Abstract
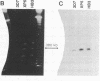
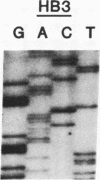
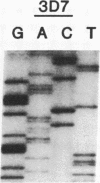
Analysis of a genetic cross of Plasmodium falciparum and of independent parasite isolates from Southeast Asia, Africa, and South America indicates that resistance to pyrimethamine, an antifolate used in the treatment of malaria, results from point mutations in the gene encoding dihydrofolate reductase-thymidylate synthase (EC 1.5.1.3 and EC 2.1.1.45, respectively). Parasites having a mutation from Thr-108/Ser-108 to Asn-108 in DHFR-TS are resistant to the drug. The Asn-108 mutation occurs in a region analogous to the C alpha-helix bordering the active site cavity of bacterial, avian, and mammalian enzymes. Additional point mutations (Asn-51 to Ile-51 and Cys-59 to Arg-59) are associated with increased pyrimethamine resistance and also occur at sites expected to border the active site cavity. Analogies with known inhibitor/enzyme structures from other organisms suggest that the point mutations occur where pyrimethamine contacts the enzyme and may act by inhibiting binding of the drug.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccanari D. P., Stone D., Kuyper L. Effect of a single amino acid substitution on Escherichia coli dihydrofolate reductase catalysis and ligand binding. J Biol Chem. 1981 Feb 25;256(4):1738–1747. [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carle G. F., Olson M. V. Separation of chromosomal DNA molecules from yeast by orthogonal-field-alternation gel electrophoresis. Nucleic Acids Res. 1984 Jul 25;12(14):5647–5664. doi: 10.1093/nar/12.14.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. X., Mueller C., Wendlinger M., Zolg J. W. Kinetic and molecular properties of the dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant clones of the human malaria parasite Plasmodium falciparum. Mol Pharmacol. 1987 Apr;31(4):430–437. [PubMed] [Google Scholar]
- Cowman A. F., Morry M. J., Biggs B. A., Cross G. A., Foote S. J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggens S. M., Gutteridge W. E., Trigg P. I. Altered dihydrofolate reductase associated with a pyrimethamine-resistant Plasmodium berghei berghei produced in a single step. Nature. 1970 Nov 7;228(5271):579–580. doi: 10.1038/228579a0. [DOI] [PubMed] [Google Scholar]
- Ferone R., Burchall J. J., Hitchings G. H. Plasmodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol. 1969 Jan;5(1):49–59. [PubMed] [Google Scholar]
- Ferone R. Dihydrofolate reductase from pyrimethamine-resistant Plasmodium berghei. J Biol Chem. 1970 Feb 25;245(4):850–854. [PubMed] [Google Scholar]
- Graves P. M., Carter R., Keystone J. S., Seeley D. C., Jr Drug sensitivity and isoenzyme type in cloned lines of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Mar;33(2):212–219. doi: 10.4269/ajtmh.1984.33.212. [DOI] [PubMed] [Google Scholar]
- HITCHINGS G. H. Daraprim as an antagonist of folic and folinic acids. Trans R Soc Trop Med Hyg. 1952 Sep;46(5):467-73; discussion, 498-508. doi: 10.1016/0035-9203(52)90038-2. [DOI] [PubMed] [Google Scholar]
- Higuchi R., von Beroldingen C. H., Sensabaugh G. F., Erlich H. A. DNA typing from single hairs. Nature. 1988 Apr 7;332(6164):543–546. doi: 10.1038/332543a0. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Bzik D. J., Horii T. Pyrimethamine resistant Plasmodium falciparum: overproduction of dihydrofolate reductase by a gene duplication. Mol Biochem Parasitol. 1987 Nov;26(1-2):121–134. doi: 10.1016/0166-6851(87)90136-8. [DOI] [PubMed] [Google Scholar]
- Kan S. C., Siddiqui W. A. Comparative studies on dihydrofolate reductases from Plasmodium falciparum and Aotus trivirgatus. J Protozool. 1979 Nov;26(4):660–664. doi: 10.1111/j.1550-7408.1979.tb04216.x. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Welsh J. A., Dame J. B., Quakyi I. A., Graves P. M., Drake J. C., Allegra C. J. Mechanism of pyrimethamine resistance in recent isolates of Plasmodium falciparum. Antimicrob Agents Chemother. 1984 Nov;26(5):656–659. doi: 10.1128/aac.26.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schoenfeld C., Most H., Entner N. Chemotherapy of rodent malaria: transfer of resistance vs mutation. Exp Parasitol. 1974 Oct;36(2):265–277. doi: 10.1016/0014-4894(74)90066-6. [DOI] [PubMed] [Google Scholar]
- Schwartz D. C., Cantor C. R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell. 1984 May;37(1):67–75. doi: 10.1016/0092-8674(84)90301-5. [DOI] [PubMed] [Google Scholar]
- Simonsen C. C., Levinson A. D. Isolation and expression of an altered mouse dihydrofolate reductase cDNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2495–2499. doi: 10.1073/pnas.80.9.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnis P., Wellems T. E. Long-range restriction maps of Plasmodium falciparum chromosomes: crossingover and size variation among geographically distant isolates. Genomics. 1988 Nov;3(4):287–295. doi: 10.1016/0888-7543(88)90117-6. [DOI] [PubMed] [Google Scholar]
- Sirawaraporn W., Yuthavong Y. Kinetic and molecular properties of dihydrofolate reductase from pyrimethamine-sensitive and pyrimethamine-resistant Plasmodium chabaudi. Mol Biochem Parasitol. 1984 Mar;10(3):355–367. doi: 10.1016/0166-6851(84)90033-1. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M., Moccio D. M., Kelleher L. E., Goutas L. J. Relative frequency and kinetic properties of transport-defective phenotypes among methotrexate-resistant L1210 clonal cell lines derived in vivo. Cancer Res. 1981 Nov;41(11 Pt 1):4447–4452. [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Thaithong S., Beale G. H., Fenton B., McBride J., Rosario V., Walker A., Walliker D. Clonal diversity in a single isolate of the malaria parasite Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78(2):242–245. doi: 10.1016/0035-9203(84)90287-6. [DOI] [PubMed] [Google Scholar]
- Thaithong S., Beale G. H. Resistance of ten Thai isolates of Plasmodium falciparum to chloroquine and pyrimethamine by in vitro tests. Trans R Soc Trop Med Hyg. 1981;75(2):271–273. doi: 10.1016/0035-9203(81)90333-3. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Volz K. W., Matthews D. A., Alden R. A., Freer S. T., Hansch C., Kaufman B. T., Kraut J. Crystal structure of avian dihydrofolate reductase containing phenyltriazine and NADPH. J Biol Chem. 1982 Mar 10;257(5):2528–2536. [PubMed] [Google Scholar]
- Walliker D., Quakyi I. A., Wellems T. E., McCutchan T. F., Szarfman A., London W. T., Corcoran L. M., Burkot T. R., Carter R. Genetic analysis of the human malaria parasite Plasmodium falciparum. Science. 1987 Jun 26;236(4809):1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- Walter R. D. Altered dihydrofolate reductase in pyrimethamine-resistant Plasmodium falciparum. Mol Biochem Parasitol. 1986 Apr;19(1):61–66. doi: 10.1016/0166-6851(86)90066-6. [DOI] [PubMed] [Google Scholar]
- Webster H. K., Thaithong S., Pavanand K., Yongvanitchit K., Pinswasdi C., Boudreau E. F. Cloning and characterization of mefloquine-resistant Plasmodium falciparum from Thailand. Am J Trop Med Hyg. 1985 Nov;34(6):1022–1027. doi: 10.4269/ajtmh.1985.34.1022. [DOI] [PubMed] [Google Scholar]
- Wellems T. E., Howard R. J. Homologous genes encode two distinct histidine-rich proteins in a cloned isolate of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6065–6069. doi: 10.1073/pnas.83.16.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellems T. E., Walliker D., Smith C. L., do Rosario V. E., Maloy W. L., Howard R. J., Carter R., McCutchan T. F. A histidine-rich protein gene marks a linkage group favored strongly in a genetic cross of Plasmodium falciparum. Cell. 1987 Jun 5;49(5):633–642. doi: 10.1016/0092-8674(87)90539-3. [DOI] [PubMed] [Google Scholar]