Abstract
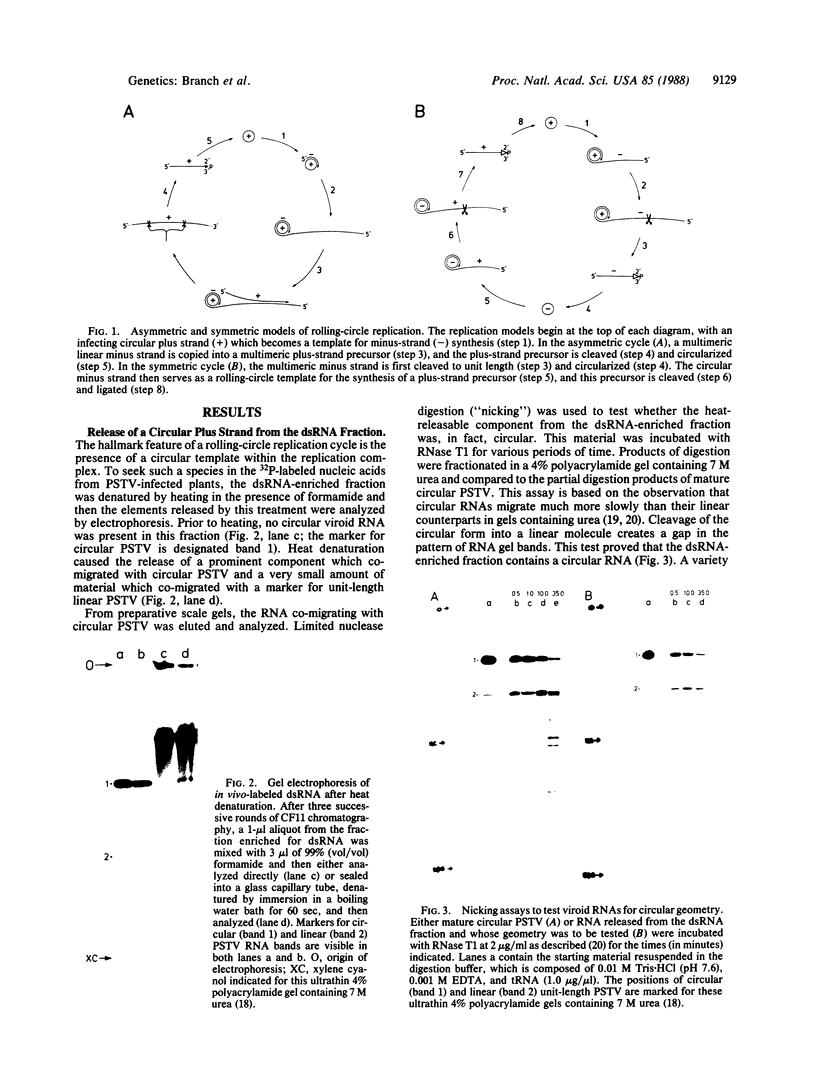
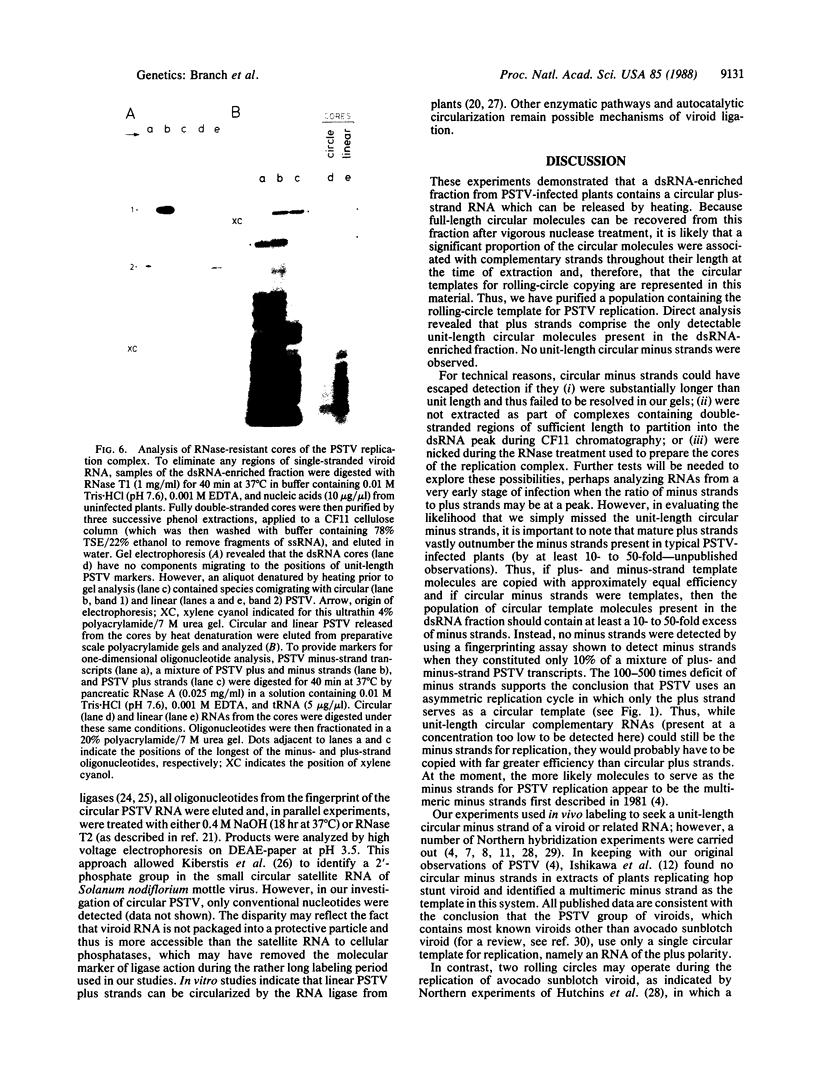
We analyzed in vivo-labeled RNA to determine which of the two proposed rolling-circle models is more likely to depict the replication cycle of potato spindle tuber viroid. A key feature distinguishing the two models is the presence of a circular monomeric minus strand in one and not the other. Chromatography on cellulose CF11 was used to purify a fraction containing the replication intermediates free from single-stranded progeny. Heat denaturation followed by gel electrophoresis was used to seek possible circular templates—species required for rolling-circle replication to take place. Upon heating, a 32P-labeled RNA was released. Limited nuclease digestion (“nicking”) revealed that this was a unitlength circular RNA. Fingerprinting identified it as a plus strand. No circular minus strands were detected in this population or in nuclease-treated samples containing RNase T1-resistant cores of the replication complex. Thus, potato spindle tuber viroid appears to use an asymmetric pathway in which minus strands are synthesized by rolling-circle copying, but plus strands are not. More details of the replication pathways used by various viroid-like RNAs are needed and will help to establish the evolutionary relationships among these infectious agents.
Keywords: double-stranded RNA, viroid-like RNAs, cellulose CF11 chromatography, in vivo RNA labeling
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Benenfeld B. J., Franck E. R., Shaw J. F., Varban M. L., Willis K. K., Rosen D. L., Robertson H. D. Interference between coinoculated viroids. Virology. 1988 Apr;163(2):538–546. doi: 10.1016/0042-6822(88)90294-2. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Dickson E. Longer-than-unit-length viroid minus strands are present in RNA from infected plants. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6381–6385. doi: 10.1073/pnas.78.10.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Greer C., Gegenheimer P., Peebles C., Abelson J. Cell-free circularization of viroid progeny RNA by an RNA ligase from wheat germ. Science. 1982 Sep 17;217(4565):1147–1149. doi: 10.1126/science.217.4565.1147. [DOI] [PubMed] [Google Scholar]
- Chen P. J., Kalpana G., Goldberg J., Mason W., Werner B., Gerin J., Taylor J. Structure and replication of the genome of the hepatitis delta virus. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8774–8778. doi: 10.1073/pnas.83.22.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson E., Diener T. O., Robertson H. D. Potato spindle tuber and citrus exocortis viroids undergo no major sequence changes during replication in two different hosts. Proc Natl Acad Sci U S A. 1978 Feb;75(2):951–954. doi: 10.1073/pnas.75.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francki R. I. Plant virus satellites. Annu Rev Microbiol. 1985;39:151–174. doi: 10.1146/annurev.mi.39.100185.001055. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer C. L., Peebles C. L., Gegenheimer P., Abelson J. Mechanism of action of a yeast RNA ligase in tRNA splicing. Cell. 1983 Feb;32(2):537–546. doi: 10.1016/0092-8674(83)90473-7. [DOI] [PubMed] [Google Scholar]
- Grill L. K., Semancik J. S. RNA sequences complementary to citrus exocortis viroid in nucleic acid preparations from infected Gynura aurantiaca. Proc Natl Acad Sci U S A. 1978 Feb;75(2):896–900. doi: 10.1073/pnas.75.2.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit length RNA in viroid replication. Mol Gen Genet. 1984;196(3):421–428. doi: 10.1007/BF00436189. [DOI] [PubMed] [Google Scholar]
- Keese P., Symons R. H. Domains in viroids: evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4582–4586. doi: 10.1073/pnas.82.14.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiberstis P. A., Haseloff J., Zimmern D. 2' phosphomonoester, 3'-5' phosphodiester bond at a unique site in a circular viral RNA. EMBO J. 1985 Mar;4(3):817–822. doi: 10.1002/j.1460-2075.1985.tb03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi Y., Tyc K., Filipowicz W., Sänger H. L., Gross H. J. Circularization of linear viroid RNA via 2'-phosphomonoester, 3', 5'-phosphodiester bonds by a novel type of RNA ligase from wheat germ and Chlamydomonas. Nucleic Acids Res. 1982 Dec 11;10(23):7521–7529. doi: 10.1093/nar/10.23.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M., Filipowicz W., Domdey H., Gross H. J. Formation of a 2'-phosphomonoester, 3',5'-phosphodiester linkage by a novel RNA ligase in wheat germ. Nature. 1981 Sep 10;293(5828):112–116. doi: 10.1038/293112a0. [DOI] [PubMed] [Google Scholar]
- Kos A., Dijkema R., Arnberg A. C., van der Meide P. H., Schellekens H. The hepatitis delta (delta) virus possesses a circular RNA. Nature. 1986 Oct 9;323(6088):558–560. doi: 10.1038/323558a0. [DOI] [PubMed] [Google Scholar]
- Nicholson A. W., Frankfort H. M., Davis N. G., Ferrari S., Lamb R. A., Robertson H. D. Direct characterization of influenza viral NS1 mRNA and related sequences from infected HeLa cells and a cell-free transcription system. Biochim Biophys Acta. 1986 Nov 13;868(2-3):153–163. doi: 10.1016/0167-4781(86)90018-7. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Cress D. E. Molecular cloning and characterization of potato spindle tuber viroid cDNA sequences. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5302–5306. doi: 10.1073/pnas.77.9.5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Spiesmacher E., Mühlbach H. P., Schnölzer M., Haas B., Sänger H. L. Oligomeric forms of potato spindle tuber viroid (PSTV) and of its complementary RNA are present in nuclei isolated from viroid-infected potato cells. Biosci Rep. 1983 Aug;3(8):767–774. doi: 10.1007/BF01120988. [DOI] [PubMed] [Google Scholar]
- Wang K. S., Choo Q. L., Weiner A. J., Ou J. H., Najarian R. C., Thayer R. M., Mullenbach G. T., Denniston K. J., Gerin J. L., Houghton M. Structure, sequence and expression of the hepatitis delta (delta) viral genome. Nature. 1986 Oct 9;323(6088):508–514. doi: 10.1038/323508a0. [DOI] [PubMed] [Google Scholar]