Abstract
The 2009 H1N1 influenza pandemic has heightened the interest of clinicians for options in the prevention and management of influenza virus infection in immunocompromised patients. Even before the emergence of the novel 2009 H1N1 strain, influenza disease was a serious complication in patients with hematologic malignancies receiving chemotherapy or undergoing hematopoietic cell transplantation. Here we review the clinical manifestations of seasonal and 2009 H1N1 influenza and discuss current diagnosis, antiviral treatment, and prophylaxis options. We also summarize infection control and vaccination strategies for patients, family members, and caregivers.
Epidemiology
In March 2009, a series of severe influenza cases were described among otherwise healthy Mexican young adults,1 followed by similar cases in the United States,2 and, within weeks, thousands of late-season influenza cases were reported throughout the world3 (Figure 1). The virologic characteristics of the 2009 H1N1 strain and events that led to its emergence have been described elsewhere.5 Pandemic 2009 H1N1 infection rates have been highest among persons younger than 25 years, but death rates have been highest among persons aged 25 to 49 years (Figure 2). One potential explanation for these trends is that exposure to strains of influenza circulating after 1957 may confer some protective immunity, resulting in neutralizing antibody titers against 2009 H1N1 likely to be protective in older persons7–9 Although older populations may be less likely to acquire 2009 H1N1, the higher prevalence of comorbidities in these populations may lead to higher morbidity and mortality rates among persons who do become infected. Studies also show that obese persons and pregnant women10 have higher mortality associated with 2009 H1N1 infection.
Figure 1.
Number of cases of influenza-like illness presenting to sentinel providers and reported to the Centers for Disease Control and Prevention. Number of visits of influenza-like illness (ILI) reported by the United States. Outpatient Influenza-Like Illness Surveillance Network (ILINet) National Summary 2008 to 2009, by age. Source: http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/data/senAllregt46.htm.4
Figure 2.
Pandemic influenza infection rates and mortality, by age (mainly immunocompetent). (A) Infection rates. (B) Mortality. Source: http://www.cdc.gov/H1N1FLU/surveillanceqa.htm.6
Patients with hematologic malignancies are likely to be at an increased risk for infection with influenza. A few small series have documented seasonal influenza outbreaks among such patients, demonstrating the susceptibility of immunocompromised populations.11–14 These limited reports suggest that cancer patients are at a high risk for acquisition of influenza in both the community and health care settings.
Natural history of influenza in patients with hematologic malignancies
Upper respiratory infection
Similar to immunocompetent patients, most patients with influenza infection and hematologic malignancies present with symptomatic upper respiratory symptoms, consisting of sore throat, nasal symptoms, malaise, and/or headache. Notably, systemic symptoms such as fever, myalgia, and fatigue may be reduced or completely absent. In the population that has received a hematopoietic cell transplant (HCT), in whom this has been studied prospectively,15 most patients were afebrile and lacked systemic symptoms. We speculate that the cytokine response associated with acute influenza infection may be decreased in these patients; use of corticosteroids may play an additional role. The symptomatic phase typically lasts for 1 to 2 weeks in immunocompromised patients, although viral shedding may be prolonged.16 Asymptomatic viral shedding of influenza is uncommon in this setting but can occur with both seasonal and 2009 H1N1 influenza (M.B., unpublished observation, December 2009).
Progression to lower respiratory disease and mortality
A devastating complication of influenza infection is lower respiratory tract disease and pneumonia, frequently leading to acute lung injury and death.17,18 Progression from upper to lower tract disease occurs after a median of 1 week in patients with hematologic malignancies,16 presenting clinically and radiographically as viral pneumonia. The radiographic appearance can range from typical diffuse ground-glass infiltrates to areas of consolidation resembling fungal or bacterial disease.17 Influenza pneumonia may be complicated by bacterial or fungal coinfection.16 Therefore, we advocate aggressive diagnostic workup with bronchoalveolar lavage (BAL) and testing for a broad range of opportunistic pathogens. The most significant risk factor for progression to lower tract disease is profound lymphopenia.16,18,19 The impacts of corticosteroids on influenza severity and outcome are conflicting, with no randomized trials assessing these effects. Although high-dose steroids seemed to prolong viral shedding in HCT recipients with upper respiratory infection16 and one study in pediatric cancer patients showed a higher rate of progression to lower tract disease,20 another study in HCT recipients suggests that progression to lower respiratory tract disease may be reduced.16 Possibly, steroids prolong viral shedding but paradoxically reduce the inflammatory cytokine response. Risk factors among hematologic malignancy patients for 2009 H1N1 influenza disease progression to lower respiratory tract disease are not known.
The dissemination of 2009 H1N1 influenza virus infection to distant organs has not been studied in humans. One report described RNA detection in the plasma in patients with lower respiratory tract disease.21 Ferret models of 2009 H1N1 influenza have not demonstrated viral dissemination into the blood or other organs,22 in contrast to H1N5 avian influenza studies showing RNA detection in blood.23 Further studies are needed.
Influenza infection may lead to acute lung injury, respiratory failure, and death. Mortality rates of influenza vary widely among HCT recipients and patients with hematologic malignancies, ranging from approximately 25% for lower tract disease in some HCT recipients to 0% in other series.16,19,24–26 One large cohort study demonstrated that seasonal influenza lower respiratory tract disease is independently associated with mortality after hematopoietic cell (HC) transplantation (adjusted hazard ratio, 2.60; 95% confidence interval, 1.40-4.86).16 Influenza-associated death can be caused either directly by influenza viral infection or by infection with secondary concomitant pathogens.16 Whether patients with influenza virus infection also have long-term sequelae, such as restrictive or obstructive airway disease as seen after infection with parainfluenza and respiratory syncytial viruses,27 is under investigation.
Differences in presentation between immunocompromised and immunocompetent patients
No comprehensive data describe whether clinical manifestations during the initial phase of 2009 H1N1 influenza infection are different from those reported with seasonal influenza infection, however, recent series have included both immunocompetent and immunocompromised patients.28,29 An initial report in cancer patients suggests that fever and cough occurs in more than 90% of patients infected with 2009 H1N1 influenza, and lower respiratory tract involvement was seen in 27% of patients.30 In immunocompetent patients, gastrointestinal and neurologic symptoms appear to be more prevalent with 2009 H1N1 infection, especially in small children.31 Therefore, we suggest a low index of suspicion during outbreak situations and favor enhanced testing for influenza.
Management of influenza
Indications for testing and treatment
Any patient with new onset of upper or lower respiratory symptoms during sustained influenza activity in the community should be evaluated for influenza. The present 2009 H1N1 strain is not reliably detected with commercial rapid tests or direct fluorescent antibody testing,32 with polymerase chain reaction (PCR) testing recommended for optimal sensitivity. PCR methods also can differentiate influenza strains and susceptibility patterns. This is important when strains with different susceptibility patterns cocirculate, as during the 2008/2009 season when different H1N1 strains (oseltamivir-resistant seasonal and oseltamivir-susceptible 2009 H1N1 influenza) were cocirculating.33 Upper respiratory samples are obtained by nasal wash or by a nasopharyngeal swab, with nasal wash being the most sensitive method.34 A BAL is typically performed to evaluate lower respiratory disease in highly immunosuppressed patients as it allows for concurrent testing for concomitant pathogens. All patients with confirmed influenza upper respiratory tract illness should have both clinical and radiographic assessments to rule out lower tract involvement.
A wide spectrum of immunosuppression exists among patients with hematologic malignancies, ranging from chemotherapy to allogeneic transplantation after myeloablative conditioning with in vivo or ex vivo T-cell depletion or refractory graft-versus-host disease. Underlying conditions that complicate influenza disease in otherwise immunocompetent persons may be present, including diabetes, obesity, and pulmonary or cardiac disease. Few studies have evaluated the outcome of influenza disease relative to the underlying immunosuppression and pre-existing conditions, although a recent meta-analysis found an average case fatality rate of 17%, with a range of 0% to 33% among patients infected with seasonal influenza with hematologic malignancies and who received a HCT.35 Neuraminidase inhibitor therapy appears to be effective when instituted early after onset of symptoms.16 A preliminary report in patients with hematologic and solid cancers by Redelman-Sidi et al30 suggests that lower respiratory tract disease occurs in 27% of 45 patients; 37% of patients were hospitalized for an average of 7 days (range, 3-15 days) and 1 person required intensive care unit care without mechanical ventilation. No deaths were reported in this series of 45 patients, most of whom received oseltamivir (n = 43).30 The 2009 H1N1 influenza may be more serious than typical seasonal influenza: as of mid-November at the Seattle Cancer Care Alliance, 21 patients had upper and 6 had lower respiratory tract influenza disease caused by 2009 H1N1, with 4 patients experiencing respiratory failure and 1 death (C.C., unpublished data, December 2009).
Choice of antiviral
Anti-influenza antiviral agents have not been studied in randomized trials specifically in patients undergoing chemotherapy or after HC transplantation. Antiviral susceptibilities of circulating influenza strains must be continually evaluated,33 with information available on websites at the US Centers for Disease Control and Prevention (CDC). Due to resistance against the M2 inhibitors in 2009 H1N1 strains, amantidine and rimantidine should not be used as single agents to treat or prevent influenza A (Table 1). Presently, 2 neuraminidase inhibitors (ie, zanamivir and oseltamivir) are licensed for the treatment of influenza. These compounds are licensed for inhaled (zanamivir) and oral (oseltamivir) use, with intravenous agents now available under special circumstances. Investigational agents including intravenous peramivir, zanamivir, and oseltamivir are under study and some are available under Emergency Use Authorization through the US Centers for Disease Control and Prevention (CDC).
Table 1.
Antiviral options for treatment of influenza
| Antiviral agent | Susceptibility |
Recommended dosing and duration in patients with hematologic malignancies |
Key toxicities | Parenteral formulation available | ||||
|---|---|---|---|---|---|---|---|---|
| 2009 H1N1 | 2008/9 seasonal H1N1 | 2008/9 seasonal H3N2 | Influenza B or C | Adults | Children | |||
| Oseltamivir | Yes | No | Yes | Yes | Treatment: 150 mg PO twice daily × 10 d*; postexposure prophylaxis: 75 mg PO once or twice daily × 10 d*; seasonal prophylaxis: 75 mg PO once daily | Dosing guidelines: see CDC guidelines: http://www.cdc.gov/A/H1N1flu/recommendations.htm36; care to be taken in dosing children < 1 y, with dose of 3-3.5 mg/kg/dose recommended | Gastrointestinal: greatly reduced if oral drug taken with food; Neurologic (children) | Yes (investigational, phase 1) |
| Zanamivir | Yes | Yes | Yes | Yes | Treatment: 10 mg twice daily (by inhaler); after exposure: 10 mg twice daily (by inhaler); prophylaxis: 10 mg twice daily (by inhaler) | Not available for children < 7 y; for children > 7 y, use adult doses | Bronchospasm | Yes (investigational, phase 2) |
| Rimantidine (amantidine) | No | Yes | No | No | Treatment: 100 mg PO twice daily | Age 1-10 y: 5 mg/kg per day PO, 1-2 divided doses, max 150 mg/day; age ≥ 10 y: 100 mg PO twice daily | Neurologic Gastrointestinal | No |
| Ribavirin | Not recommended as monotherapy37 | Not recommended as monotherapy37 | Not recommended as monotherapy37 | Not recommended as monotherapy37 | Treatment: 200-400 mg PO 3× daily† | No dosing guidelines available | Hematologic Gastrointestinal | Yes (investigational in the US) |
| Peramivir | Yes | No | Yes | Yes | Treatment: 600 mg IV once daily | Up to 30 d: 6 mg/kg; 31-90 d: 8 mg/kg; 91-180 d: 10 mg/kg; 181 d to 5 y: 12 mg/kg; 6-17 y: 10 mg/kg (maximum 600 mg/day) | Gastrointestinal; Neutropenia | Yes (EUA in the US) |
All doses stated are for patients with normal renal function. Dose reductions may be necessary in patients with reduced renal clearance.
PO indicates by mouth; IV, intravenous; and EUA, emergency use authorization (granted by US Food and Drug Administration).
Authors' recommendation; see “Management of upper and lower respiratory infection” for rationale.
Management of upper and lower respiratory infection
Nonrandomized studies suggest that preemptive therapy with oral or inhaled neuraminidase inhibitors (ie, oseltamivir, zanamivir) is effective in preventing progression to lower tract disease.16,18,20,41–43 Monotherapy appears to be effective in these nonrandomized studies. For lower respiratory tract disease, a more aggressive approach with intravenous drug administration, high-dose oral regimens, and/or combination therapy approaches in the case of respiratory failure can be considered (Table 2).
Table 2.
Treatment strategies for influenza disease at the Seattle Cancer Care Alliance
| First-line treatment | Second-line treatment | Research protocols | |
|---|---|---|---|
| Asymptomatic shedding | Consider neuraminidase inhibitors (monotherapy) | No treatment, isolation, observe for development of symptoms | None |
| Upper respiratory infection | Oral/inhaled neuraminidase inhibitors (monotherapy)—dependent on circulating strain susceptibility | Oral/inhaled neuraminidase inhibitors (monotherapy)—alternative agent | Triple combination therapy vs monotherapy (phase 2) |
| Lower respiratory tract disease (no respiratory failure) | Oseltamivir (high dose) Consider IV peramivir. Consider combination therapy | Consider IVIG | IV zanamivir (phase 2) |
| Lower respiratory tract disease (respiratory failure, mechanical ventilation) | IV peramivir/zanamivir combination therapy | IVIG | None |
Institutions comprising the Seattle Cancer Care Alliance are Fred Hutchinson Cancer Research Center, University of Washington, and Seattle Children's Hospital.
IVIG indicates intravenous immune globulin.
Choice of neuraminidase inhibitors.
If the susceptibility pattern of the circulating strain is not available, an antiviral effective against all influenza strains (ie, zanamivir) or a drug combination should be used until this information is available. For instance, most seasonal H1N1 influenza viruses circulating during 2008 to 2009 were resistant to oseltamivir, and empiric treatment consisting of zanamivir or oseltamivir plus rimantidine was recommended by the CDC. As of mid-December 2009, the predominant circulating strain in the United States is the 2009 H1N1 strain, which to date is almost universally susceptible to oseltamivir, and always susceptible to zanamivir.
Dose and duration of treatment.
One issue causing considerable debate among physicians caring for highly immunocompromised patients with influenza is that of appropriate dosing and duration of antiviral therapy. Oseltamivir has been studied at doses of 75 mg or 150 mg twice daily in immunocompetent subjects with seasonal influenza, in which no advantage of the higher dose was demonstrated but a somewhat higher rate of adverse effects noted.44,45 The relevance of these studies for highly immunosuppressed patients or with highly virulent strains46 is questionable. Complicating factors include higher viral load in immunocompromised patients, prolonged shedding, uncertain drug absorption especially in patients with chemotherapy-associated gastrointestinal malabsorption or gastrointestinal graft-versus-host disease in HCT recipients, and the propensity to antiviral drug resistance in a setting of viral replication and low-level drug pressure. With 2009 H1N1 influenza, the issue of prolonged viral shedding may be even more pressing.22 Taken together, these factors may favor using a higher dose in immunosuppressed patients, especially when absorption is uncertain.43 We have adopted this strategy (Table 1) for patients with influenza (both seasonal and 2009 H1N1) and lower respiratory tract disease. We also advocate consideration of the higher dose in patients with upper respiratory tract infection due to 2009 H1N1 in a setting of HCT and profound lymphopenia. Whether a high-dose strategy is necessary or beneficial in other immunocompromised patients is unknown.
Original studies of oseltamivir were conducted in healthy patients symptomatic for fewer than 48 hours,44 and there is minimal data concerning treatment of immunosuppressed patients who have had a longer duration of symptoms before treatment. We believe that symptomatic immunocompromised patients with influenza require antiviral therapy regardless of the duration of symptoms before diagnosis. The rationale for this is that these patients have ongoing viral replication, clinical symptoms can be deceivingly mild at presentation and disease onset is often uncertain,15 and progression to lower tract disease may occur even after 1 week of symptoms.16
Little guidance exists regarding the duration of treatment in immunocompromised patients. The recommended duration of treatment of influenza in immunocompetent patients is 5 days, based on clinical studies in previously healthy persons.44 However, longer treatment could be considered in immunosuppressed patients. The median time from diagnosis of upper respiratory tract to lower tract disease is 7 days in HCT recipients.16 Thus, a 5-day course may be too short to prevent cases of late progression. Importantly, prolonged shedding frequently occurs in immunosuppressed patients,47 and may serve as a source of progression of influenza illness and health care-associated disease transmission. We therefore advocate an initial course of 10 days with prolonged treatment for pneumonia and if patient remains symptomatic or if viral shedding is still ongoing (Table 1). We recognize that prolonged treatment may cause extended drug pressure and thus predispose to resistance, especially if the drug levels are low. This needs to be balanced against the potential of recurrence and infection control issues. Therefore, we recommend treatment at higher doses until cessation of shedding. To date, we have not seen an emergence of resistance with this approach but more prospective study of this issue is needed.
Single versus combination therapy.
To date, no data from adequately powered randomized trials are available to demonstrate a benefit from combination antiviral influenza therapy, however, a trend toward less resistance was seen in one relatively small comparative trial.48 Recommendations to use combination therapy in 2008 to 2009 were based on the uncertainty of antiviral susceptibility. This recommendation was not based on synergy but rather on the premise that at least one active agent should be given. However, in vitro and animal synergy data have been used to support the hypothesis that combination therapy may be beneficial.49–52 A triple combination of oseltamivir, ribavirin, and amantidine has been shown to inhibit seasonal, oseltamivir-resistant, and 2009 H1N1 influenza strains more efficiently than single drugs or the combination of 2 drugs in vitro.53 Randomized trials are ongoing in both immunosuppressed and immunocompetent patients with severe infection to test these hypotheses. If patients have severe lower respiratory tract disease, we advocate considering combination therapy when possible, given the poor outcome in immunocompromised patients and the potential for the development of resistance, acknowledging that supporting data from randomized trials are still lacking (Table 2). Combination therapy is also recommended when multiple strains with different sensitivities are circulating.
No evidence exists from randomized trials that pooled intravenous immunoglobulin (IVIG) is beneficial as adjunctive treatment. However, IVIG may be beneficial due to pre-existing neutralizing antibodies in approximately one third of donors7,8 and the potential role of immunoglobulin G2 subclass deficiencies.54 We reserve IVIG for critically ill patients with lower respiratory tract disease and acute lung injury.
Route of administration.
Oseltamivir is commercially available for oral administration and zanamivir, as an inhalation powder. Intravenous administration may be preferable for critically ill patients and those with uncertain absorption or other conditions preventing oral administration (eg, nausea, altered mental status). Peramivir, a novel neuraminidase inhibitor available for intravenous administration, has received an Emergency Use Authorization by the Food and Drug Administration for patients with laboratory-confirmed or untypable but suspected 2009 H1N1 virus infection.55 Specifically, intravenous peramivir is authorized for patients not responding to either oral or inhaled antiviral therapy, or when drug delivery by a route other than intravenous (eg, enteral oseltamivir or inhaled zanamivir) is dependable or feasible, or if the clinician judges intravenous therapy is appropriate. Pediatric patients for whom an intravenous agent is clinically appropriate for similar reasons may also receive this drug under Emergency Use Authorization, although pharmacokinetics in this age group is not yet known. An intravenous form of zanamivir is undergoing phase 2 evaluation in both immunocompetent and immunosuppressed patients. This drug is available through an emergency use program administered by GlaxoSmithKline.
Supportive care.
Bacterial infections are a common complication of seasonal and 2009 H1N1 influenza infection. No evidence exists on the prophylactic use of antibiotics in immunocompromised patients infected in influenza. We routinely evaluate patients with lower respiratory tract disease with BAL and blood cultures for the presence of copathogens. Our threshold to start antibiotics is generally low, especially in critically ill patients, although data from randomized trials are lacking.
The role of steroids is controversial and no data from randomized trials exist.16,20,56 We have no consistent approach with regard to steroids but some clinicians try to reduce immunosuppression if possible. High doses of steroids (≥ 2 mg/kg) prolong viral shedding,16 but there also seems to be an anti-inflammatory effect with moderate doses (up to 1 mg/kg of prednisone) that may be beneficial in patients with acute lung injury or acute respiratory distress syndrome.57 Clearly, more data are needed to make firm recommendations.
Delay of transplantation or chemotherapy.
In HC transplantation candidates with active influenza infection, we administer antiviral therapy (Tables 1–2) and delay myeloablative HC transplantation until resolution of symptoms and cessation of shedding, an approach consistent with recent international guidelines.58 Delay of chemotherapy or nonmyeloablative conditioning regimens is recommended whenever feasible, although supportive data are not available. One study has suggested that progression to lower respiratory tract disease is uncommon after nonmyeloablative conditioning.59 If transplantation or chemotherapy cannot be delayed because of progressive underlying disease, our experience supports that high-dose antiviral therapy, and possibly even combination antiviral therapy, should be given. A less aggressive conditioning regimen should be considered if HC transplantation must proceed.
Pipeline.
The need for innovative approaches could not be more urgent. Evidence suggests that antiviral therapy is associated with reduced morbidity and mortality attributable to influenza in patients receiving chemotherapy or HCT.47,60 However, emergence of drug resistance continues to be a problem,47 and novel influenza strains with unknown virulence continue to emerge. Novel drugs and treatment strategies are critically needed to overcome resistance issues and limited routes of administration, and to improve effectiveness. A novel neuraminidase inhibitor, peramivir, is presently in advanced clinical development (Table 3). Intravenous zanamivir is being studied in a phase 2 clinical trial. An emergency use program exists for both intravenous peramivir and zanamivir.61 An intravenous form of oseltamivir is in early clinical evaluation. Other neuraminidase inhibitors62 as well as compounds targeting the viral polymerase63 and hemaglutinin64 are under investigation (Table 3).
Table 3.
Drugs and drug combinations with activity against influenza virus in clinical development or evaluation
| Drug | Drug target | Manufacturer/sponsor | Route of administration | Clinical development stage | Emergency use mechanism available |
|---|---|---|---|---|---|
| Peramivir | Viral neuraminidase | Biocryst Pharmaceuticals | IV, IM | Phase 3 | EUA |
| Zanamivir | Viral neuraminidase | GlaxoSmithKline | IV | Phase 2 | Emergency use program |
| Oseltamivir | Viral neuraminidase | Roche Pharmaceuticals | IV | Phase 1 | No |
| T-705 (Favipiravir) | Viral polymerase | Toyama Chemical | Oral | Phase 2 (US), phase 3 (Japan) | No |
| CS-8958 (R-118958) | Viral neuraminidase | Daiichi Sankyo Co, Ltd | Inhaled | Phase 3 | No |
| DAS181 | Sialic acid receptor of the respiratory epithelial cells | Nexbio Inc | Inhaled | Phase 2 | No |
| Nitazoxanide | Viral hemaglutinin | Romark Laboratories | Oral | In vitro; no animal or human data | Licensed for treatment of cryptosporidiosis (no indication for influenza treatment) |
| Neuraminidase inhibitor + amantidine + ribavirin | In vitro synergistic combination targeting viral neuraminidase, M2 and depletion of intracellular phosphate of influenza | Adamas Pharmaceuticals | Oral | Phases 2-3 | Licensed drugs (no indication for combination) |
| Oseltamivir + zanamivir | Viral neuraminidase | GlaxoSmithKline Roche Pharmaceuticals | Oral and inhaled | Phases 3-4 | Licensed drugs (no indication for combination) |
EUA indicates emergency use authorization granted by US Food and Drug Administration; and IM, intramuscular.
Due to emergence of resistance, a general plea for combination therapy for influenza disease has been made, and in vitro and animal data support this approach.49,52,53,65 Clinical trials evaluating this are under way. The combination of 2 different neuraminidase inhibitors is also being studied to examine potential drug-drug interactions and possible additive effects.
Treatment approaches for influenza have been directed almost exclusively at viral targets. A novel approach is to target the host respiratory epithelial cell. The compound DAS181 is a novel inhaled blocker of sialic acid receptors in the airway epithelium that prevents viral entry, thereby accelerating viral clearance and preventing progression of viral-induced lung disease.66 DAS181 has activity against influenza viruses, parainfluenza viruses, and human metapneumovirus.67 It has been shown to be active even against highly resistant strains of influenza (including the presently circulating 2009 H1N1 strain and avian H5N1) in vitro and in animal models.66,68
Prevention of influenza
Three principle strategies exist to prevent the acquisition of influenza: infection control practices, vaccination, and chemoprophylaxis (Table 4).
Table 4.
Estimated effectiveness of prevention techniques for influenza virus acquisition in institutions caring for patients with hematologic malignancies
| Target population | Technique |
||||
|---|---|---|---|---|---|
| Hand hygiene | Social distancing | Vaccination | Chemoprophylaxis (after exposure) | Chemoprophylaxis (long-term prophylaxis throughout the season) | |
| Patients | ++++ | ++ | ++ | +++ | ++/+++ |
| Caregivers or family members | ++++ | +++ (if feasible) | ++++ | ++++ | No data |
| Medical staff | ++++ | +++ | ++++ | +++ | No data |
| Other institutional staff | ++++ | + | ++++ | +++ | No data |
++++ indicates highly likely to be effective (data from randomized trials or high quality cohort data); +++, likely to be effective; ++, possibly effective; and +, unlikely to be effective.
Infection control
Personal infection control practices.
Two basic tenets of infection control have been strict attention to hand hygiene and social distancing. A small body of evidence supports the efficacy of hand hygiene (defined as either the use of soap and water or alcohol-based hand sanitizer to cleanse the hands) in reducing influenza virus acquisition.69,70 A recent meta-analysis found that the frequent performance of hand hygiene was associated with a 55% reduction in the risk of acquiring respiratory virus infections.71
Evidence supporting benefits of social distancing is more challenging to find in the medical literature. Typical social distancing measures include reminding patients to keep at least 6 feet from persons who are sick, isolating or cohorting patients with respiratory symptoms in clinical areas, and strictly enforcing a “no-sick-at-work” policy for health care workers.
Infection control for influenza in health care settings.
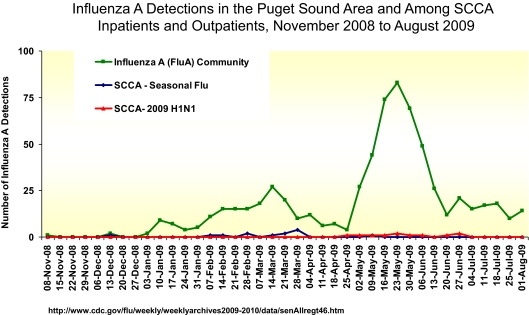
The prevention of influenza in health care settings relies on the early detection of persons with suspected or confirmed illness, and assuring that these patients do not infect susceptible patients and medical staff. The Infection Control Program at the Seattle Cancer Care Alliance has been highly successful in preventing respiratory virus infections among its highly vulnerable cancer patients by adapting the CDC infection control recommendations. As seen in Figure 3, despite a nearly 100-fold increase in influenza cases in the Seattle area during spring of 2009, no corresponding increase in influenza cases among Seattle Cancer Care Alliance (SCCA) patients was seen. The components of our plan include:
Figure 3.
Aggressive implementation of a novel Infection Control Program at the Seattle Cancer Care Alliance assisted in controlling influenza during the H1N1 pandemic of spring 2009. Influenza A detections in the Puget Sound Area and among SCCA inpatients and outpatients, November 2008 to August 2009.
Elimination of potential exposures.
Early identification of persons with potential influenza infection is a cornerstone of institutional infection control. All persons entering the outpatient clinic or inpatient wards are met with hand hygiene stations, replete with alcohol-based hand sanitizer, tissues, and information about respiratory infections and respiratory etiquette. Between October 1 and April 30 at SCCA, an 11-point symptom survey is administered by licensed practical nurses or volunteers to all who enter clinical areas. A sticker color-coded for the day of the week documents the completion of the survey. No person without a sticker is admitted to these areas, and all employees are empowered to enforce this policy. Attempts are made to reschedule patients with respiratory symptoms; those who cannot are given a mask and placed in either an isolation area or private rooms until they can be assessed by their clinical care team. Isolation lists are maintained electronically as part of each patient's medical record. Staff with any symptoms of respiratory infection are furloughed until completely asymptomatic. Respiratory virus testing by multiplex PCR is offered for staff who have minimal residual symptoms but feel well enough to work after an absence of more than 4 days; a negative test is sufficient for return to work.
Engineering controls.
Patients with suspected or confirmed respiratory infections in the outpatient clinic are placed in single rooms as soon as possible or in isolation zones in the waiting area when a room is not immediately available. The inpatient facilities allow for placement of patients with suspected or proven influenza into single rooms, and patients in respiratory isolation follow special protocols when needing to leave their room for procedures, tests, and so forth.
Administrative controls.
A comprehensive isolation plan has been developed and widely distributed for both inpatient and outpatient facilities. Didactic sessions to familiarize staff with policies are conducted on an ongoing basis. The infection control program regularly monitors adherence to hand hygiene and compliance with isolation guidelines, and provides monthly feedback to staff. An electronic surveillance system allows for real-time quantification of the numbers of patients and staff who are infected with influenza, which is scrutinized daily by the infection control team. Other measures include a sick-leave policy that is tolerant of absences for respiratory illnesses, the design of redundant work plans for staff at all levels should absences be required, the requirement to either receive annual influenza vaccination or sign a written declination waiver, an institutional commitment both to educate patients, families, and caregivers about influenza but also to assist with identifying resources for furloughing ill caregivers, and a plan for postexposure prophylaxis of exposed patients and staff.
Personal protective equipment.
For standard seasonal influenza, most organizations agree with the CDC recommendations for droplet precautions for health care personnel (donning of gloves, gowns, and surgical masks when working in close contact with patients with suspected or proven influenza and adding goggles or face shields when appropriate).72 However, some agencies are recommending N95 masks or respirators for either some or all close contact with patients with 2009 H1N1 influenza (Table 5). These recommendations seem inconsistent to us, as no research to date would support a different mode of transmission/acquisition for 2009 H1N1 influenza compared with seasonal influenza. A recent trial randomized nurses caring for patients with acute febrile respiratory illnesses in the 2008 to 2009 season to either fit-tested N95 respirators or surgical masks, and found no significant difference in the proportion in each group who developed laboratory-confirmed influenza.78 This same study also found no difference in the incidence of other laboratory-confirmed respiratory viruses between nurses randomized to either arm, and found a nonsignificant trend toward more influenza-like illness in the nurses with surgical masks (9 vs 1 in the N95 group, P = .06). Taken together, we currently advocate the use of standard surgical masks for all standard exposures (Table 5). We note that these recommendations may change as more information becomes available or United States Occupational Safety & Health Administration rulings become clearer.
Table 5.
Summary of recommendations on mask use in caring for patients with 2009 H1N1 influenza
| Device | CDC73 | WHO74/Canada Public Health75/IOM76 | SHEA/IDSA/APIC/ACOEM77 |
|---|---|---|---|
| Standard surgical masks | Never | Acceptable, except for ″aerosol generating procedures″ | Acceptable, including the following procedures: collection of nasopharyngeal specimens, closed suctioning of airway secretions, and administration of nebulized medications |
| N95 or PAPR | All health care personnel who are in close contact with patients in isolation with confirmed, suspected, or probable 2009 H1N1 influenza should wear a fit-tested disposable N95 respirator or better | Use for ″aerosol generating procedures,″ including ″obtaining specimens by nasopharyngeal aspirate, nasopharyngeal swab, throat swab or bronchial aspirate″ (per WHO, not defined by Canadian Public Health Service) | For bronchoscopy, open suctioning of airway secretions, resuscitation involving emergency intubation or cardiac pulmonary resuscitation, and endotracheal intubation |
CDC indicates Centers for Disease Control and Prevention; WHO, World Health Organization; IOM, Institute of Medicine; SHEA, Society of Healthcare Epidemiologists of America; IDSA, Infectious Diseases Society of America; APIC, Association of Professionals in Infection Control and Epidemiology; and ACOEM, American College of Occupational and Environmental Medicine.
Vaccination.
Two seasonal influenza vaccines are available: trivalent inactivated virus (TIV; injectable) and live attenuated trivalent influenza virus (LAIV; nasal spray). Both are designed to protect against 2 strains of influenza A and 1 strain of influenza B. This year, monovalent inactivated and live attenuated preparations for prevention against 2009 H1N1 influenza have been introduced. These vaccines rely on the growth of a laboratory strain of virus in fertilized chicken eggs. Manufacturing and distribution of seasonal influenza vaccine generally take more than 6 months. Because the current 2009 H1N1 strain was not recognized until April 2009, this year's seasonal influenza vaccine does not contain the 2009 H1N1 strain, and immunization against both seasonal and influenza are recommended. Antibody responses to the 2009 H1N1 vaccine in healthy adults indicate excellent immunogenicity from several different manufacturers.8,9
Vaccination of patients with hematologic malignancies: The ability of patients with hematologic malignancies to generate a protective response after immunization depends on both the patient's underlying disease and its therapy. In general, patients treated with high doses of systemic corticosteroids or recent HC transplantation are least likely to develop protective antibodies after vaccination. Two reviews summarize the existing data on vaccine efficacy in hematologic malignancy patients,35,79 with immune responses ranging from a low of 19% in adults with multiple myeloma to nearly 100% of general hematology-oncology patients. Patients, immediately preceding or in the 6 months after myeloablative conditioning for HC transplantation,80 or those who are within 7 days after receipt of conventional chemotherapy,81,82 are unlikely mount a protective response and should have vaccination deferred, but all others are recommended to receive both seasonal and 2009 H1N1 vaccines. Importantly, contacts of these patients and particularly children in close contact with these patients need appropriate immunization to prevent spread of influenza within the family setting. Patients with hematologic malignancies and their close household contacts should preferentially receive inactivated influenza vaccines due to the theoretic risk for mutation, dissemination, and morbidity from LAIV. In select cases where the inactivated vaccine is unavailable, the risk of complications from acquiring influenza should be weighed against the theoretic risks from the LAIV and its use could be considered.
Vaccination of health care workers: Vaccination of health care workers is an essential component of protecting vulnerable patients with impaired immunity to influenza. Comprehensive vaccination of health care workers reduces the all-causes mortality of the elderly patients by approximately 40%.83 One report of an influenza outbreak on a bone marrow transplant ward suggested that increased rates of vaccination of health care workers in the subsequent year was associated with markedly reduced number of nosocomial cases of influenza.14 Accordingly, health care institutions caring for immunocompromised patients should make 100% staff compliance with influenza vaccination a goal in the control of influenza. Mandatory vaccination of health care workers has been upheld in courts of law and promoted as an ethical imperative.84 Alternatively, a mandatory influenza vaccination declination form, acknowledging the risk posed to vulnerable patients by declining vaccination, can be used.85 The combination of increased influenza educational sessions, easier access to vaccination clinics, and vaccine declination forms has resulted in a greater than 2-fold increase in the uptake of staff receiving influenza vaccine in our institution (Tables 4, 6).
Table 6.
Influenza vaccination guidelines at the Seattle Cancer Care Alliance
| Population | Inactivated vaccine (injection)* | Live-attenuated vaccine (nasal spray)† | Comments |
|---|---|---|---|
| Hematologic malignancy patients (non-HCT) | Yes | No (within 3 mo of chemotherapy‡) | |
| HCT recipients | Yes (6 mo after transplantation) | No | During shortage of inactivated vaccine, LAIV vaccine may be considered late after hematopoietic cell transplantation > 6 mo after discontinuation of all immunosuppressive agents |
| Health care workers | Yes | Yes (second line) | LAIV only during shortage of inactivated vaccine; should not have direct patient contact for at least 3 d |
| Caregivers | Yes | Yes (second line) | If LAIV recipients develop fever (particularly in children), nasal stuffiness, or runny nose (rhinorrhea), they are at higher risk of shedding the vaccine virus, and should remain sequestered from the patient for at least 3 d |
| Visitors | Yes | Yes (second line) |
Contraindications (obtained from package insert): Persons with a history of Guillain-Barré syndrome that occurred after receiving influenza vaccine; persons who have a severe allergy to chicken eggs.
Contraindications (obtained from package insert): persons younger than 2 or older than 50 years; persons with a medical condition that places them at high risk for complications from influenza, including those with chronic heart or lung disease, such as asthma or reactive airways disease; people with medical conditions such as diabetes or kidney failure; or people with illnesses that weaken the immune system, or who take medications that can weaken the immune system; children younger than 5 years with a history of recurrent wheezing; children or adolescents receiving aspirin; persons with a history of Guillain-Barré syndrome that occurred after receiving influenza vaccine; pregnant women; and persons who have a severe allergy to chicken eggs or who are allergic to any of the nasal spray vaccine components.
Based on authors' opinion.
Use of the LAIV in hematologic malignancy patients and staff: LAIV contains strains of influenza that are both cold-adapted (therefore unable to replicate in the lower airways) and have reduced pathogenicity. Although illness after receipt of LAIV is very unusual, rare cases of transmission of LAIV but without symptomatic disease to close contacts have been documented. Transmission of LAIV is very rare, with an estimated rate of 0.58% in a daycare setting where young influenza-naive children received either vaccine or placebo, and secretions were liberally shared.86 Health care workers administering the vaccine have also been infected.87,88 Recent studies find the LAIV to be more effective than TIV in producing protection among children younger than 6 years89 but may be less effective than TIV in adults,90 perhaps due to incomplete pre-existing immunity in adults that neutralizes the vaccine strain. Shedding of vaccine strain in healthy children and adults generally lasts 3 to 7 days. This vaccine is not recommended for immunocompromised patients, although safety data in HIV-infected persons and children on maintenance chemotherapy have demonstrated safety.91
More concern about the administration of LAIV to health care workers has emerged recently with the availability of 2009 H1N1 live vaccine and a simultaneous shortage of 2009 H1N1 monovalent inactivated vaccine. This concern is based on theoretic concerns about lack of control of replication in immunocompromised hosts, potential for recombination with wild-type virus during prolonged periods of shedding, and documentation of rare cases of secondary transmission after vaccination. The CDC recommends that LAIV may be given to persons, including health care workers, who are not involved in the care of patients requiring protective isolation.92 Until recently, our institution has restricted the use of LAIV to persons without direct patient contact for a period of at least 7 days. However, we have recently modified our approach due to the shortage of the TIV, the high risk of transmission of wild-type strain (10%–37% transmission rate93; J.E., personal oral communication, December 2009), and the overall low risk of transmission with LAIV (0.58%-2.4%), and permitted use of LAIV in health care workers in outpatient settings and family members of patients more than 100 days after transplantation (Table 6).
Chemoprophylaxis.
Vaccination is our primary prophylaxis modality. However, persons with significant exposure (ie, close face-to-face contact for more than just a few seconds or direct contact with direct secretion) to confirmed cases of influenza who have not been vaccinated (or who received the vaccination less than 3 weeks before the time of exposure) should be considered for prophylactic antiviral therapy with either oseltamivir or zanamivir based on the susceptibility of the circulating strain. The CDC has also endorsed “preemptive” therapy as an option for persons exposed to influenza, where antiviral use is deferred until the exposed person manifests symptoms. We do not endorse the preemptive approach for hematologic malignancy and HCT patients, but have considered it to be a useful alternative, for example, in certain health care workers with limited patient contact. Postexposure therapy should not be delayed until influenza testing is returned on exposed persons.
Antiviral prophylaxis given to all patients at risk during a community or institutional outbreak is another option.94 Initial results from a randomized trial in mildly to moderately immunosuppressed (mainly solid organ) transplant recipients has been presented95 and suggests a beneficial effect for laboratory-confirmed seasonal influenza with little drug resistance. Whether such approach is effective without increased emergence of resistance in severely immunocompromised patients with the current pandemic strain is unknown, although resistance has been described even in normal hosts receiving prophylaxis.61 We would consider this option if a nosocomial outbreak were to occur. Such a strategy has been successfully used in a seasonal influenza outbreak at an outpatient living facility.94 The 2008 global HC transplantation infection prevention guidelines endorse the use of antiviral prophylaxis during community outbreaks that lead to nosocomial transmission.58
Conclusions and future directions
The initial experience with 2009 H1N1 influenza suggests that this virus can cause serious disease in immunosuppressed patients. Clinical findings include respiratory failure, treatment failures due to drug resistance, and death28,61 (and unpublished data). Published data are currently unavailable but based on the course of the pandemic at our center and elsewhere several concepts appear to emerge. First, rapid diagnosis using PCR is critical for identifying infected patients, initiating antiviral treatment, and implementing appropriate infection controls. Second, effective antiviral treatment should be started early. In critically ill patients with compatible symptoms and influenza circulating at high levels in the community, empiric treatment while awaiting test results seems justified. We also favor a higher dose of oseltamivir in analogy to recommendations for H5N1 avian influenza23,46 and the questionable absorption in patients with chemotherapy- or graft-versus-host disease–associated malabsorption. Third, our experience shows that aggressive infection control procedures can minimize transmission within the immunocompromised patient population and also reduce acquisition from sources outside the system.
Future work should focus on conducting randomized trials in immunocompromised patients, the development and evaluation of drugs with new mechanisms of action (Table 3), and the evaluation of combination therapies and adjunctive anti-inflammatory agents. To fully understand the impact of the 2009 H1N1 and seasonal influenza, studies of the epidemiology, risk factors, and factors associated with outcome should be initiated using existing networks to increase sample size and allow for multivariable analyses. Initial observations suggest a wide spectrum of disease severity and that patients with hematologic malignancies are not a homogeneous group. Therefore, pathogenesis studies are needed to define the role of the underlying immunosuppression, viral load, and cytokine responses, and to evaluate RNA detection in blood and other biomarkers that may correlate with disease severity.
Acknowledgment
This work was supported by the National Institutes of Health (CA1809, HL 093294, and HL 081595).
Authorship
Contribution: C.C., J.E., and M.B. researched the topic and wrote the paper.
Conflict-of-interest disclosure: J.E. received research support from MedImmune, Novartis, Sanofi Pasteur, and Adamas Pharmaceuticals. M.B. served as a consultant for Roche Pharmaceuticals and Novartis, and received research support from Roche Pharmaceuticals, Adamas Pharmaceuticals, GlaxoSmithKline, and Nexbio Inc (expected). C.C. declares no competing financial interests.
Correspondence: Michael Boeckh, Vaccine and Infectious Disease Institute, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, Mailstop D3-100, Seattle, WA 98109; e-mail: mboeckh@fhcrc.org.
References
- 1.Domínguez-Cherit G, Lapinsky SE, Macias AE, et al. Critically ill patients with 2009 influenza A(H1N1) in Mexico. JAMA. 2009;302(17):1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 2.Swine influenza A (H1N1) infection in two children—Southern California, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58(15):400–402. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2009 H1N1 Flu: International Situation Update. [Accessed October 17, 2009]. http://www.cdc.gov/h1n1flu/updates/international.
- 4.US Centers for Disease Control and Prevention. Percentage of Visits for Influenza-like-Illness Reported by Sentinel Providers 2009-2010. [Accessed January 21, 2010]. http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/data/senAllregt46.htm.
- 5.Morens DM, Taubenberger JK, Fauci AS. The persistent legacy of the 1918 influenza virus. N Engl J Med. 2009;361(3):225–229. doi: 10.1056/NEJMp0904819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Centers for Disease Control and Prevention. 2009 H1N1 Early Outbreak and Disease Characteristics. [Accessed January 21, 2010]. http://www.cdc.gov/H1N1FLU/surveillanceqa.htm.
- 7.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–1952. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg ME, Lai MH, Hartel GF, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361(25):2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 9.Zhu FC, Wang H, Fang HH, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361(25):2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 10.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14(33):19309. doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 11.Whimbey E, Elting LS, Couch RB, et al. Influenza A virus infections among hospitalized adult bone marrow transplant recipients. Bone Marrow Transplant. 1994;13(4):437–440. [PubMed] [Google Scholar]
- 12.Elting LS, Whimbey E, Lo W, Couch R, Andreeff M, Bodey GP. Epidemiology of influenza A virus infection in patients with acute or chronic leukemia. Support Care Cancer. 1995;3(3):198–202. doi: 10.1007/BF00368891. [DOI] [PubMed] [Google Scholar]
- 13.Yousuf HM, Englund J, Couch R, et al. Influenza among hospitalized adults with leukemia. Clin Infect Dis. 1997;24(6):1095–1099. doi: 10.1086/513648. [DOI] [PubMed] [Google Scholar]
- 14.Weinstock DM, Eagan J, Malak SA, et al. Control of influenza A on a bone marrow transplant unit. Infect Control Hosp Epidemiol. 2000;21(11):730–732. doi: 10.1086/501726. [DOI] [PubMed] [Google Scholar]
- 15.Peck AJ, Englund JA, Kuypers J, et al. Respiratory virus infection among hematopoietic cell transplant recipients: evidence for asymptomatic parainfluenza virus infection. Blood. 2007;110(5):1681–1688. doi: 10.1182/blood-2006-12-060343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nichols WG, Guthrie KA, Corey L, Boeckh M. Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis. 2004;39(9):1300–1306. doi: 10.1086/425004. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28(2):222–242. doi: 10.1055/s-2007-976494. [DOI] [PubMed] [Google Scholar]
- 18.Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine (Baltimore) 2006;85(5):278–287. doi: 10.1097/01.md.0000232560.22098.4e. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7(suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 20.Shah J, VigilL K, Adachi J, Granwehr B, Raad I, Chemaly R. Influenza virus infections in pediatric cancer patients—emphasis on preemptive therapy [abstract].. Presented at the Annual Meeting of the Infectious Disease Society of America; October 31, 2009; Philadelphia, PA. Abstract 1098. [Google Scholar]
- 21.Campbell A, Chien J, Kuypers J, et al. Respiratory virus pneumonia after hematopoietic cell transplantation: viral load in bronchoalveolar lavage and RNA detection in serum as possible predictors of outcome. J Infect Dis. doi: 10.1086/651662. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munster VJ, de Wit E, van den Brand JM, et al. Pathogenesis and transmission of swine-origin 2009 A(H1N1) influenza virus in ferrets. Science. 2009;325(5939):481–483. doi: 10.1126/science.1177127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143(4):455–467. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chemaly RF, Torres HA, Aguilera EA, et al. Neuraminidase inhibitors improve outcome of patients with leukemia and influenza: an observational study. Clin Infect Dis. 2007;44(7):964–967. doi: 10.1086/512374. [DOI] [PubMed] [Google Scholar]
- 26.Machado CM, Boas LS, Mendes AV, et al. Low mortality rates related to respiratory virus infections after bone marrow transplantation. Bone Marrow Transplant. 2003;31(8):695–700. doi: 10.1038/sj.bmt.1703900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erard V, Chien JW, Kim HW, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193(12):1619–1625. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302(17):1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 29.Lapinsky SE. H1N1 novel influenza A in pregnant and immunocompromised patients. Crit Care Med. 2009 Nov 23; doi: 10.1097/CCM.0b013e3181c85d5f. [DOI] [PubMed] [Google Scholar]
- 30.Redelman-Sidi G, Kamboj M, Huang C-K, et al. Pandemic H1N1 Influenza Infection in Cancer Patients.. Presented at the Annual Meeting of the Infectious Disease Society of America; November 1, 2009; Philadelphia, PA. Abstract LB-51. [Google Scholar]
- 31.Dawood FS, Jain S, Finelli L, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 32.Ginocchio CC, Zhang F, Manji R, et al. Evaluation of multiple test methods for the detection of the novel 2009 influenza A (H1N1) during the New York City outbreak. J Clin Virol. 2009;45(3):191–195. doi: 10.1016/j.jcv.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooskens J, Jonges M, Claas EC, Meijer A, van den Broek PJ, Kroes AM. Morbidity and mortality associated with nosocomial transmission of oseltamivir-resistant influenza A (H1N1) virus. JAMA. 2009;301(10):1042–1046. doi: 10.1001/jama.2009.297. [DOI] [PubMed] [Google Scholar]
- 34.Kaiser L, Briones MS, Hayden FG. Performance of virus isolation and Directigen Flu A to detect influenza A virus in experimental human infection. J Clin Virol. 1999;14(3):191–197. doi: 10.1016/s1386-6532(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 35.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9(8):493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.US Centers for Disease Control and Prevention. [Accessed __].
- 37.Chan-Tack KM, Murray JS, Birnkrant DB. Use of ribavirin to treat influenza. N Engl J Med. 2009;361(17):1713–1714. doi: 10.1056/NEJMc0905290. [DOI] [PubMed] [Google Scholar]
- 38.Shima T, Yoshimoto G, Nonami A, et al. Successful treatment of parainfluenza virus 3 pneumonia with oral ribavirin and methylprednisolone in a bone marrow transplant recipient. Int J Hematol. 2008;88(3):336–340. doi: 10.1007/s12185-008-0148-6. [DOI] [PubMed] [Google Scholar]
- 39.Chakrabarti S, Collingham KE, Holder K, Fegan CD, Osman H, Milligan DW. Pre-emptive oral ribavirin therapy of paramyxovirus infections after haematopoietic stem cell transplantation: a pilot study. Bone Marrow Transplant. 2001;28(8):759–763. doi: 10.1038/sj.bmt.1703216. [DOI] [PubMed] [Google Scholar]
- 40.Sparrelid E, Ljungman P, Ekelof-Andstrom E, et al. Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplant. 1997;19(9):905–908. doi: 10.1038/sj.bmt.1700752. [DOI] [PubMed] [Google Scholar]
- 41.Machado CM. Influenza infections after hematopoietic stem cell transplantation. Clin Infect Dis. 2005;41(2):273–274. doi: 10.1086/431304. [DOI] [PubMed] [Google Scholar]
- 42.Johny AA, Clark A, Price N, Carrington D, Oakhill A, Marks DI. The use of zanamivir to treat influenza A and B infection after allogeneic stem cell transplantation. Bone Marrow Transplant. 2002;29(2):113–115. doi: 10.1038/sj.bmt.1703343. [DOI] [PubMed] [Google Scholar]
- 43.Khanna N, Steffen I, Studt JD, et al. Outcome of influenza infections in outpatients after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis. 2009;11(2):100–105. doi: 10.1111/j.1399-3062.2008.00362.x. [DOI] [PubMed] [Google Scholar]
- 44.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial: US Oral Neuraminidase Study Group. JAMA. 2000;283(8):1016–1024. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 45.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial: Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355(9218):1845–1850. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 46.Govorkova EA, Ilyushina NA, Boltz DA, Douglas A, Yilmaz N, Webster RG. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob Agents Chemother. 2007;51(4):1414–1424. doi: 10.1128/AAC.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis. 2009;199(10):1435–1441. doi: 10.1086/598684. [DOI] [PubMed] [Google Scholar]
- 48.Ison MG, Gnann JW, Jr, Nagy-Agren S, et al. Safety and efficacy of nebulized zanamivir in hospitalized patients with serious influenza. Antivir Ther. 2003;8(3):183–190. [PubMed] [Google Scholar]
- 49.Nguyen JT, Hoopes JD, Smee DF, et al. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother. 2009;53(10):4115–4126. doi: 10.1128/AAC.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smee DF, Hurst BL, Wong MH, Bailey KW, Morrey JD. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob Agents Chemother. 2009;53(5):2120–2128. doi: 10.1128/AAC.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smee DF, Hurst BL, Wong MH, et al. Effects of the combination of favipiravir (T-705) and oseltamivir on influenza a virus infections in mice. Antimicrob Agents Chemother. 2010;54(1):126–133. doi: 10.1128/AAC.00933-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poland GA, Jacobson RM, Ovsyannikova IG. Influenza virus resistance to antiviral agents: a plea for rational use. Clin Infect Dis. 2009;48(9):1254–1256. doi: 10.1086/598989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nguyen J, Hoopes J, Le M, Prichard M, Went G. InVitro Activity of Triple Combination Therapy against Drug-Resistant InfluenzaViruses.. Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. 2009. [Google Scholar]
- 54.Gordon L, Johnson P, PermezelL M, et al. Association between Severe Swine-origin Influenza A Virus (S-OIV) Infection and Immunoglobulin G2 Subclass Deficiency Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco. 2009. [Google Scholar]
- 55.US Centers for Disease Control and Prevention. Emergency Use Authorization of Peramivir IV Fact Sheet For Health Care Providers. 2009. Nov 19, [Accessed January 21, 2010]. http://www.cdc.gov/h1n1flu/eua/Final%20HCP%20Fact%20sheet%20Peramivir%20IV_CDC.pdf.
- 56.Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 57.Quispe-Laime AM, Bracco JD, Barberio PA, et al. H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med. 2010;36(1):33–41. doi: 10.1007/s00134-009-1727-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplant recipients: a global perspective: preface. Bone Marrow Transplant. 2009;44(8):453–455. doi: 10.1038/bmt.2009.254. [DOI] [PubMed] [Google Scholar]
- 59.Schiffer JT, Kirby K, Sandmaier B, Storb R, Corey L, Boeckh M. Timing and severity of community acquired respiratory virus infections after myeloablative versus non-myeloablative hematopoietic stem cell transplantation. Haematologica. 2009;94(8):1101–1108. doi: 10.3324/haematol.2008.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee N, Chan PK, Choi KW, et al. Factors associated with early hospital discharge of adult influenza patients. Antivir Ther. 2007;12(4):501–508. [PubMed] [Google Scholar]
- 61.Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58(32):893–896. [PubMed] [Google Scholar]
- 62.Koyama K, Takahashi M, Oitate M, et al. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long-retention profile in the mouse respiratory tract. Antimicrob Agents Chemother. 2009;53(11):4845–4851. doi: 10.1128/AAC.00731-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Furuta Y, Takahashi K, Shiraki K, et al. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009;82(3):95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284(43):29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hayden F. Developing new antiviral agents for influenza treatment: what does the future hold? Clin Infect Dis. 2009;48(suppl 1):S3–S13. doi: 10.1086/591851. [DOI] [PubMed] [Google Scholar]
- 66.Triana-Baltzer GB, Gubareva LV, Klimov AI, et al. Inhibition of neuraminidase inhibitor-resistant influenza virus by DAS181, a novel sialidase fusion protein. PLoS One. 2009;4(11):e7838. doi: 10.1371/journal.pone.0007838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Malakhov MP, Aschenbrenner LM, Smee DF, et al. Sialidase fusion protein as a novel broad-spectrum inhibitor of influenza virus infection. Antimicrob Agents Chemother. 2006;50(4):1470–1479. doi: 10.1128/AAC.50.4.1470-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan RW, Chan MC, Wong AC, et al. DAS181 inhibits H5N1 influenza virus infection of human lung tissues. Antimicrob Agents Chemother. 2009;53(9):3935–3941. doi: 10.1128/AAC.00389-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grayson ML, Melvani S, Druce J, et al. Efficacy of soap and water and alcohol-based hand-rub preparations against live H1N1 influenza virus on the hands of human volunteers. Clin Infect Dis. 2009;48(3):285–291. doi: 10.1086/595845. [DOI] [PubMed] [Google Scholar]
- 70.Cowling BJ, Chan KH, Fang VJ, et al. Facemasks and hand hygiene to prevent influenza transmission in households: a cluster randomized trial. Ann Intern Med. 2009;151(7):437–446. doi: 10.7326/0003-4819-151-7-200910060-00142. [DOI] [PubMed] [Google Scholar]
- 71.Jefferson T, Del Mar C, Dooley L, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. BMJ. 2009;339:b3675. doi: 10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siegel JD, Rhinehart E, Jackson M, Chiarello L and the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings ( http://www.cdc.gov/ncidod/dhqp/pdf/isolation2007.pdf). [Accessed January 21, 2010].
- 73.Centers for Disease Control and Prevention. Interim Guidance on Infection Control Measures for 2009 H1N1 Influenza in Healthcare Settings, Including Protection of Healthcare Personnel ( http://www.cdc.gov/h1n1flu/guidelines_infection_control.htm). [Accessed January 21, 2010]. [PubMed]
- 74.World Health Organization. Infection prevention and control in health care for confirmed or suspected cases of pandemic (H1N1) 2009 and influenza-like illnesses ( http://www.who.int/csr/resources/publications/swineflu/swineinfinfcont/en/index.html). [Accessed January 21, 2010].
- 75.Public Health Agency of Canada. Guidance: Infection prevention and control measures for Health Care Workers in Acute Care Facilities ( http://www.phac-aspc.gc.ca/alert-alerte/h1n1/hpps/ig_acf-ld_esa-eng.php). [Accessed October 18, 2009].
- 76.Institute of Medicine. Respiratory Protection for Healthcare Workers in the Workplace Against Novel H1N1 Influenza A ( http://www.iom.edu/en/Reports/2009/RespProtH1N1.aspx). [Accessed October 18, 2009]. [PubMed]
- 77. [Accessed January 21, 2010]. www.sheaonline.org/…/FINAL_Joint_SHEA_APIC_IDSA_ACOEM_Position_Statement_High_Risk_HCW.pdf.
- 78.Loeb M, Dafoe N, Mahony J, et al. Surgical mask vs N95 respirator for preventing influenza among health care workers: a randomized trial. JAMA. 2009;302(17):1865–1871. doi: 10.1001/jama.2009.1466. [DOI] [PubMed] [Google Scholar]
- 79.Ring A, Marx G, Steer C, Harper P. Influenza vaccination and chemotherapy: a shot in the dark? Support Care Cancer. 2002;10(6):462–465. doi: 10.1007/s00520-001-0337-9. [DOI] [PubMed] [Google Scholar]
- 80.Ljungman P, Avetisyan G. Influenza vaccination in hematopoietic SCT recipients. Bone Marrow Transplant. 2008;42(10):637–641. doi: 10.1038/bmt.2008.264. [DOI] [PubMed] [Google Scholar]
- 81.Ortbals DW, Liebhaber H, Presant CA, Van Amburg AL, III, Lee JY. Influenza immunization of adult patients with malignant diseases. Ann Intern Med. 1977;87(5):552–557. doi: 10.7326/0003-4819-87-5-552. [DOI] [PubMed] [Google Scholar]
- 82.Robertson JD, Nagesh K, Jowitt SN, et al. Immunogenicity of vaccination against influenza, Streptococcus pneumoniae and Haemophilus influenzae type B in patients with multiple myeloma. Br J Cancer. 2000;82(7):1261–1265. doi: 10.1054/bjoc.1999.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas RE, Jefferson T, Demicheli V, Rivetti D. Influenza vaccination for healthcare workers who work with the elderly. Cochrane Database Syst Rev. 2006;3:CD005187. doi: 10.1002/14651858.CD005187.pub2. [DOI] [PubMed] [Google Scholar]
- 84.Tilburt JC, Mueller PS, Ottenberg AL, Poland GA, Koenig BA. Facing the challenges of influenza in healthcare settings: the ethical rationale for mandatory seasonal influenza vaccination and its implications for future pandemics. Vaccine. 2008;26(suppl 4):D27–D30. doi: 10.1016/j.vaccine.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 85.Ribner BS, Hall C, Steinberg JP, et al. Use of a mandatory declination form in a program for influenza vaccination of healthcare workers. Infect Control Hosp Epidemiol. 2008;29(4):302–308. doi: 10.1086/529586. [DOI] [PubMed] [Google Scholar]
- 86.Kamboj M, Sepkowitz KA. Risk of transmission associated with live attenuated vaccines given to healthy persons caring for or residing with an immunocompromised patient. Infect Control Hosp Epidemiol. 2007;28(6):702–707. doi: 10.1086/517952. [DOI] [PubMed] [Google Scholar]
- 87.Izurieta HS, Haber P, Wise RP, et al. Adverse events reported following live, cold-adapted, intranasal influenza vaccine. JAMA. 2005;294(21):2720–2725. doi: 10.1001/jama.294.21.2720. [DOI] [PubMed] [Google Scholar]
- 88.Vesikari T, Karvonen A, Korhonen T, et al. A randomized, double-blind study of the safety, transmissibility and phenotypic and genotypic stability of cold-adapted influenza virus vaccine. Pediatr Infect Dis J. 2006;25(7):590–595. doi: 10.1097/01.inf.0000220229.51531.47. [DOI] [PubMed] [Google Scholar]
- 89.Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25(10):860–869. doi: 10.1097/01.inf.0000237797.14283.cf. [DOI] [PubMed] [Google Scholar]
- 90.Monto AS, Ohmit SE, Petrie JG, et al. Comparative efficacy of inactivated and live attenuated influenza vaccines. N Engl J Med. 2009;361(13):1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 91.King JC, Jr, Fast PE, Zangwill KM, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J. 2001;20(12):1124–1131. doi: 10.1097/00006454-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 92.Fiore AE, Shay DK, Broder K, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58(RR-8):1–52. [PubMed] [Google Scholar]
- 93.Witkop CT, Duffy MR, Macias EA, et al. Novel Influenza A (H1N1) Outbreak at the U.S. Air Force Academy Epidemiology and Viral Shedding Duration. Am J Prev Med. 2009 Oct 21; doi: 10.1016/j.amepre.2009.10.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 94.Vu D, Peck AJ, Nichols WG, et al. Safety and tolerability of oseltamivir prophylaxis in hematopoietic stem cell transplant recipients: a retrospective case-control study. Clin Infect Dis. 2007;45(2):187–193. doi: 10.1086/518985. [DOI] [PubMed] [Google Scholar]
- 95.Ison M, Szakaly P, Shapira M, Kriván G, Nist A, Dutkowski R. Oseltamivir prophylaxis significantly reduces the incidence of seasonal influenza infection in immunocompromized patients.. XI International Symposium on Respiratory Viral Infections.; Bangkok, Thailand. 2009. [Google Scholar]