 clinical research centers in Korea (College of Medicine in University of Ulsan, Seoul National University, Yonsei University, Sungkyunkwan University, Korea University, Kyungpook National University, Soonchunhyang University, Gachon University, Hallym University, and Inha University). Patients with PAP were identified by review of the Korean Interstitial Lung Disease Study Group. The medical records of eligible patients were reviewed by one investigator of each institution, and data were extracted using a standard data collection instrument. The diagnosis of PAP was confirmed by a physician diagnosis and pathologic specimens were obtained by video-assisted thoracoscopic surgery or transbronchial lung biopsy with bronchoalveolar lavage (BAL). The symptoms and signs present at the initial assessment at each institution were used as data. We contacted patients by telephone to ascertain follow-up information and outcomes. Arterial blood gas analysis, spirometry, and lung volume measurements were estimated at diagnosis and if possible, estimated again after whole lung lavage. We assessed the efficacy of whole lung lavage based on these parameters.
RESULTS
We identified 38 patients (24 males and 14 females) with PAP. The mean age at diagnosis was 52 yr (Table 1). There were no cases of PAP due to occupational exposure.
Table 1.
Demographic and clinical features upon diagnosis of idiopathic pulmonary alveolar proteinosis in Korean patients
WBC, white blood cell; Hb, hemoglobin; LDH, lactate dehydrogenase; CEA, carcinoembryogenic antigen; PaO2, partial pressure of oxygen in arterial blood; D(A-a)O2, alveolar-arterial O2 gradient; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
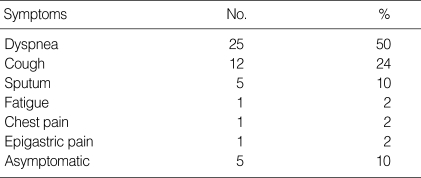
Eighty six percent of patients were symptomatic at diagnosis and the most common symptom at presentation was dyspnea, and followed by cough (Table 2). The most prominent finding on physical examination was crackles in 50% of patients, with cyanosis present in one patient. The chest radiographs for all but one patient showed bilateral air-space disease with an ill-defined nodular or confluent pattern. High resolution computed tomography showed patchy ground-glass opacifications with superimposed interlobular septal thickening in all patients.
Table 2.
Symptoms of Korean patients with idiopathic pulmonary alveolar proteinosis at presentation
*Some patients presented with more than one symptom.
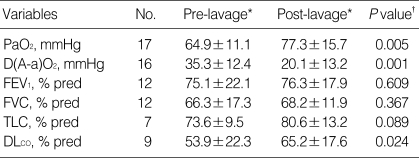
On pulmonary function testing, a mild restrictive ventilatory defect was common, the mean percent predicted forced vital capacity (FVC) was slightly decreased at 77%, the mean percent predicted forced expiratory volume in one second (FEV1) was normal at 84.6%, but the mean percent predicted total lung capacity (TLC) of 81% was not decreased. However, the percent predicted diffusing capacity of the lung for carbon monoxide (DLCO) was disproportionately reduced (mean±SD; 67.7±31.6) among 29 patients. Arterial blood gas analysis showed hypoxemia with a mean room air partial pressure of oxygen (PaO2) of 69.0±14.2 mmHg and a mean alveolar arterial O2 gradient; D(A-a)O2 of 36.0±4.4 mmHg. The serum lactate dehydrogenase (LDH) level was elevated with a mean value of 455.4±183.1 IU/L (range 153 to 834 IU/L). Transbronchial lung biopsy was performed in 35 patients (92%) and video-assisted thoracoscopic surgery was performed in 16 patients (42%). Diagnosis was established by transbronchial lung biopsy with BAL or surgical lung biopsy. On light microscopic examination, we observed the characteristic finding of alveoli filled with a granular, eosinophilic material that stained with periodic acid-Schiff, and a well-preserved alveolar architecture. Whole lung lavage was indicated for patients who presented with dyspnea or hypoxemia and deteriorated chest radiography. Whole lung lavage was performed in 26 patients (68%), of whom 11 patients underwent another lavage. We defined the recurrence as significant progression of respiratory symptoms attributable to PAP or the application of further therapeutic interventions such as repeated lavage. Five patients (13.2%) showed spontaneous resolution after unspecified periods of observation. We analyzed oxygenation/hypoxemia, spirometric measurements, lung volume parameters, and radiologic findings before and after whole lung lavage (Table 3). Oxygenation improved dramatically after therapeutic lavage, and hypoxemia was corrected in 16 of 17 patients for whom data were available. D(A-a)O2 also improved after lung lavage. Spirometric results before and after whole lung lavage were available for 12 patients. FVC improved in eight patients and worsened in four patients. There was no significant difference in the mean FVC before and after whole lung lavage (P=0.388), as analyzed using the Wilcoxon signed rank test. FEV1 was increased in six patients and decreased in six patients. The difference in FEV1 before and after whole lung lavage was not statistically significant (P=0.609). Eight of nine patients showed improvement in lung volume measurements following therapeutic lavage. DLCO after whole lung lavage showed significant improvement (P=0.015). TLC showed a slight improvement after lavage, but the difference before and after lavage was not significant (P=0.089). Using radiologic evaluation with chest radiography or chest computed tomography, we found that 22 of 26 patients (85%) had improved after whole lung lavage therapy. Radiologic studies after lavage was unavailable in 2 patients, no changes in 2 patients. The mean follow-up period was 3 yr (range, 0.04 to 11.5 yr). At the end of the study, 20 patients were alive, 2 patients had died, and 16 patients were no longer available for follow-up. One patient died soon after the whole lung lavage and the other patient died of advanced gastric cancer. The definition of disease recurrence varied between reports, but significant progression of respiratory symptoms due to PAP and the application of further therapeutic lavage were considered as recurrences. PAP recurred in 4 out of 24 patients (16.7%) during the follow-up period. Of the four patients with a recurrence of PAP, three underwent another whole lung lavage and the fourth patient was closely observed without lavage.
Table 3.
Pre-lavage and post-lavage pulmonary parameters for patients with idiopathic PAP
*Mean±SD (standard deviation); †P value is from the Wilcoxon signed rank test for comparison of pre-lavage versus post-lavage data for each parameter from each patient for whom data were available.
PaO2, partial pressure of oxygen in arterial blood; D(A-a)O2, alveolar-arterial O2 gradient; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
DISCUSSION
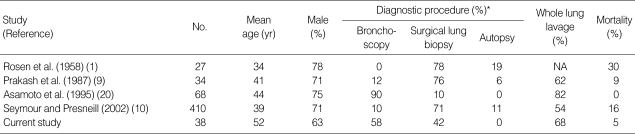
PAP was first recognized in 1958 by Rosen and colleagues (1). Since then, there have been several reports on the clinical respects of idiopathic PAP. Seymour and Presneill (10) analyzed 410 identifiable separate cases of PAP in 241 separate initial publications; most of these cases involved patients from western countries. Asamoto and colleagues (20) reported on the clinical manifestations of PAP in a Japanese population. The Japanese report was unique because it focused on an Asian population with idiopathic PAP, but it was different from the retrospective meta-analysis by Seymour and colleagues (10) in several respects. First, two-thirds of the pathologic diagnoses were established by bronchoscopic biopsy instead of surgical lung biopsy. Further, it reported that the therapeutic outcomes of whole lung lavage were superior to those of other studies (Table 4). Before our study, however, this was the only data available on idiopathic PAP in Asian population. In this study, we retrospectively reviewed 38 patients with idiopathic PAP in Korea. All the Korean patients were born and had grown up in Korea. The median age at diagnosis was 52 yr for both genders, which is in contrast to the distribution of patients from the report by Seymour and Presneill (10) who reported a median age of 39 yr and a different median age in men and women (Table 4). The median age in our study was close to that of the recent report on autoimmune PAP in Japan by Inoue and colleagues (23).
Table 4.
Comparison of published pulmonary alveolar proteinosis studies
*Cases establishing diagnosis by autopsy, not shown.
NA=not available.
Recently, the use of surgical lung biopsy has decreased (24, 25) because a diagnosis of PAP can be established in approximately 75% of clinically suspected cases based on the typical findings of a milky fluid from the BAL (25) that contains large and foamy alveolar macrophages or monocyte-like alveolar macrophage and an increased numbers of lymphocytes (26, 27). There are relatively few inflammatory cells of other cell types, unless superinfection is present. There is also a large amount of amorphous, lipoproteinaceous material that is characteristically eosinophilic, granular, and positive with a periodic acid-Schiff stain (25, 27). In this study, pathologic diagnosis was established by surgical lung biopsy (42%) or transbronchial biopsy with BAL (58%). Milky fluid on BAL and consistent transbronchial lung biopsy specimens provided diagnosis, such as surgical lung biopsy.
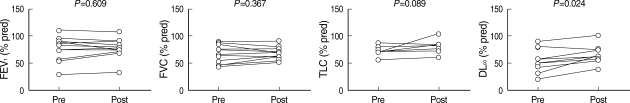
Pulmonary function test at diagnosis showed results that were similar to those of previous studies: a restrictive defect, with a disproportionate reduction in diffusing capacity, a slightly decreased mean FVC of 77.3% and a moderately decreased DLCO of 67.7%. Arterial blood gas analysis at the initial presentation also showed the same results; PaO2 was decreased to 69.0 mmHg and D(A-a)O2 was increased to 35.3 mmHg at the initial presentation. The efficacy of whole lung lavage was evaluated with radiologic findings and pulmonary function tests. After the therapeutic lavage, oxygenation parameters such as PaO2 and D(A-a)O2 improved significantly in comparison to the initial examinations (Fig. 1). DLCO also increased dramatically. FVC and TLC improved slightly, but this improvement was not statistically significant (Fig. 2). These results are similar to those from previous studies on idiopathic PAP.
Fig. 1.
Paired pre-lavage and post-lavage arterial blood gas analysis data from patients with idiopathic PAP. The P value is for the comparison of pre-lavage versus post-lavage data for individual patients for each parameter for only those patients with available data using a Wilcoxon signed rank test. The number of evaluable patients for each parameter: PaO2 (n=17), D(A-a)O2 (n=16).
Fig. 2.
Paired pre-lavage and post-lavage pulmonary function data from patients with idiopathic PAP. The P value is for the comparison of pre-lavage versus post-lavage data for individual patients for each parameter for only those patients with available data using a Wilcoxon signed rank test. The number of evaluable patients for each parameter: FEV1 (n=12), FVC (n=12), TLC (n=7), DLCO (n=9).
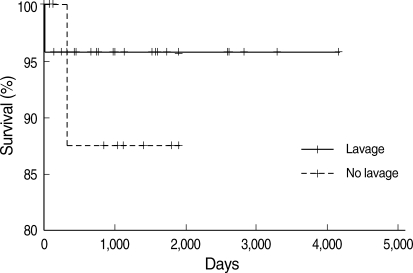
The possibility of spontaneous resolution was initially proposed in the Rosen et al. report (1). They described the patients as either having stable persistent symptoms, progressive deterioration in their process, or spontaneous improvement. Other authors also have made similar observations. Kariman and colleagues (28) reported that spontaneous resolution occurred in 24% of a series of 23 patients. Seymour and Presneill (10) reported that 24 of 303 patients (7.9%) showed a significant degree of spontaneous improvement. The reason for the variation in the amount of spontaneous resolution reported by different studies and authors may be because authors used different individual criteria. In our study, we defined spontaneous resolution as radiographic and symptomatic improvement until follow-up. Based on these criteria, 5 of 38 patients (13%) showed spontaneous resolution. In the survival analysis, there was no difference between the lavage and no lavage groups (Fig. 3). The mean follow-up period from the time of whole lung lavage of 3 yr was too short to compare the survival between the lavage and no lavage groups. Moreover, one patient died soon after therapeutic lavage in the lavage group and one patient died of stomach cancer in the no lavage group, but neither death was directly related to PAP.
Fig. 3.
Overall survival from the time of diagnosis of idiopathic PAP. There was no survival difference between two groups (lavage, n=26; no lavage, n=12, P=0.524).
This study has several limitations; it was a retrospective study, the follow-up period was too short to analyze survival outcomes, and the smoking history of the patients was unavailable.
This is the first multicenter study to analyze 38 Korean patients with idiopathic PAP. The clinical features and pulmonary parameters of Korean patients with idiopathic PAP are consistent with reports in other published studies. Whole lung lavage appears to be the most effective form of treatment.
References
- 1.Rosen SH, Castleman B, Liebow AA. Pulmonary alveolar proteinosis. N Engl J Med. 1958;258:1123–1142. doi: 10.1056/NEJM195806052582301. [DOI] [PubMed] [Google Scholar]
- 2.Teja K, Cooper PH, Squires JE, Schnatterly PT. Pulmonary alveolar proteinosis in four siblings. N Engl J Med. 1981;305:1390–1392. doi: 10.1056/NEJM198112033052305. [DOI] [PubMed] [Google Scholar]
- 3.Nogee LM, de Mello DE, Dehner LP, Colten HR. Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med. 1993;328:406–410. doi: 10.1056/NEJM199302113280606. [DOI] [PubMed] [Google Scholar]
- 4.Nogee LM, Dunbar AE, 3rd, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 5.Kavuru MS, Bonfield TL, Thompson MJ. Pulmonary alveolar proteinosis. In: Mason RJ, Broaddus VC, Murray JF, Nadel JA, editors. Textbook of pulmonary medicine. 4th ed. Philadelphia, PA: Elsevier; 2006. pp. 1716–1734. [Google Scholar]
- 6.Ben-Dov I, Kishinevski Y, Roznman J, Soliman A, Bishara H, Zelligson E, Grief J, Mazar A, Perelman M, Vishnizer R, Weiler-Ravel D. Pulmonary alveolar proteinosis in Israel: ethnic clustering. Isr Med Assoc J. 1999;1:75–78. [PubMed] [Google Scholar]
- 7.deMello DE, Lin Z. Pulmonary alveolar proteinosis: a review. Pediatr Pathol Mol Med. 2001;20:413–432. [PubMed] [Google Scholar]
- 8.Goldstein LS, Kavuru MS, Curtis-McCarthy P, Christie HA, Farver C, Stoller JK. Pulmonary alveolar proteinosis: clinical features and outcomes. Chest. 1998;114:1357–1362. doi: 10.1378/chest.114.5.1357. [DOI] [PubMed] [Google Scholar]
- 9.Prakash UB, Barham SS, Carpenter HA, Dines DE, Marsh HM. Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review. Mayo Clin Proc. 1987;62:499–518. doi: 10.1016/s0025-6196(12)65477-9. [DOI] [PubMed] [Google Scholar]
- 10.Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215–235. doi: 10.1164/rccm.2109105. [DOI] [PubMed] [Google Scholar]
- 11.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, Mulligan RC. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 12.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seymour JF, Dunn AR, Vincent JM, Presneill JJ, Pain MC. Efficacy of granulocyte-macrophage colony-stimulating factor in acquired alveolar proteinosis. N Engl J Med. 1996;335:1924–1925. doi: 10.1056/NEJM199612193352513. [DOI] [PubMed] [Google Scholar]
- 14.Bonfield TL, Kavuru MS, Thomassen MJ. Anti-GM-CSF titer predicts response to GM-CSF therapy in pulmonary alveolar proteinosis. Clin Immunol. 2002;105:342–350. doi: 10.1006/clim.2002.5301. [DOI] [PubMed] [Google Scholar]
- 15.Seymour JF, Presneill JJ, Schoch OD, Downie GH, Moore PE, Doyle IR, Vincent JM, Nakata K, Kitamura T, Langton D, Pain MC, Dunn AR. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic idiopathic alveolar proteinosis. Am J Respir Crit Care Med. 2001;163:524–531. doi: 10.1164/ajrccm.163.2.2003146. [DOI] [PubMed] [Google Scholar]
- 16.Tazawa R, Hamano E, Arai T, Ohta H, Ishimoto O, Uchida K, Watanabe M, Saito J, Takeshita M, Hirabayashi Y, Ishige I, Eishi Y, Hagiwara K, Ebina M, Inoue Y, Nakata K, Nukiwa T. Granulocyte-macrophage colony-stimulating factor and lung immunity in pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2005;171:1142–1149. doi: 10.1164/rccm.200406-716OC. [DOI] [PubMed] [Google Scholar]
- 17.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 18.Carraway MS, Ghio AJ, Carter JD, Piantadosi CA. Detection of granulocyte-macrophage colony-stimulating factor in patients with pulmonary alveolar proteinosis. Am J Respir Crit Care Med. 2000;161:1294–1299. doi: 10.1164/ajrccm.161.4.9906080. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, Nakata K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190:875–880. doi: 10.1084/jem.190.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asamoto H, Kitaichi M, Nishimura K, Itoh H, Izumi T. Primary pulmonary alveolar proteinosis: clinical observations of 68 patients in Japan. Nihon Kyobu Shikkan Gakkai Zasshi. 1995;33:835–845. [PubMed] [Google Scholar]
- 21.Kim HT, Chung HS, Han SK, Shim Y, Kim KY, Han YC. A case of pulmonary alveolar proteinosis. Korean J Intern Med. 1987;33:668–674. [Google Scholar]
- 22.Kim G, Lee SJ, Lee HP, Yoo CG, Han SK, Shim YS, Kim YW. The clinical characteristics of pulmonary alveolar proteinosis: experience at Seoul National University Hospital, and review of the literature. J Korean Med Sci. 1999;14:159–164. doi: 10.3346/jkms.1999.14.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP, Nakata K. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–762. doi: 10.1164/rccm.200708-1271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah PL, Hansell D, Lawson PR, Reid KB, Morgan C. Pulmonary alveolar proteinosis: clinical aspects and current concepts on pathogenesis. Thorax. 2000;55:67–77. doi: 10.1136/thorax.55.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang BM, Stern EJ, Schmidt RA, Pierson DJ. Diagnosing pulmonary alveolar proteinosis. A review and an update. Chest. 1997;111:460–466. doi: 10.1378/chest.111.2.460. [DOI] [PubMed] [Google Scholar]
- 26.Iyonaga K, Suga M, Yamamoto T, Ichiyasu H, Miyakawa H, Ando M. Elevated bronchoalveolar concentrations of MCP-1 in patients with pulmonary alveolar proteinosis. Eur Respir J. 1999;14:383–389. doi: 10.1034/j.1399-3003.1999.14b24.x. [DOI] [PubMed] [Google Scholar]
- 27.Schoch OD, Schanz U, Koller M, Nakata K, Seymour JF, Russi EW, Boehler A. BAL findings in a patient with pulmonary alveolar proteinosis successfully treated with GM-CSF. Thorax. 2002;57:277–280. doi: 10.1136/thorax.57.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kariman K, Kylstra JA, Spock A. Pulmonary alveolar proteinosis: prospective clinical experience in 23 patients for 15 years. Lung. 1984;162:223–231. doi: 10.1007/BF02715650. [DOI] [PubMed] [Google Scholar]