Abstract
Periventricular-intraventricular hemorrhage (PV-IVH) is a major cause of neurological disabilities in preterm newborns. This study aimed to determine the perinatal factors associated with PV-IVH. We conducted a retrospective case-control study from preterm infants born at ≤34 weeks of gestation and admitted to Neonatal Intensive Care Units of Seoul National University Children's Hospital and Seoul National University Bundang Hospital between June 2003 and December 2007. Neonates with no cranial sonographic data or infants transferred from other centers after three days of age were excluded. Of 1,044 eligible subjects, 59 infants with PV-IVH grade 2, 3, and 4 were allocated to the case group. The control group consisted of 118 infants without PV-IVH who were matched for gestational age and birth weight to each case of PV-IVH. At the multivariate logistic regression model, metabolic acidosis (odds ratio [OR]: 6.94; 95% confidence interval [CI]: 1.12-43.23) and use of inotropes (OR: 3.70; 95% CI: 1.16-11.84) were associated with an increased risk of PV-IVH. Maternal use of antenatal corticosteroids decreases the risk of PV-IVH (OR: 0.36; 95% CI: 0.14-0.92).
Keywords: Intracranial Hemorrhages; Infant, Premature; Intensive Care, Neonatal; Risk Factors
INTRODUCTION
During the last few decades, the survival of preterm infants has increased dramatically (1, 2). This improvement is mainly due to advances in perinatal medicine and neonatal intensive care. Nevertheless, the incidence of neurological impairment remains high among preterm survivors. The most important neurological manifestations of brain damage in preterm infants are cognitive and motor disabilities. Periventricular-intraventricular hemorrhage (PV-IVH) is one of the major causes of the development of cerebral palsy and mental retardation, and the incidence ranges from 15% to 40%, depending on the center in spite of the many efforts to reduce the incidence (3, 4).
Identifying the risk factors and underlying mechanisms for PV-IVH has the potential to allow for the development of effective strategies for prevention of many neurodevelopmental problems of premature infants. A number of perinatal risk factors such as a low gestational age and birth weight, intrauterine infection, vaginal delivery, low Apgar score, acidosis and sepsis have been proposed as associated with the pathogenesis of PV-IVH (5-7). However, only a few studies have undertaken a multivariate analysis to identify the independent risk factors associated with PV-IVH. Furthermore, although prematurity is the major risk factor for PV-IVH, many studies have not adequately controlled for birth weight and gestational age, and the results of the studies may have been confounded by these variables. We have therefore conducted an age- and birth weight-matched control study to adjust for these factors and to increase the sensitivity of the detection of the potential risk factors associated with the development of PV-IVH.
MATERIALS AND METHODS
Subjects
We conducted a retrospective case-control study from a cohort of 1,074 preterm infants born at ≤34 weeks of gestation and admitted to the Neonatal Intensive Care Units of Seoul National University Children's Hospital and Seoul National University Bundang Hospital between June 2003 and December 2007. Neonates with no cranial sonographic data obtained by three days after birth and/or neonates that transferred from other centers after three days of age were excluded from the study. Of 1,044 eligible subjects, 59 infants with PV-IVH grade 2, grade 3, and grade 4 were allocated to the case group of infants.
To match infants without PV-IVH to the case group, gestational age and birth weight were separately assigned as criteria. A control group consisted of 118 infants that were selected; 59 infants closely matched for gestational age and 59 infants closely matched for birth weight to each case of PV-IVH.
Cranial ultrasounds, according to the clinical protocol of the neonatal intensive care unit (NICU), were performed with a 2.2-5 MHz transducer during the first three days, on the seventh day and at the end of the third week of life. When there was clinical suspicion of bleeding, additional ultrasound examinations were performed. Both coronal and sagittal sections were obtained through the anterior fontanelles. The severity of PV-IVH was graded according to classification described by Papile et al. (8). When there was more than one report, or if there was bilateral hemorrhage, the highest grade was selected. Other intracranial bleedings, such as a subarachnoid, subdural and epidural hemorrhage were not concerned in assessing PV-IVH. The first day of bleeding was defined as the day when the hemorrhage was first identified.
Measurements
For patients that were selected for the study, the following data were collected from both the maternal and neonatal medical records. Maternal characteristics and obstetric parameters included maternal age, parity, fertility treatment (especially the use of in vitro fertilization), gestational age (established or confirmed by ultrasonographic measurement of fetal biometry), amount of amniotic fluid evaluated by ultrasound, presence of histological chorioamnionitis (confirmed on a pathological report of the placenta), mode of delivery (Caesarian section versus vaginal delivery), preeclampsia, premature rupture of membranes (more than 18 hr before delivery), use of tocolytic agents and use of antenatal corticosteroids (betametasone or dexamethasone) in either an incomplete or a full course. Neonatal outcome and postnatal progress factors included birth weight, gender, Apgar score, use of an umbilical artery catheter or umbilical vein catheter, results of cranial ultrasound, use of inotropic agents for hypotension, metabolic acidosis and treatment of acidosis (NaHCO3), patent ductus arteriosus, development of respiratory distress syndrome (RDS), mechanical ventilation, development of sepsis (clinically highly suspicious or bacteriologically proven), experience of any surgical procedure and transfusion of red blood cells or a platelet concentrate.
Neonatal progress factors and events developed only before the first day of bleeding of each case of PV-IVH were collected in the case group. In control group, the same period with the matched case was applied and neonatal factors occurred within that period were collected.
The use of inotropes were indicated for infants with hypotension, defined as a lower mean arterial blood pressure than the value equivalent to the gestational age in weeks or below 30 mmHg with at least one of the following clinical presentations: a significantly decreased urine output, metabolic acidosis and/or impairment of peripheral perfusion. Metabolic acidosis was defined as a capillary pH value lower than 7.25 with normal pCO2 and a capillary HCO3- value lower than 20.0 mM/L. Suspicious sepsis was diagnosed when the patient presented clinical symptoms or signs of sepsis with laboratory findings suspected systemic inflammation such as leukocytosis or leukopenia, thrombocytopenia and elevated C-reactive protein even though negative blood cultures. Clinical symptoms and signs included fever or hypothermia, newly developed or increased apnea or bradycardia, hypoglycemia or hyperglycemia. The study protocol was approved by our institutional ethical committee.
Statistical analysis was performed with the use of SPSS version 16.0 (SPSS, Chicago, IL, USA). The Student's t test was used for comparison of the continuous variables. Pearson's chi-square test or Fisher's exact test (both two-sided) were used for comparison of the categorical variables as appropriate. Logistic regression analysis was used to evaluate the independent relationship among variables found to be significantly different between the two groups. Only variables that reached P≤0.10 as determined by univariate analysis were entered into a stepwise logistic regression. The odds ratios (ORs) with a 95% confidence intervals (CIs) was calculated and a P value <0.05 was considered as statistically significant.
RESULTS
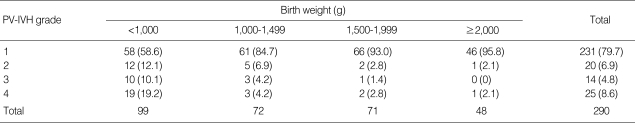
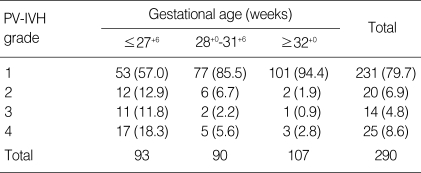
Between June 2003 and December 2007, 1,074 infants born at ≤34 weeks of gestation were admitted to the NICUs of Seoul National University Children's Hospital and Seoul National University Bundang Hospital. Excluded patients included 30 infants with no cranial ultrasound data obtained by three days after birth and/or patients transferred from other centers after three days of age. Of the 1,044 eligible subjects, PV-IVH was diagnosed in 290 cases (27.8%); 231 cases (79.7%) with grade 1, 20 cases (6.9%) with grade 2, 14 cases (4.8%) with grade 3, and 25 cases (8.6%) with grade 4. The incidence of PV-IVH and its severity as a function of birth weight and gestational age are shown in Tables 1, 2.
Table 1.
The incidence and severity of PV-IVH according to birth weight
Data presented as number (%).
PV-IVH, periventricular-intraventricular hemorrhage.
Table 2.
The incidence and severity of PV-IVH according to gestational age
Data presented as number (%).
PV-IVH, periventricular-intraventricular hemorrhage.
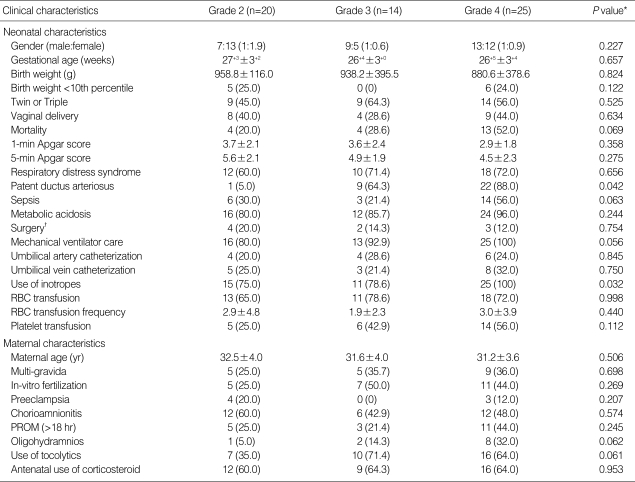
The case group consisted of 59 infants with PV-IVH grade 2, grade 3, and grade 4. The mean gestational age and birth weight of the case group infants were 26+6±3+2 weeks and 920.8±428.7 g, respectively. After three months from the onset of bleeding, 10 out of 59 cases developed post-hemorrhagic hydrocephalus, with six infants requiring ventriculoperitoneal shunt insertion and one infant needing only transient external ventricular drainage. Nine (45%) of 20 cases of grade 2 hemorrhage resolved and only one (4%) of 25 cases of grade 4 hemorrhage resolved after three months. The mortality rate of infants with grade 4 hemorrhage was 52%.
In the case group, demographic data, including gestational age and birth weight showed no significant differences between PV-IVH grade 2, grade 3, and grade 4. Patent ductus arteriosus (P=0.042, ANOVA) and inotrope treatment (P=0.032, ANOVA) were significantly different for PV-IVH grade 2, grade 3, and grade 4 as shown in Table 3. In all infants with grade 4 hemorrhage, inotropes were administered before bleeding commenced.
Table 3.
Comparison of neonatal and maternal characteristics among different PV-IVH grades
*One-way ANOVA analysis; †Explorative laparotomy or PDA ligation.
Data presented as number (%) or mean±standard deviation.
PV-IVH, periventricular-intraventricular hemorrhage; RBC, red blood cell; PROM, premature rupture of membrane.
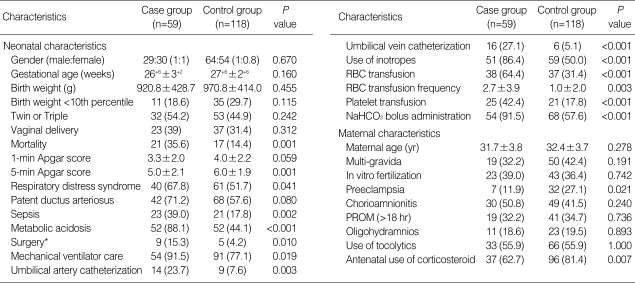
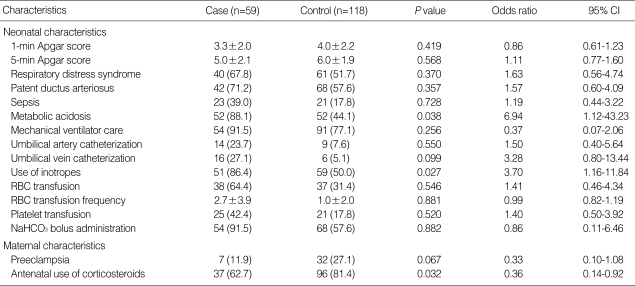
The results of univariate analysis of the neonatal and maternal demographic data for the case and the control groups are shown in Table 4. There was no difference in terms of the demographic data for gestational age, birth weight, proportions of gender, percentage of small for gestational age, rate of multiple pregnancies and the mode of delivery. The mortality rate was significantly high in the case group, 35.6% compared to the 4.4% in the control group (P<0.001, χ2-test). Many clinical factors were significantly higher in the case group than in the control group. The factors included the use of mechanical ventilation, the use of umbilical artery catheterization, the use of umbilical vein catheterization, respiratory distress syndrome, use of inotropes, surgery, sepsis, red blood cell transfusion and frequency, platelet transfusion, metabolic acidosis and treatment for acidosis by NaHCO3 administration. Negative associations were observed for the 5-min Apgar score (5.0±2.1 vs. 6.0±1.9 min; P=0.001, χ2-test), maternal preeclampsia (11.9% vs. 27.1%; P=0.021, χ2-test) and antenatal use of corticosteroids (62.7% vs. 81.4%; P=0.007, χ2-test) for the case group and control group, respectively.
Table 4.
Univariate analysis of neonatal and maternal characteristics of the case and control groups
*Explorative laparotomy or PDA ligation.
Data presented as number (%) or mean±standard deviation.
RBC, red blood cell; PROM, premature rupture of membrane.
The multivariate logistic regression analysis showed that metabolic acidosis (OR: 6.94; 95% CI: 1.12-43.23) and the use of inotropes (OR: 3.70; 95% CI: 1.16-11.84) were associated with an increased risk of PV-IVH. Maternal use of antenatal corticosteroids decreased the risk of PV-IVH (OR: 0.36; 95% CI: 0.14-0.92). The use of stepwise logistic regression analysis demonstrated that the use of an umbilical vein catheter was likely to increase the risk of PV-IVH (OR: 3.28; 95% CI: 0.8-13.44), as shown in Table 5.
Table 5.
Multivariate logistic regression analysis of neonatal and maternal characteristics of the case and control groups
Data presented as number (%) or mean±standard deviation.
RBC, red blood cell; CI, confidence interval.
DISCUSSION
The main goal of this study was to determine the perinatal risk factors associated with the development of PV-IVH. As a low birth weight and low gestational age are unquestionable major risk factors for PV-IVH, we performed a case-control study matching birth weight and gestational age.
We excluded grade 1 hemorrhage for the case group patients because in general, infants with a grade 1 hemorrhages have been shown to have similar outcomes to infants that do not develop a PV-IVH (9). Therefore, we allocated only infants with grade 2, grade 3, and grade 4 hemorrhages to the case group for comparison with non-hemorrhage infants.
PV-IVH is caused by bleeding from the primitive germinal matrix, which gradually involutes with advancing gestation (9, 10). Blood vessels in the germinal matrix next to the ventricles are very fragile and vulnerable to fluctuations in blood flow, which can cause the vessels to rupture and bleed (11). A number of risk factors have been proposed for the development of PV-IVH; the factors include gender (3), Apgar score (3, 5, 12, 13), premature rupture of membranes (14), intrauterine infection (12), vaginal delivery (5, 6), in vitro fertilization (15), mechanical ventilation (6, 16) with recurrent endotracheal suctioning (17), a large patent ductus arteriosus (18), development of the respiratory distress syndrome (6, 13) or sepsis (6, 15), transfusion of blood products (15), metabolic acidosis (12) and rapid bicarbonate infusion (13). Maternal preeclampsia or gestational hypertension has been associated with a lower rate of PV-IVH (6, 19). To reduce the incidence of PV-IVH, several pharmacological interventions have been proposed, including the administration of antenatal steroids, prenatal tocolytic therapy (16), postnatal administration of low-dose indomethacin and surfactants (20-22).
We found no significant difference in this study for the following risk factors: gender, restricted intrauterine growth, multiple pregnancies, mode of delivery, premature rupture of membrane, in vitro fertilization, tocolysis, oligohydramnios and chrioamnionitis. However a significant association was found for metabolic acidosis and the use of inotropes based on multivariate logistic regression analysis. Even though found as statistically not significant based on logistic regression, red blood cell transfusion, platelet concentrate transfusion and bolus NaHCO3 parental administration were all significantly high in the case group based on the use of univariate analysis (64.4% vs. 31.4%; P≤0.001, 42.2% vs. 17.8%; P≤0.001 and 91.5% vs. 57.6%; P≤0.001, χ2-test, respectively for the case group and the control group). The use of an umbilical artery and vein catheters were likely to contribute to an increased risk for PV-IVH (23.7% vs. 7.6%; P=0.003 and 27.1% vs. 5.1%; P≤0.001, respectively, for the case group and the control group).
These results contribute to the understanding of the pathogenesis of PV-IVH. Metabolic acidosis, use of inotropes, transfusion of blood products and sodium bicarbonate bolus infusions are all related as influencing a change of cerebral circulation (23). Furthermore, the withdrawal and infusion of blood via umbilical catheters can cause a significant rapid change of cerebral blood flow of preterm infants (24). Perlman et al. have demonstrated that the alteration from auto-regulation to a pressure-passive circulatory pattern appears to be an important step in the development of PV-IVH (23). When a pressure-passive circulatory pattern is challenged with fluctuations of cerebral blood flow and pressure, hemorrhage can occur in the immature germinal matrix (23-25). In animal models, hypoxia-ischemia compromises cerebral auto-regulation of premature infants (26). Specific metabolic derangements (e.g., hypocarbia, hypercarbia, hypoxemia and acidosis) also can disrupt the auto-regulatory abilities in preterm infants (27). Rapid alteration in the acid-base status as well as hypoxia can overwhelm the ability of the neonate to protect cerebral circulation and can result in PV-IVH.
In this study, we evaluated the use of inotropes for involvement in circulatory instability. As demonstrated in the new-born beagle puppy model, cerebral hyperperfusion following cerebral hypoperfusion and ischemia is fundamental to the hemodynamic basis of PV-IVH (26). In the absence of autoregulation, the systemic blood pressure becomes the primary determinant of cerebral blood flow and pressure, which is a pressure-passive circulatory situation. Rapid volume expansion with blood products or hypertonic solutions and excessive use of inotropes for the correction of hypotension results in a rapid increase of cerebral blood flow and can cause injury to fragile germinal matrix capillaries (5, 23, 28, 29). More research remains to be performed to determine proper criteria or detailed methods for neonatal intensive care that minimize the rapid alteration of hemodynamics of preterm infants.
In the analysis of the prenatal factors, maternal preeclampsia and the use of antenatal corticosteroids were significantly low in the case group as compared to the control group. When analyzed with the use of multivariate logistic regression, only corticosteroid use was associated with a decreased risk of PV-IVH. Increasing evidence suggests that the incidence of PV-IVH is lower in infants born to mothers with pregnancy-induced hypertension. The mechanism that accounts for this reduction remains unclear, but may be related to medication used to treat the mother (e.g., magnesium sulfate) or to the obstetrical management (19). Consistent with the results of this study, antenatal steroid treatment has been reported as conferring protection against the development of PV-IVH (30). The mechanisms by which corticosteroids decrease the risk of hemorrhage are unclear, but they appear to be independent of enhanced pulmonary maturation. The postulated effects include an anti-angiogenic effect with inhibition of microvessel morphogenesis in the germinal matrix capillary network and stabilization of the microvasculature of the developing germinal matrix (30).
In contrast to previous reports which demonstrated causes of PV-IVH irrelevant to each other, the results of this study exhibit factors related to hemodynamic changes as risk factors of PV-IVH. Many factors which are not statistically significant in this study could be related to influence circulatory blood flow. However metabolic acidosis and its treatment with bolus NaHCO3, use of inotropes for hypotension, use of umbilical catheters and transfusion of blood products are all related to circulatory volume changes directly. These findings suggest that hemodynamic changes of systemic and cerebral circulation are important for the development of PV-IVH in preterm newborns. As the survival rate of extremely preterm infants has remarkably increased recently, more delicate hemodynamic balancing has come to be more essential than any other perinatal factors that have been proposed in previous studies for the prevention of PV-IVH.
We were unable to differentiate early and late PV-IVH in this study. In general, it is likely that early PV-IVH has risk factors that relate to the intrapartum period, whereas late PV-IVH that occurs after a few days of life may well relate to early postnatal hemodynamic factors. However, there is no established definition to distinguish between early and late PV-IVH (5, 12). An additional investigation is needed to determine the timing of the cerebral injury in premature infants and to define an accepted definition distinguishing early and late PV-IVH. Furthermore, future research should be directed at identifying the distinct hemodynamic risk factors for early and late of PV-IVH in premature infants.
Footnotes
This study was supported by KT&G Grant-in Aid for neonatal research (SNUCH 20060102).
References
- 1.Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Kusuda S, Fujimura M, Sakuma I, Aotani H, Kabe K, Itani Y, Ichiba H, Matsunami K, Nishida H. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics. 2006;118:e1130–e1138. doi: 10.1542/peds.2005-2724. [DOI] [PubMed] [Google Scholar]
- 3.Heuchan AM, Evans N, Henderson Smart DJ, Simpson JM. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995-97. Arch Dis Child Fetal Neonatal Ed. 2002;86:F86–F90. doi: 10.1136/fn.86.2.F86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Synnes AR, Chien LY, Peliowski A, Baboolal R, Lee SK. Variations in intraventricular hemorrhage incidence rates among Canadian neonatal intensive care units. J Pediatr. 2001;138:525–531. doi: 10.1067/mpd.2001.111822. [DOI] [PubMed] [Google Scholar]
- 5.Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003;112:33–39. doi: 10.1542/peds.112.1.33. [DOI] [PubMed] [Google Scholar]
- 6.Babnik J, Stucin-Gantar I, Kornhauser-Cerar L, Sinkovec J, Wraber B, Derganc M. Intrauterine inflammation and the onset of peri-intraventricular hemorrhage in premature infants. Biol Neonate. 2006;90:113–121. doi: 10.1159/000092070. [DOI] [PubMed] [Google Scholar]
- 7.Vural M, Yilmaz I, Ilikkan B, Erginoz E, Perk Y. Intraventricular hemorrhage in preterm newborns: risk factors and results from a University Hospital in Istanbul, 8 years after. Pediatr Int. 2007;49:341–344. doi: 10.1111/j.1442-200X.2007.02381.x. [DOI] [PubMed] [Google Scholar]
- 8.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weighs less than 1500g. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 9.Enzmann D, Murphy-Irwin K, Stevenson D, Ariagno R, Barton J, Sunshine P. The natural history of subependymal germinal matrix hemorrhage. Am J Perinatol. 1985;2:123–133. doi: 10.1055/s-2007-999929. [DOI] [PubMed] [Google Scholar]
- 10.Volpe JJ. Intracranial hemorrhage: germinal matrix hemorrhage of the premature infant. Neurology of the Newborn. Philadelphia: WB Saunders; 2001. pp. 435–448. [Google Scholar]
- 11.Chey MJ, Chang YP, Choi JH, Hwang YS, Yun CK, Kim IO, Yeon KM. A clinical study of periventricular-intraventricular hemorrhage in very low birth weight infants. J Korean Pediatr Soc. 1990;33:1341–1352. [Google Scholar]
- 12.Vergani P, Patane L, Doria P, Borroni C, Cappellini A, Pezzullo JC, Ghidini A. Risk factors for neonatal intraventricular haemorrhage in spontaneous prematurity at 32 weeks gestation or less. Placenta. 2000;21:402–407. doi: 10.1053/plac.1999.0499. [DOI] [PubMed] [Google Scholar]
- 13.Ertan AK, Tanriverdi HA, Meier M, Schmidt W. Perinatal risk factors for neonatal intracerebral hemorrhage in preterm infants. Eur J Obstet Gynecol Reprod Biol. 2006;127:29–34. doi: 10.1016/j.ejogrb.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Verma U, Tejani N, Klein S, Reale MR, Beneck D, Figueroa R, Visintainer P. Obstetric antecedants of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate. Am J Obstet Gynecol. 1997;176:275–281. doi: 10.1016/s0002-9378(97)70485-x. [DOI] [PubMed] [Google Scholar]
- 15.Linder N, Haskin O, Levit O, Klinger G, Prince T, Naor N, Turner P, Karmazyn B, Sirota L. Risk factors for intraventricular hemorrhage in very low birth weight premature infants: a retrospective case-control study. Pediatrics. 2003;111:e590–e595. doi: 10.1542/peds.111.5.e590. [DOI] [PubMed] [Google Scholar]
- 16.Vermeulen GM, Bruinse HW, de Vries LS. Perinatal risk factors for adverse neurodevelopmental outcome after spontaneous preterm birth. Eur J Obstet Gynecol Reprod Biol. 2001;99:207–212. doi: 10.1016/s0301-2115(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 17.Mancini MC, Barbosa NE, Banwart D, Silveira S, Guerpelli JL, Leone CR. Intraventricular hemorrhage in very low birth weight infants: associated risk factors and outcome in the neonatal period. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:151–154. doi: 10.1590/s0041-87811999000500004. [DOI] [PubMed] [Google Scholar]
- 18.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F183–F186. doi: 10.1136/fn.75.3.f183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlman JM, Risser RC, Gee JB. Pregnancy-induced hypertension and reduced intranventricular hemorrhage in preterm infants. Pediatr Neurol. 1997;17:29–33. doi: 10.1016/s0887-8994(97)00073-8. [DOI] [PubMed] [Google Scholar]
- 20.Ment LR, Vohr BR, Allan W, Westerveld M, Sparrow SS, Schneider KC, Katz KH, Duncan CC, Makuch RW. Outcome of children in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2000;105:485–491. doi: 10.1542/peds.105.3.485. [DOI] [PubMed] [Google Scholar]
- 21.Fowlie PW, Davis PG. Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev. 2002:CD000174. doi: 10.1002/14651858.CD000174. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- 23.Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. N Engl J Med. 1983;309:204–209. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]
- 24.Bray M, Stucchi I, Fumagalli M, Pugni L, Ramenghi L, Agosti M, Mosca F. Blood withdrawal and infusion via umbilical catheters: effect on cerebral perfusion and influence of ibuprofen. Biol Neonate. 2003;84:187–193. doi: 10.1159/000072301. [DOI] [PubMed] [Google Scholar]
- 25.Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312:1353–1357. doi: 10.1056/NEJM198505233122104. [DOI] [PubMed] [Google Scholar]
- 26.Ment LR, Stewart WB, Duncan CC, Lambrecht R. Beagle puppy model of intraventricular hemorrhage. J Neurosurg. 1982;57:219–223. doi: 10.3171/jns.1982.57.2.0219. [DOI] [PubMed] [Google Scholar]
- 27.Short BL, Walker LK, Traystman RJ. Impaired cerebral autoregulation in the newborn lamb during recovery from severe, prolonged hypoxia, combined with carotid artery and jugular vein ligation. Crit Care Med. 1994;22:1262–1268. doi: 10.1097/00003246-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, Magill HL, Runyan W, 3rd, Somes GW, Clark FC. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhgae. J Pediatr. 1990;117:607–614. doi: 10.1016/s0022-3476(05)80700-0. [DOI] [PubMed] [Google Scholar]
- 29.Goddard-Finegold J, Armstrong D, Zeller RS. Intraventricular hemorrhage, following volume expansion after hypovolemic hypotension in the newborn beagle. J Pediatr. 1982;100:796–799. doi: 10.1016/s0022-3476(82)80596-9. [DOI] [PubMed] [Google Scholar]
- 30.Wells JT, Ment LR. Prevention of intraventricular hemorrhage in preterm infants. Early Hum Dev. 1995;42:209–233. doi: 10.1016/0378-3782(95)01651-i. [DOI] [PubMed] [Google Scholar]