Abstract
Aloe has been widely used in phytomedicine. Phytomedicine describes aloe as a herb which has anti-inflammatory, anti-proliferative, anti-aging effects. In recent years several cases of aloe-induced hepatotoxicity were reported. But its pharmacokinetics and toxicity are poorly described in the literature. Here we report three cases with aloe-induced toxic hepatitis. A 57-yr-old woman, a 62-yr-old woman and a 55-yr-old woman were admitted to the hospital for acute hepatitis. They had taken aloe preparation for months. Their clinical manifestation, laboratory findings and histologic findings met diagnostic criteria (RUCAM scale) of toxic hepatitis. Upon discontinuation of the oral aloe preparations, liver enzymes returned to normal level. Aloe should be considered as a causative agent in hepatotoxicity.
Keywords: Aloe; Hepatitis, Toxic
INTRODUCTION
The demand for dietary supplements has continually increased in recent years as the concept of 'well being' widely spread in Korea. The market value for dietary supplements in Korea was approximately 600 billion won (600 million USD) in year 2005 (1), and personal spending was approximately 950,000 won (950 USD) per year in 2005 (2). One of the leading products in Korea's dietary supplements market is aloe.
Aloe has been purported to have positive effects on wound healing, recovery from burn injury, cell growth, and immune modulation. However, cases of aloe-induced toxic hepatitis have been reported since 2005. In particular, there have been one each in Germany (3), Turkey (4), and USA (5). In Switzerland (6), 10 cases of hepatotoxicity associated with dietary supplements from Herbalife® products were reported. Although 2 of those 10 cases took aloe preparation, the causality assessment between aloe and toxic hepatitis could not be done due to multiple Herbalife® products.
We report 3 cases of aloe-induced toxic hepatitis in Korea.
CASE REPORTS
Case 1
A 57-yr-old female patient with a 2 month history of dyspepsia was presented to our department. Past medical history and family history did not reveal any significant disease. She also used drugs for arthralgia intermittently for several years. She did not take any alcohol. She had taken aloe tablets containing 250 mg of an extract of Aloe arborescens and 28.5 mg of an extract of Aloe vera (Fig. 1) for about 6 months prior to the admission. On admission, the patient's physical examination was normal.
Fig. 1.
Preparation of aloe which the patient had taken. (A) Container bottle and tablets. (B) Packs of aloe extract.
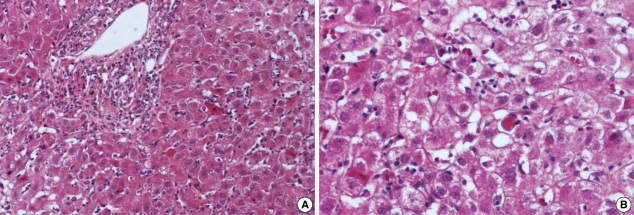
Laboratory abnormalities included aspartate aminotransferase (AST) 331 IU/L, alanine aminotransferase (ALT) 565 IU/L, alkaline phosphatase (AP) 309 IU/L. Anti-HAV IgM, anti-HCV, HCV PCR, and anti-HEV IgM were negative. Anti-HBs IgG was positive. There was no serologic evidence for recent infections with cytomegalovirus (CMV), Epstein-Barr-virus (EBV), or herpes simplex virus (HSV). Autoimmune markers were all negative. Abdominal ultrasonography showed reduced echogenicity of liver. Dilatation of intra- or extrahepatic bile ducts was absent. Liver biopsy revealed moderate portal infiltrates consisting of eosinophilis, neutrophils, and monocytes. There were inflammatory cell infiltration and acidophil body on the hepatic lobule. There was no bile stasis (Fig. 2).
Fig. 2.
Histopathological findings of the liver. (A) The portal area and lobular area show inflammatory cell infiltration (H&E, ×100). (B) The acidophilic body and ballooning cell change are noted (H&E, ×200).
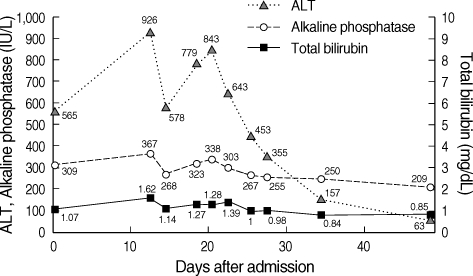
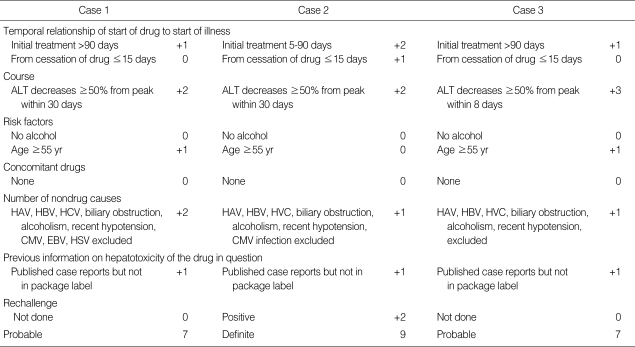
Aloe tablets was immediately discontinued. ALT was highest (926 IU/L) on the 12th day of admission and gradually decreased to 452 IU/L on the 25th day of admission. ALT as well as total bilirubin gradually returned to normal level over several weeks (Fig. 3). Using the Roussel Uclaf Causality Assessment Method for determining drug hepatotoxicity (RUCAM) scale, this case was scored as 'probable' (Table 1). The type of liver injury was determined as 'hepatocellular' since R ratio (serum activity of ALT/serum activity of AP) was 10 (7).
Fig. 3.
Case 1. Upon discontinuation of the oral aloe preparation, liver enzymes returned to normal level.
Table 1.
RUCAM score of these cases
RUCAM, Roussel Uclaf Causality Assessment Method for determining drug hepatotoxicity; ALT, alanine aminotransferase; HAV, hepatitis A virus; HBV, hepatitis B virus, HCV, hepatitis C virus; CMV, cytomegalovirus; EBV, epstein-barr-virus; HSV, herpes simplex virus.
Case 2
A 62-yr-old female patient was presented to our department with a week history of fatigue. Past medical history and family history did not reveal any significant disease. The patient did not take any alcohol or durgs. She had taken aloe powder containing 420 mg of an extract of Aloe vera (Fig. 1B) for about 3 months prior to the admission. Physical examination revealed jaundice on her sclera. She was the sales person of the aloe product she was taking.
Laboratory abnormalities included AST 1,477 IU/L, ALT 1,564 IU/L, total bilirubin 14.64 mg/dL. Anti-HAV IgM, anti-HCV, HCV PCR, and anti-HEV IgM were negative. Anti-HBs IgG was positive. IgM antibody for HSV was in trace, IgM antibody for CMV was negative. Abdominal ultrasonography was normal. Liver biopsy revealed severe portal and lobular infiltrates consisting of neutrophils and monocytes. There were several acidophilic bodies and ballooning cell change in hepatic lobule. There were bile-stasis and bile stained Kupffer cells (Fig. 2B).
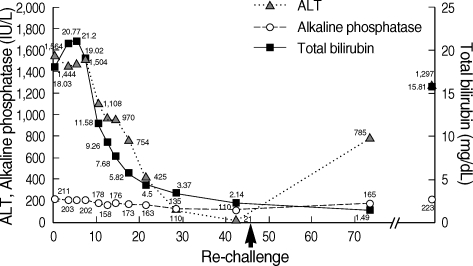
Aloe extract was immediately discontinued. ALT gradually decreased to 452 IU/L which was lower than half of the peak value on the 17th day of admission. When she was discharged ALT and AST were normal. We explained to her about the aloe-induced hepatotoxity and advised not to take it anymore. However, the patient started taking the same aloe extract again 1 month after her discharge from the hospital. A month later, follow-up liver function test showed AST 477 IU/L, ALT 785 IU/L, and AP 165 IU/L (Fig. 4). Since a hepatitis recurred after re-challenge of aloe extract, we could confirm her diagnosis as aloe-induced toxic hepatitis. The RUCAM scale was scored as 'definite' (Table 1). The type of liver injury was determined as 'hepatocellular' since R ratio was 39.
Fig. 4.
Case 2. Upon discontinuation of the oral aloe preparation, liver enzymes returned to normal level. After re-challenge (arrow), liver enzymes go up again.
Six months later, the patient was presented to our department with a 2 week history of jaundice. Laboratory test showed AST 752 IU/L, ALT 1,135 IU/L, AP 243 IU/L and total bilirubin 15.81 mg/dL. We recommended admission, but she refused to be admitted and she never visited our hospital again.
Case 3
A 55-yr-old female patient was presented to our department with a 3 month history of epigastric discomfort. Past medical history and family history did not reveal any significant disease. The patient did not take any alcohol or drugs. She had taken aloe extracts (Fig. 1) for about 5 months prior to the admission. On admission, the patient's physical examination was normal except mild tenderness on epigastric area.
Laboratory abnormalities included AST 344 IU/L, ALT 666 IU/L, AP 298 IU/L. Anti-HAV IgM, anti-HCV, HCV PCR, and anti-HEV IgM were negative. Anti-HBs IgG was positive. Autoimmune markers were negative. Abdominal ultrasonography showed increased echogenicity of liver. Dilatation of intra- or extrahepatic bile ducts was absent.
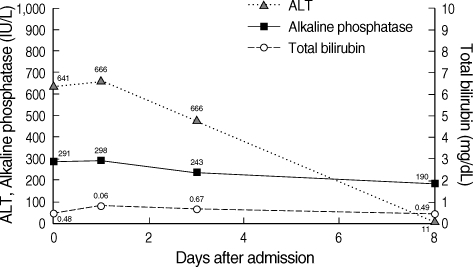
Aloe extract was immediately discontinued. ALT was highest (666 IU/L) on the 2nd day of admission, and gradually decreased. ALT was 484 IU/L on her discharge (on the 5th day of admission). After 4 days, she visited our department for follow-up. Liver function test was normal at that time (AST 29 IU/L, ALT 11 IU/L, AP 190 IU/L) (Fig. 5). The RUCAM scale was scored as 'probable' (Table 1). The type of liver injury was determined as 'hepatocellular' since R ratio was 11.
Fig. 5.
Case 3. Upon discontinuation of the oral aloe preparation, liver enzymes returned to normal level.
DISCUSSION
The major driving force for the growth of the dietary supplements market is the perception that 'they are safe because they are natural'. However, the recently reported cases of hepatotoxicity induced by natural substances (8) indicate that natural substances may not be entirely safe.
There are about 400 species of aloe. Among them, particularly aloe vera has been used in phytomedicine. There have been positive reports on aloe vera as anti-inflammatory, anticancer, analgesic, anti-aging as well as liver protective. But, clinical effectiveness of aloe vera was not sufficiently defined because there were no large and randomized studies (9). In 1994, Korea's National Institute of Safety Research conducted an experiment on the efficacy and toxicity of aloe (10). There was no difference of natural killer cell activity between the aloe vera gel treated and control animals. To observe the toxicity of aloe gel, rats were given the high dose aloe orally. Any adverse effects were not detected in hematological test, serum biochemistry, and histopathological examination.
There are no specific tests or diagnostic criteria for herbinduced hepatic injury. Careful history taking, laboratory finding, and histopathology are used to diagnose it. The best way to determine causing agent is re-challenging. But it is not ethical and not applicable. Instead, the RUCAM scale is used (7, 11).
Since patients usually do not regard dietary supplements as 'real' medicine, they may fail to mention it when physicians query medication history. Physicians should keep in mind that dietary supplements can be the cause of hepatotoxicity when querying medication history, and should educate the lay public.
There are three types of acute liver injury by drug or herb (12): hepatocellular, cholestatic, and mixed type. Our cases are characterized as hepatocellular; there is a predominant initial elevation of the ALT level. There are two proposed pathogeneses of drug induced liver disease (13): direct toxicity and idiosyncratic mechanism. It is more likely that an idiosyncratic immunological mechanism (hypersensitivity) is responsible for the cases. A role for hypersensitivity is further supported by the presence of eosinophilic granulocytes in the periportal fields seen in the biopsy. Hypersensitivity to aloe has been described in humans (14), and the patch test or allergic skin test showed positive results (15).
Herb induced liver injury is an important problem in clinical setting, because it can be an etiology of undiagnosed acute hepatitis. However, there are few available data about the incidence and clinical manifestation of dietary supplements such as aloe. Our cases emphasize that physicians should consider various dietary supplements as causative agents for hepatotoxicity.
References
- 1.Food and Drug Statistical Yearbook. Korea Food and Drug Administration. 2006. Aug, [accessed 2008 0ct 27]. Available at: URL: http://www.kfda.go.kr/open_content/main/main.php.
- 2.Yoo TW, Kim BI, Kim JB, Kim DJ, Kim JW, Baik SK, Kim KS, Cheon GJ. The survey for the actual condition of drug medication and development of health care cost associated with toxic liver injury in Korean: a multicenter study for the detection and the development of nationwide reporting system of toxic liver injury. Korean J Hepatol. 2007;13:34–43. [PubMed] [Google Scholar]
- 3.Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. Acute hepatitis induced by an aloe vera preparation: a case report. World J Gastroenterol. 2005;11:303–304. doi: 10.3748/wjg.v11.i2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanat O, Ozet A, Ataergin S. Aloe vera-induced acute toxic hepatitis in a healthy young man. Eur J Intern Med. 2006;17:589. doi: 10.1016/j.ejim.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Bottenberg MM, Wall GC, Harvey RL, Habib S. Oral aloe vera-induced hepatitis. Ann Pharmacother. 2007;41:1740–1743. doi: 10.1345/aph.1K132. [DOI] [PubMed] [Google Scholar]
- 6.Schoepfer AM, Engel A, Fattinger K, Marbet UA, Criblez D, Reichen J, Zimmermann A, Oneta CM. Herbal does not mean innocuous: ten cases of severe hepatotoxicity associated with dietary supplements from herbalife products. J Hepatol. 2007;47:521–526. doi: 10.1016/j.jhep.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Benichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11:272–276. doi: 10.1016/0168-8278(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 8.Stickel F, Patsenker E, Schuppan D. Herbal hepatotoxicity. J Hepatol. 2005;43:901–910. doi: 10.1016/j.jhep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Vogler BK, Ernst E. Aloe vera: a systematic review of its clinical effectiveness. Br J Gen Pract. 1999;49:823–828. [PMC free article] [PubMed] [Google Scholar]
- 10.Jang DD, Cho JC, Choi KS, Kil HI. Studies of the effectiveness and toxicity of aloe vera gel. The report of National Institute of Safety Research. 1994;7:225–233. [Google Scholar]
- 11.Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- 12.Chae HB. Clinical features and diagnosis of drug induced liver injury. Korean J Hepatol. 2004;10(Suppl1):7–18. [Google Scholar]
- 13.Kang DY. Pathologic features of toxic and drug induced liver injury. Korean J Hepatol. 2004;10(Suppl1):19–29. [Google Scholar]
- 14.Morrow DM, Rapaport MJ, Strick RA. Hypersensitivity to aloe. Arch Dermatol. 1980;116:1064–1065. [PubMed] [Google Scholar]
- 15.Lee EG, Kwon SH, Kim SH, Myung SJ, Choi JW, Kim YJ, Ahn Y. A case of hypersensitivity associated with oral aloe agent. J Asthma Allergy Clin Immunol. 2003;23:833–836. [Google Scholar]