Abstract
Engineered vascularized adipose tissue could serve as an alternative to traditional tissue reconstruction procedures. Adipose formation occurs in a coordinated fashion with neovascularization. Previous studies have shown that extracellular matrix-based materials supplemented with factors that stimulate neovascularization promote adipogenesis in a number of animal models. The present study examines the ability of fibroblast growth factor (FGF-1) delivered from alginate microbeads to induce neovascularization and adipogenesis in type I collagen gels in a vascular pedicle model of adipose tissue engineering. FGF-1 loaded microbeads stimulated greater vascular network formation in an in vitro 3D co-culture model compared than a single bolus of FGF-1. In in vivo studies, FGF-1 loaded beads suspended in collagen and implanted in a chamber surrounding the exposed femoral pedicle of a rat resulted in a significant increase in vascular density at 1 and 6 weeks in comparison to bolus administration of FGF-1. Staining for smooth muscle actin showed that over 48% of vessels had associated mural cells. While an increase in neovascularization was achieved, there was less than 3% adipose under any condition. These results show that delivery of FGF-1 from alginate beads stimulated a more persistent neovascularization response than bolus FGF-1 both in vitro and in vivo. However, unlike previous studies, this increased neovascularization did not result in adipogenesis. Future studies need to provide a better understanding of the relationship between neovascularization and adipogenesis in order to design advanced tissue engineering therapies.
Introduction
A significant challenge to the creation of clinically functional tissue engineering products is appropriate vascularization that provide oxygen and nutrients and promote integration of the tissue constructs.[1, 2] Despite almost 2 decades of extensive research in tissue engineering, clinical successes consist primarily of tissues which are thin enough that the normal neovascularization process is sufficient or tissues that can largely survive via diffusion of nutrients form existing vasculature.[3, 4] One strategy that has been explored to improve the function of engineered tissue is to stimulate neovascularization through the use of soluble, naturally-occurring growth factors. Stimulating or accelerating vascular formation using growth factors is a promising solution; however therapeutic effect of the proteins can be enhanced through use of appropriate delivery method. The delivery of a single, high concentration of proteins can stimulate a response, but sustained delivery of proteins has been demonstrated to be the most effective use of these proteins for stimulating a persistent neovascularization response. [5–7]
Engineered adipose tissue could eventually serve as a material for use in the replacement of lost or damaged tissue due to tumor removal, trauma, lipoatrophy, or congenital defects. However, the growth of adipose tissue is tightly coupled to neovascularization. In adulthood, increases in adipose tissue are accompanied by increases in the microcirculation. [8] The concomitant relationship between angiogenesis and adipogenesis is not fully understood, albeit, but the dependency of adipogenesis on neovascularization been observed during embryonic development [9, 10] and in wound healing where vascular formation precedes adipogenesis [1]. This information has been used to design approaches to adipose tissue engineering that incorporate factors that stimulate neovascularization.
Previous studies has demonstrated that Matrigel, a laminin-111 rich extract from a mouse sarcoma, induces adipogenesis when supplemented with fibroblast growth factor 2 [11, 12], vascular endothelial growth factor-120 (VEGF120,) and platelet derived growth factor (PDGF-BB). [13]. The adipogenic properties of these materials are thought to primarily result from their ability to induce neovascularization. Similarly type 1 collagen supplemented with FGF-2 generates vascularized adipose tissue in fat pads defects [14] or when implanted around the epigastric vein and artery in a rodent [15]. Without exogenous growth factors adipose formation was not achieved in either Matrigel or collagen. It has been suggested that supplementing these matrices with growth factors which promote angiogenesis leads to an environment rich in nutrients and oxygen which fosters the development and survival of adipose tissue.
Fibroblast growth factor 1 (FGF-1) is a potent modulator of a variety of cells. [16–18] It induces formation of vessels in vitro as well as in vivo [19–21] and has been shown to increase preadipocyte differentiation and proliferation[10]. We have previously shown that delivering FGF-1 from alginate microbeads increases vessel development in an experimental model useful for islet transplantation. [7, 22] Delivering angiogens using alginate microbeads not only aids in containing the protein to the targeted area but, more importantly, allows for sustained delivery of proteins in order to induce persistent vascularization. The goal of the present studies was to investigate the effects of sustained local delivery of FGF-1 from alginate microbeads on neovascularization and subsequent adipogenesis in an in vivo model of vascularized adipose formation.
Materials and Methods
Fabrication of Microbeads
Microbeads for experiments were fabricated as previously described [7, 22] based on a modification of a technique developed for islet encapsulation [23]. Briefly, 2% (w/v) low viscosity alginate of high guluronic acid content (LVG, G:M ratio > 1.5) was loaded into a custom-made air droplet microencapsulator and extruded through a 25 gauge needle (at air jacket pressure of 10 pounds per square inch (psi) and air jacket pressure of 15 psi) into a 1.1% CaCl2 solution resulting in gelled spherical microbeads. For some studies, heparin (5U/ml) was added to the LVG solution prior to gelation. For all in vitro and in vivo, studies microbeads were sterilized by autoclaving the beads at 110°C for 20 minutes. Microbeads were then allowed to re-swell in sterile saline for 2 days prior to incubation with FGF-1.
FGF-1 Activity Assay
Alginate microbeads of 2% LVG with and without heparin were incubated in FGF-1 solution (10 ng/µl) for 2 days. FGF-1 loaded microbeads were placed in a solution of sterile saline with physiological levels of calcium in a humid incubator at 37°C and samples of release were taken at 4 and 8 hours. Activity of released FGF-1 was tested using an enzyme immunoassay for quantification of proliferating cells. Human umbilical vein endothelial cells (HUVEC) were seeded in 96-well-plates (7,000 cells/well) and grown in endothelial basal media with supplements (2% fetal bovine serum, bovine brain extract, gentamicin, hydrocortisone, EGF) for 3 days to approximately 80% confluence. The media was replaced with 0.5% serum in basal media with gentamicin for 24 hours before stimulation with the test samples. All groups were studied in quadruplicate, with negative control of stimulation with PBS and positive controls of 20% serum and FGF-1 not released from the microbeads.
After 24 hours of stimulation, wells were incubated with bromodeoxyuridine (BrdU). Twenty-four hours after addition of BrdU, the proliferative response was determined by measuring BrdU incorporation using a BrdU cell proliferation assay kit (Calbiochem). Briefly, samples were incubated for 30 minutes in fixative/denaturing solution prior to adding anti-BrdU for 1 hour. Wells were then incubated for another hour with detector anti-BrdU antibody followed by a 30-minute incubation with a horseradish peroxidase (HRP)-conjugated antibody. Tetra methyl benzidine was added to the wells for 15 minutes where it reacted with the HRP forming a blue solution. The reaction was stopped and color development measured using a spectrophotometer at 450 nm and at a reference wavelength of 540nm. Data are presented normalized to the response of HUVECs to the positive control of 20% serum stimulation.
Three-Dimensional Angiogenesis in vitro
Sprouting angiogenesis in response to FGF-1 loaded microbeads was studied using a modified version of a previously published three-dimensional angiogenesis assay.[5, 24] Rat tail type 1 collagen (BD Bioscience, Franklin Lakes, NJ) was neutralized with 1N NaOH and diluted with 10× PBS and dH2O to yield a final concentration of 2.5 mg/ml. Microbeads (250 beads/well) suspended in 150 µl/well of collagen were used to form a first layer of collagen in 48-well plates. Beads were loaded with FGF-1 to yield final concentrations of 2.5, 0.5 and 0 (“empty beads”) µg/ml of collagen. A fourth study group consisting of empty FGF-1 beads along with a bolus injection of FGF-1 (2.5 µg/ml) was also evaluated (“beads and bolus”). For the bolus group, FGF-1 solution was added to the collagen prior to gelation. The collagen layer containing the beads was allowed to assemble at 37°C for 30 minutes before adding the top layer containing the cell aggregate.
Cell aggregates were prepared by suspending 2,500 cells/well of HUVECs along with 2,500 cells/well of human umbilical artery smooth muscle cells (HUASMCs) in EC culture medium containing 0.25% (w/v) carboxymethylcellulose in nonadherent round-bottom-96-well plates (Griener, Germany). After 24 hours of incubation at 37°C a co-culture spheroid formed at the bottom of each well. The aggregates were then suspended in 200µl of 2.5 mg/ml collagen and placed on top of the 1st assembled collagen/bead layer. The entire plate was incubated for 45 minutes to allow for complete assembly of the top collagen layer.
Serum free media was added to the wells. The aggregates were monitored for the formation of networks and assay media changed every two days. Images of aggregates (5×, 1.25 µm/pixel) were taken daily using an Axiovert 200 inverted microscope (Carl Zeiss MicroImaging, Inc) to monitor sprout formation. Sprout lengths were quantified using a grid at 36 even 10° intervals aligned with the digital photograph. Results are presented as mean radial distance (MRD) which was calculated using the following equation:
| (1) |
where N=36 and li is the intersecting length between a line drawn from the aggregate at an angle of 2πi/N and the greatest invasion distance.
In vivo model of vascularized adipose formation
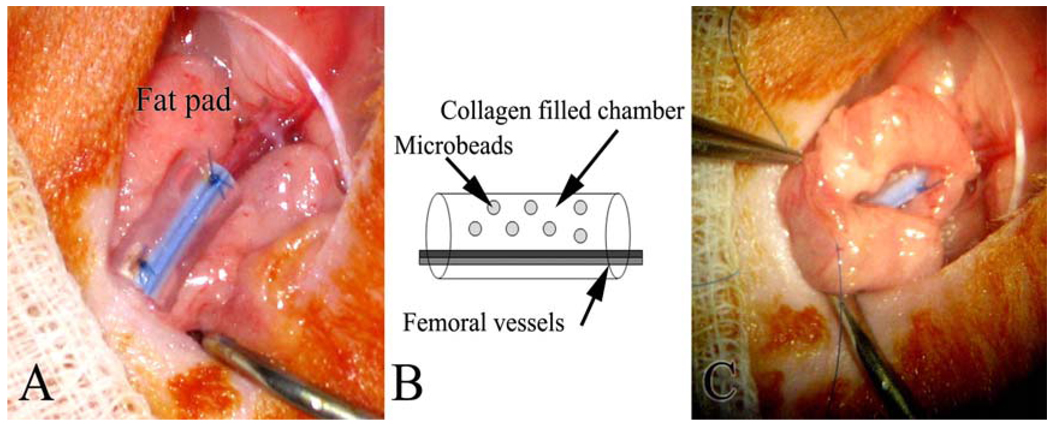
The in vivo pedicle model of vascularized adipose tissue formation was performed as described previously.[25] Briefly, Sprague Dawley rats (170–260 grams, n=5 per group per time point) were anesthetized using Isoflurane and the femoral vessels surgically exposed. A silicon chamber (length = 7mm, ID=3.3 mm) was placed around the vessels and collagen gels (50 µl) containing microbeads were transferred into the chamber. (Figure 1) Twelve microbeads fabricated with 2% LVG + heparin loaded with FGF-1 protein were dispersed in 50 µl solution of collagen (2.5 mg/ml) which was then allowed to gel at 37°C for a minimum of 30 minutes prior to implantation. These conditions yielded final FGF-1 concentrations of 2.5, 0.5, and 0 µg/ml (“empty beads”). For the bolus FGF-1 group (“beads and bolus”), FGF-1 (2.5 µg/ml) was added to collagen with empty microbeads prior to gelation. The silicone tube containing the gels was sealed using 6-0 suture nylon suture. To immobilize the tube, a local fat pad supplied by the inferior epigastric vessels was wrapped around the tube and sutured to the abdominal muscle. The incision was closed using 4-0 nylon suture. Animals were sacrificed at 1, 3 and 6 weeks post-implantation using 2% Xylocaine. At the time of harvest, tissue inside the chambers was explanted, fixed in formalin, paraffin embedded and serially sectioned (4µm thickness) from the centerline of the tube for histological analysis. Specimens were stained with hematoxylin and eosin (H&E) and Masson’s Trichrome stain to examine general histological structure.
Figure 1.
Model of vascularized adipose tissue formation. A silicone tube is cut open and wrapped around exposed femoral artery and vein (A). The tube is then filled with a collagen gel containing alginate microbeads and sutured closed. (B). A local fat pad is wrapped around the tube to seal the ends (C).
Immunohistochemistry and image analyses
Serial sections were stained for CD31, a sensitive marker of ECs, and smooth muscle alpha actin (SMA), a mural cell marker [26]. Deparaffinized and rehydrated sections underwent steam antigen retrieval using Dako target retrieval solution (Dako, Carpinteria, CA) prior to immunohistological staining. Specimens were stained following an indirect procedure using rabbit anti-human CD31 (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-SMA (Abcam) and a biotinylated anti-rabbit secondary antibody using the Vectastain Elite ABC kit (Vector Labs, Burlingame, CA). Sections were digitally imaged (20× objective, 0.17 µm/pixel) using an Axiovert 200 inverted microscope. Vessels stained positive for CD31 and SMA were manually selected using Axiovision AC (Carl Zeiss, Germany). The vessel density (number of vessels per area) was calculated using the following formula:
| (2) |
Similarly, for SMA stained section the density of SMA stained vessels was calculated using the following formula:
| (3) |
Four images (328 µm × 485 µm) per section were quantified for both CD31 and SMA analysis. Capillaries were defined as vessels less than 20 µm in diameter. Additionally adipose formation was quantified by manual selection of adipose area stained sections and is presented as percent of adipose tissue per total tissue area.
Statistical Analyses
To determine significant differences between the groups of data, analysis of variance (ANOVA) was performed with a Fisher LSD post-hoc test. In all cases, differences were considered significant for p < 0.05. For 3D culture and animal studies the data are expressed as mean ± standard error of the mean. Sample size analyses of preliminary data were used to determine group sizes for comparison.
Results
Activity of Released Protein
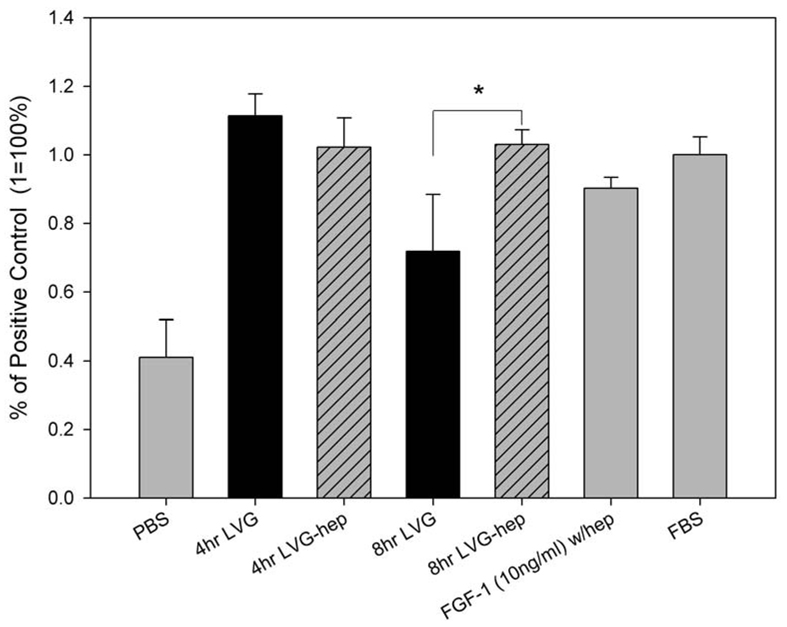
The biological activity of FGF-1 released from alginate microbeads was evaluated for its ability to stimulate EC proliferation. FGF-1 protein released from microbeads after 4 hours and 8 hours stimulated the proliferation of ECs (Figure 2). At 4 hours there were no differences in proliferation between FGF-1 released from microbeads with or without heparin. At 8 hours, FGF-1 released from microbeads made with heparin induced greater proliferation when compared to beads made without heparin. For all future studies heparin was included in the microbeads to enhance the biological activity of the FGF-1.
Figure 2.
FGF-1 released from alginate microbeads at 4 and 8 hours had significantly stimulated greater proliferation than negative control cells (PBS) (p<0.05, n=4). Black bars are FGF-1 released from alginate in the absence of heparin. Hashed bars are FGF-1 release from microbeads containing heparin. At 8 hours FGF-1 released from alginate beads containing heparin caused a significantly greater proliferation (*) than FGF-1 released from beads without heparin.
Angiogenesis Assay
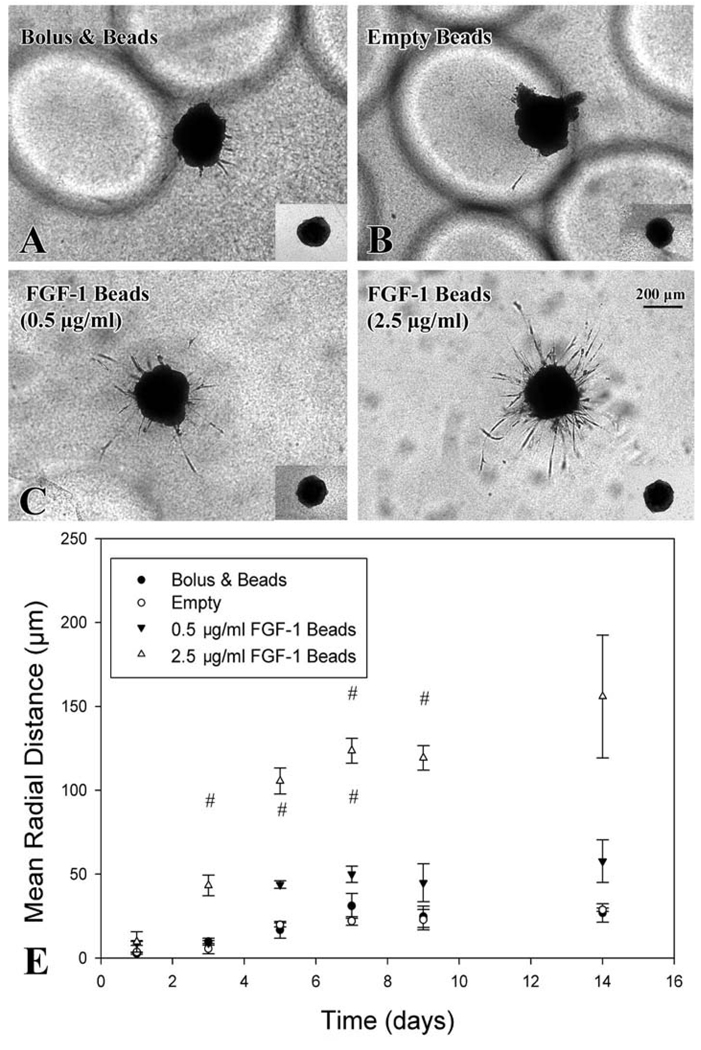
A 3D in vitro co-culture model was used to determine the ability of FGF-1 loaded microbeads to promote sprouting angiogenesis. Cells migrated from pellets invading the collagen to form distinct vessel-like structures by day 1 in all groups. By day 3 differences in mean radial distance (MRD) of invasion were present between beads loaded with FGF-1 (2.5 µg/ml, MRD=43.3 ± 6.1 µm) and all other groups (0.5 µg/ml group: 9.0 ± 1.3 µm; Bolus group: 9.8 ± 2.0 µm; Empty group: 5.6 ± 3.1 µm) (Figure 3). Beads loaded with FGF-1 at 2.5 µg/ml had greater MRD than empty control beads from day 3 through day 9. For FGF-1 loaded beads with the lower level of FGF-1 (0.5 µg/ml)sprouting was significant from empty control beads from day 5 through day 7. By day 14 vessel sprout formation had stabilized for all treatment groups.
Figure 3.
Sprouting angiogenesis from co-culture cell pellet stimulated by FGF-1 released from alginate microbeads. (A, B, C, D) Images taken at day 9 (inset images are of pellets at day 0) show vascular networks formation for groups with FGF-1 loaded beads. FGF-1 (2.5 µg/ml) beads showed increase and persistent vessel formation from day 3 to day 14 (E) # signifies statistical difference (p<0.05) from empty group.
Animal Studies
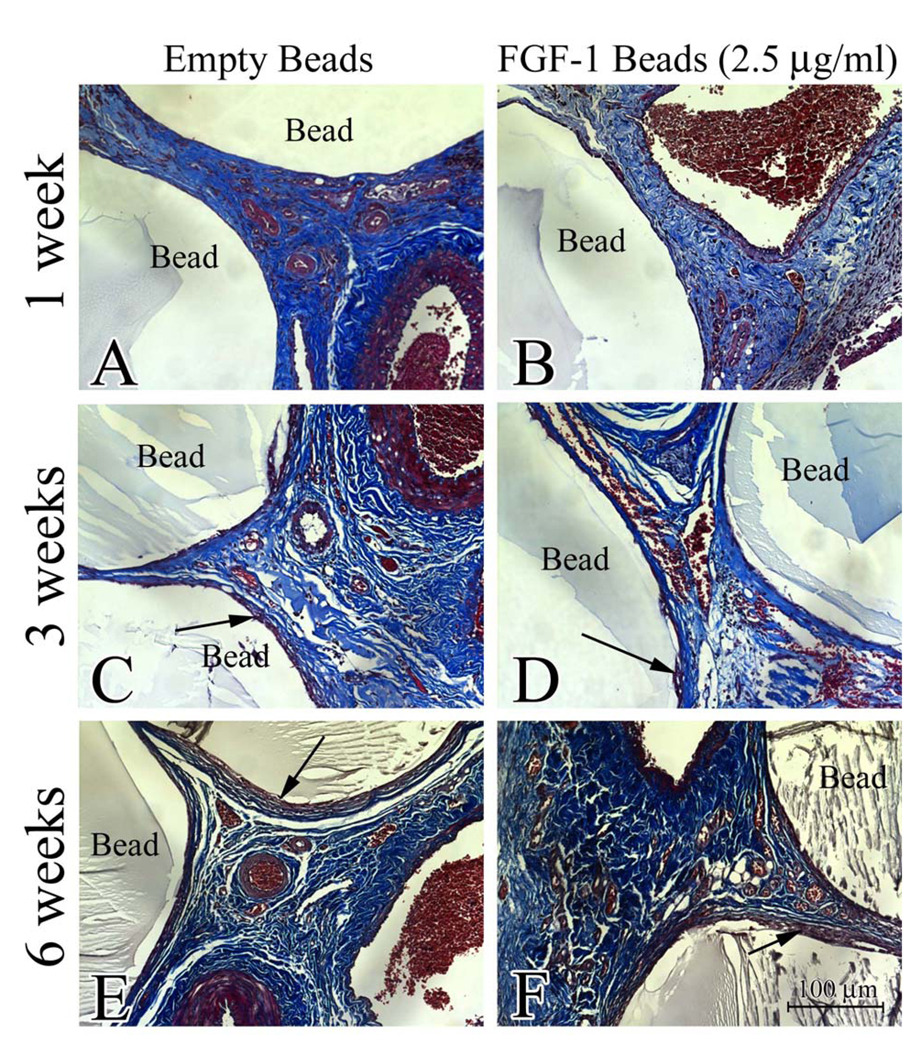
Microbeads synthesized with 2% LVG and 5 U/ml heparin were loaded with two different concentrations of FGF-1 and implanted in an in vivo model used to study strategies for engineering vascularized adipose tissue. Chambers were harvested at 1, 3 and 6 weeks post implantation. Two groups were included as controls: empty microbeads and empty microbeads injected simultaneously with a bolus of FGF-1. Bolus injection of FGF-1 was administered at the same total dose of FGF-1 as loaded into the highest concentration of FGF-1 loaded microbeads (2.5 µg/ml). Stains revealed that microbeads were still intact at all time points for all 4 four groups. Cellular and vessel invasion into the collagen gels could be seen at week 1 (Figure 4). By week 3 the majority of the collagen had degraded and was replaced by fibrovascular tisue
Figure 4.
Cellular and vessel invasion into the collagen gels could be seen at week 1. Samples harvested at 1 week and stained with Masson’s showed that collagen gel had been degraded and replaced fibrovascular tissue. (arrow indicates a region of non-degraded gel).
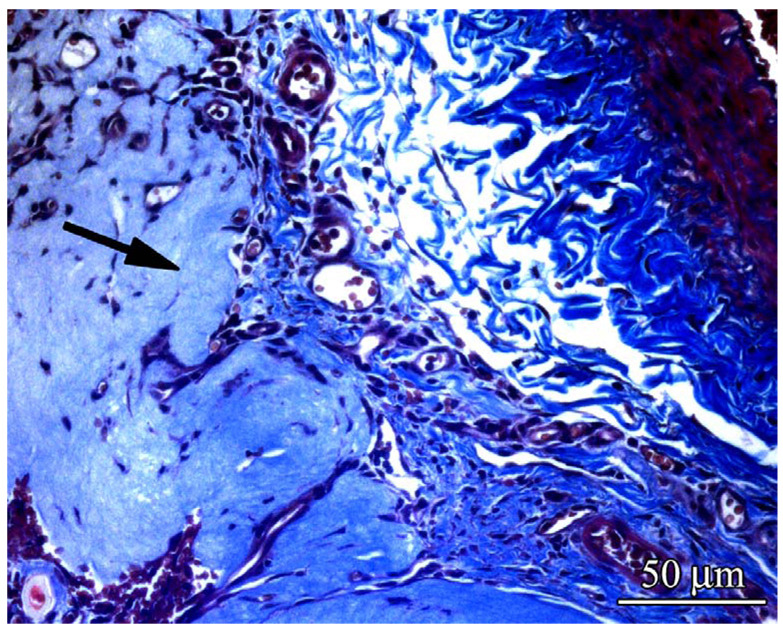
To evaluate fibrous encapsulation of the implanted alginate beads, the specimens were stained with Masson’s Trichrome. Microscopic evaluation of the stains showed minimal fibrous capsule formation at 1 week. (Figure 5) At 3 and 6 weeks a small fibrous capsule around the beads could be seen around all the beads but the size of the capsule did not vary with treatment conditions.
Figure 5.
Sections of specimens were stained with Masson’s trichrome and imaged (20×, 0.17µm/pixel) to evaluate the presence of fibrosis around the alginate microbeads. No fibrosis was apparent at 1 week (A & D). Mild fibrosis (arrows) was visible at 6 weeks (C & F) regardless of whether beads were empty or loaded with FGF-1
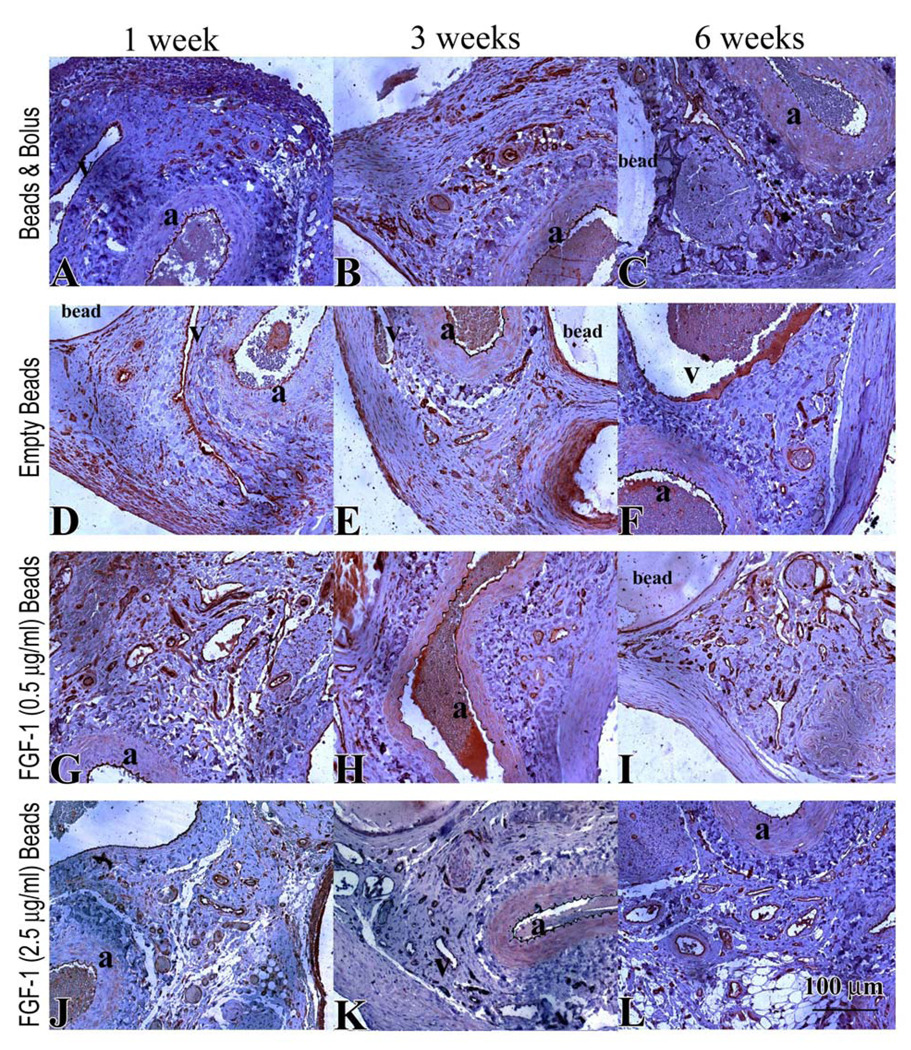
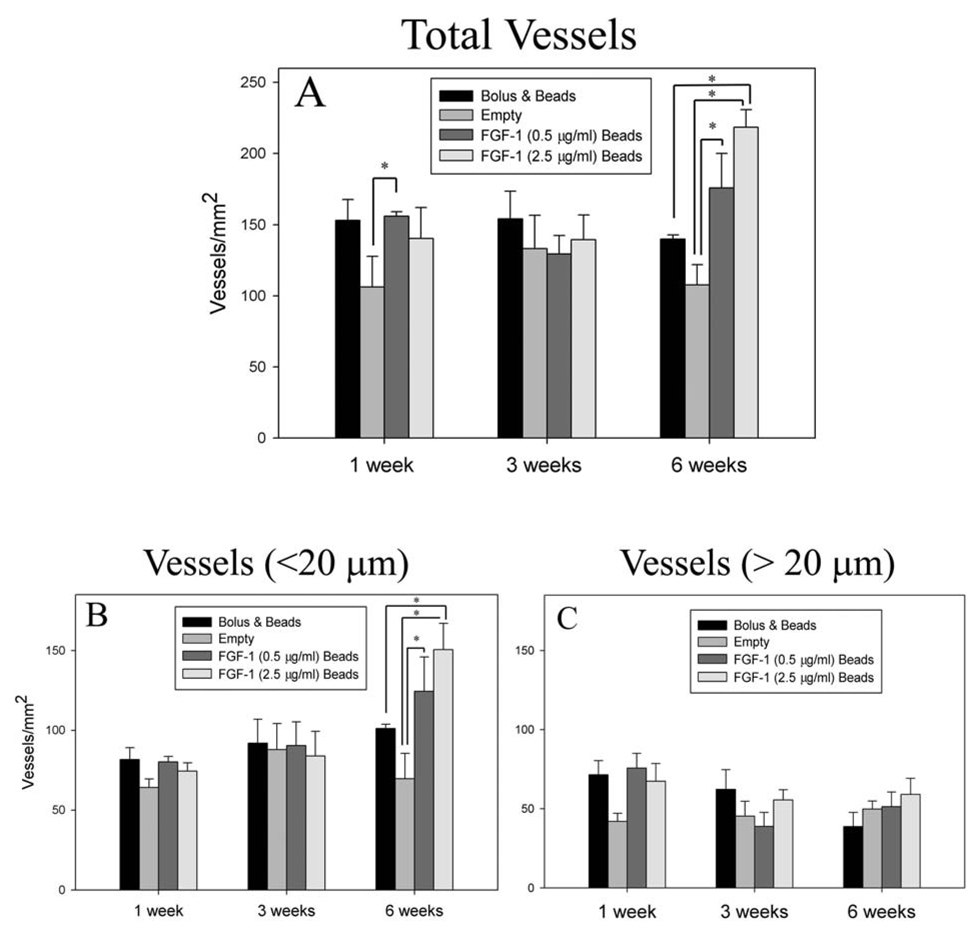
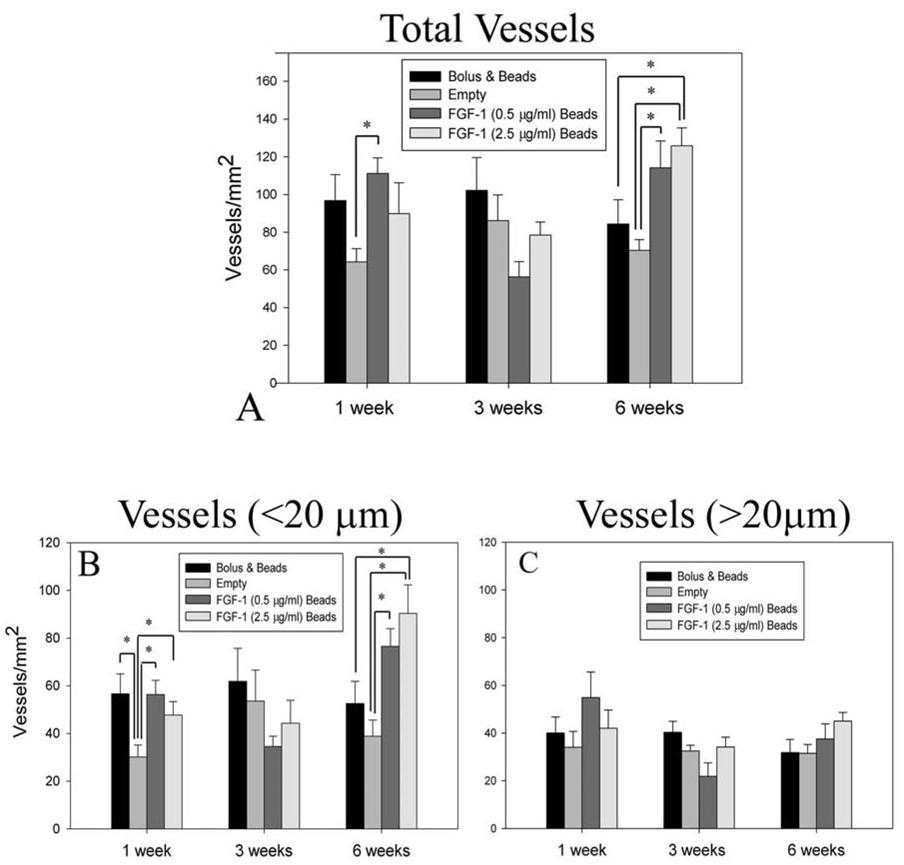
Vascular Density
Numerous blood vessels were observed for all groups at all time points (Figure 6 A–L). At 1 week, vessel density in the groups that had FGF-1 was higher in comparison to control microbeads without FGF-1 (Figure 7A); however, only FGF-1 loaded beads at 0.5 µg/ml, exhibited a statistically significant difference (156 ± 12 vs 106 ±3 vessels/mm2 , p=0.045). At 3 weeks there were no differences between the groups. Vessel number density increased from 1 week to 6 weeks for FGF-1 loaded beads at the higher concentration of 2.5 µg/ml. These beads induced a higher number density at 6 weeks than both empty beads (210 ± 48 vs 108 ± 54 vessels/mm2, p< 0.001) and bolus injection of FGF-1 (139 ± 6 vessels/mm2, p=0.004). Microbeads loaded with a lower concentration (0.5 µg/ml) of FGF-1 had a higher number density in comparison to both bolus (175 ± 48 vs 139 ± 6 vessels/mm2, p=0.007) and empty beads (108 ± 54 vessels/mm2, p=0.143) groups but the density was lower than the beads loaded at the higher FGF-1 concentration (p=0.152). Bolus injection of FGF-1 resulted in higher vessel density at 1 week relative to controls (153 ± 33 vs 106 ± 7 vessels/mm2, p=0.059), but by 6 weeks the vessel density had decreased to control levels (139 ± 7 vs 108 ± 54 vessels/mm2, p=0.192).
Figure 6.
CD31 stain of endothelial cells (brown) in specimens from rats treated for 1, 3 and 6 weeks with bolus FGF-1 (A–C), empty beads (D–F), FGF-1 (0.5 µg/ml) beads (G–I) FGF-1 (2.5 µg/ml) beads (J–L). The femoral artery and vein are indicated by “a” and “v” respectively.
Figure 7.
Animals treated with FGF-1 loaded beads showed a higher total vessel number density (A) at 6 weeks. When the total vessels were separated into vessels less than 20µm (B) and vessels greater than 20 µm (C), the increase in the number of vessels appeared to primarily result from an increase in number of vessels less than 20 µm in diameter. * Indicates statistical significance p<0.05
The effects of FGF-1 on vascularization were further examined by isolating influence on the density of capillaries (Figure 7B). Similar to the pattern seen with overall vessel density, at 1 week mean capillary density in tissues with FGF-1 delivered from microbeads or bolus injection of FGF-1 appeared to have a higher vessel density than in the absence of FGF-1; however, no statistically significant differences in capillary density were noted. At 6 weeks, groups with FGF-1 loaded beads had a higher capillary density than beads without FGF-1. Beads loaded with 2.5 µg/ml of FGF-1 also showed an increase in vessel number when compared to the bolus injection of FGF-1 (151 ± 17 vs 101 ± 3 vessels/mm2, p=0.009) and control (70 ± 16 vessels/mm2, p<0.001). Density of vessels greater than 20µm in diameter was not different between the groups at any of the time points. (Figure 7C) These results suggest that the increase in vessel density caused by FGF-1 delivery was primarily due to an increase in capillary density.
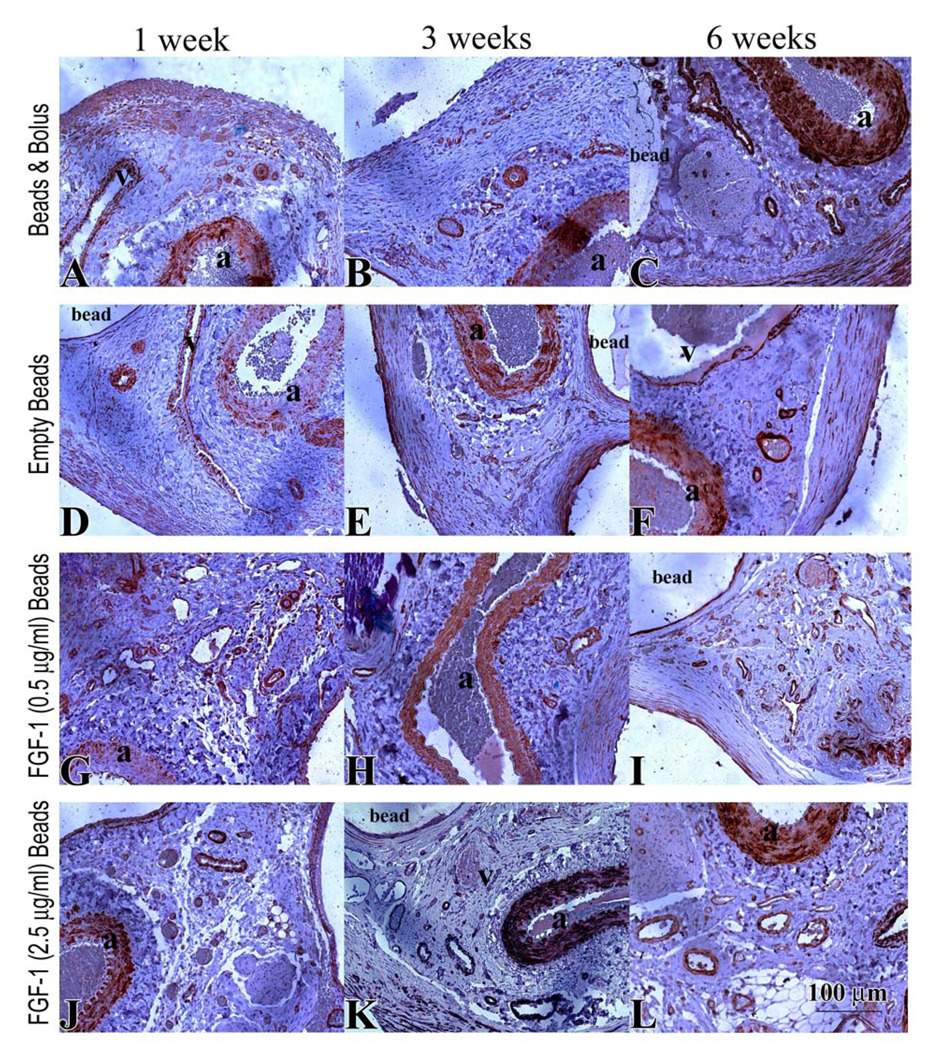
Mural Cells
Staining for SMA was used to assess the presence of mural cells in contact with ECs as an indication of vessel maturity (Figure 8 A–L). At 1 week, similar to the CD31 stained vessels, beads with FGF-1 (injected or loaded) had a higher number of SMA coated vessels than empty beads. This result was only statistically significant for beads loaded with 0.5 µg/ml of FGF-1 (111 ± 16 vs 64 ± 7, p=0.012) (Figure 9 A). At 6 weeks FGF-1 loaded microbeads at both concentrations showed a greater number of SMA positive vessels in comparison to the empty beads. Also, microbeads loaded with FGF-1 at 2.5 µg/ml had a significant increase in vessel number in comparison to the bolus FGF-1 group (120 ± 16 vs 84 ±13 vessels/mm2, p=0.044).
Figure 8.
SMA stain (brown) in specimens from rats treated for 1, 3 and 6 weeks with bolus FGF-1 (A–C), empty beads (D–F), FGF-1 (0.5 µg/ml) beads (G–I) FGF-1 (2.5 µg/ml) beads (J–L). Artery and vein are indicated by “a” and “v” respectively.
Figure 9.
Animals treated with FGF-1 loaded beads showed a higher total number of SMA positive vessels per area (A) at 6 weeks. When the total vessels were separated into vessels less than 20µm (B) and vessels greater than 20 µm (C), the increase in the number of SMA positive vessels appeared to primarily result from an increase in number of positive vessels less than 20 µm in diameter. * Indicates statistical significance p<0.05
When the number of capillaries stained for SMA was quantified, similar trends were observed. At 1 week a higher number of SMA positive capillaries was observed for all groups containing FGF-1 in comparison to empty beads. (Figure 9 B). However, by 6 weeks the bolus injection group of FGF-1 had decreased to levels found with the empty bead whereas beads loaded with FGF-1 still had a significantly higher number of SMA stained capillaries. As with CD31 stained vessels, the effect of increased number of SMA-associated vessels appeared to be primarily due to an increase in the number of stained capillaries. No differences in the density of vessels greater than 20 µm was observed between any of the groups at any time point. (Figure 9 C)
In order to normalize for differences in total vessel number between the groups, the ratio of SMA to CD31 stained vessels was quantified. At all time points, between 48 and 71% of vessels were associated with SMA positive cells in all groups (Table 1). No significant difference in the ratio of SMA to CD31 positive stained vessels was observed between any of the groups at any of the time points.
Table 1.
Mural cell coverage as % of SMA positive vessels to CD31 vessels
| Bolus & Beads | Empty Beads | FGF-1 (0.5 µ g/ml) Beads |
FGF-1 (2.5 µ g/ml) Beads |
|
|---|---|---|---|---|
| 1 week | 67 ± 12 | 60 ± 10 | 67 ± 1 | 74 ± 9 |
| 3 weeks | 68 ± 12 | 71 ± 10 | 64 ± 7 | 48 ± 9 |
| 6 weeks | 55 ± 17 | 68 ± 16 | 67 ± 0.13 | 69 ± 12 |
Adipose tissue formation
Previous studies have shown that stimulation of neovascularization in type I collagen gels results in increased adipogenesis in both pedicle and subcutaneous implantation models. [14, 15] Tissue sections showed only scattered amounts of adipose present in only a few of the samples in this study (< 3% adipose area in all groups). Adipose tissue formation was not greater than controls under any of the FGF-1 conditions. The percentage of adipose formation showed there was no significant difference between any group and any time point with mean adipose levels less than 1% under all conditions. (Table 2)
Table 2.
Generation of adipose tissue as % of total tissue area
| Bolus & Beads |
Empty Beads |
FGF-1 (0.5 µ g/ml) Beads |
FGF-1 (2.5 µ g/ml) Beads |
|
|---|---|---|---|---|
| 1 week | 1.67 ± 2.4 | 1.86 ± 3.9 | 1.06 ± 1.7 | 2.63 ± 2.8 |
| 3 weeks | 0.01 ± 0.0 | 0.25 ± 0.6 | 0.05 ± 0.1 | 1.1 ± 2.5 |
| 6 weeks | 0.96 ± 2.1 | 0.39 ± 0.5 | 0.0 ± 0.0 | 0.85 ± 1.6 |
Discussion
Therapeutic neovascularization using growth factors holds promise for clinical application; however, an optimal delivery approach is still needed. Methods that could avoid the need for repeated injection and high short term doses may improve clinical outcomes. The use of alginate to control the local levels of growth factors may help progress in neovascularization therapies as well as increase the viability of implanted tissue constructs or transplanted cells. In the present study, controlled release of FGF-1 protein from alginate microbeads was investigated for its ability to increase the persistence of vascular network formation over bolus delivery in vitro and in vivo.
FGF-1 released from the beads retained its biological activity as indicated by the stimulation of HUVEC proliferation after release. This response was enhanced by the incorporation of heparin into the beads (5U/ml). The improvement in activity in the presence of heparin was not observed after 4 hours of release presumably due to the initial high burst release in this system.[27] Four hours later when the amount of protein released was lower, the effects of heparin on proliferation was apparent. For this reason heparin was incorporated into the alginate for further in vitro and in vivo studies. Heparin acts to enhance the activity of FGF-1 in two ways, facilitating dimerization of the growth factor with its receptor in order to activate its tyrosine kinase [28], and protection from inactivation due to heat, acid or proteolysis. [29] In a study by Schroeder-Tefft et al, transforming growth factor beta 2 (TGF-β2) mixed with heparin formed a complex that remained biologically active when stored at physiologic conditions (PBS at 37°C) compared to TGF-β2 alone which had a drastic decrease in activity. [30] In this same study, TGF-β2+heparin mixed with fibrillar collagen also remained biologically active in vivo. Other researchers have incorporated heparin into matrices for protein release and demonstrated an added benefit of heparin aiding to slow down the release of the protein. [31–33]
A 3D in vitro model of angiogenesis was used to investigate the ability of the released protein to stimulate the formation of microvascular networks. Sprouting occurred in all the groups but only in the groups with FGF-1 released from microbead did the sprouting invasion in the gel continue to increase. A one time bolus administration of FGF-1 at the same dose as loaded in the alginate was not sufficient to promote significant sprout formation. In the present study, the beads loaded at a higher amount of FGF-1 showed persistent vascular network formation in vitro. In addition the release of FGF-1 from beads loaded with a lower total amount of FGF-1, was able to stimulate a significant response. These results show that when delivered from alginate a lower total dose of FGF-1 may be able to stimulate a significant response. We have previously shown that persistently high concentrations of FGF-1 cause vessels to regress.[5] There is an initial burst release from the microbeads, but the initial high amount of FGF-1 released, did not appear to adversely affect the formation of sprouts to form. These results suggest that total protein load is not the only factor involved in promoting a persistent response.
Similar increases in neovascularization were observed in response to FGF-1 delivered from microbeads in vivo. Results from these experiments demonstrated that FGF-1 loaded beads can stimulate a more persistent response than bolus delivery. The results presented here show that at equal total FGF-1 amounts, encapsulation of FGF-1 in alginate microbeads and bolus delivery increased vascular density at one week, but only delivery from alginate showed an increase in vascular density at 6 weeks. Also, these results show a persistent vascular response can be achieved using lower doses when the protein is delivered from alginate microbeads.
Along with an increase in vessel number, animals treated with FGF-1 loaded microbeads had an accompanied increase in SMA coated vessels. Mural cells, commonly stained for using SMA, surround tubes on endothelial cells in capillaries and their presence indicates vessel maturity. Vessels consisting of mural cells have been shown to be less dependent on angiogenic factors for survival. [34] While FGF-1 stimulated an increase in the number of SMA positive vessels, the percentage of positive vessels did not vary between groups. These results are consistent with was previously observed in another in vivo animal model study using alginate microbeads to deliver FGF-1 locally. [7, 22] When combined with our finding that the increased vessel density was due to formation of capillaries, it appears that the sustained delivery of FGF-1 does not improve the remodeling and stabilization process, or that 6 weeks is too early a time to examine this process. Delivery of additional growth factors involved in the process of blood vessel maturation, such as PDGF-BB, angiopoietin-1, or TGF-β [35–37], may be required to directly increase recruitment of mural cells and density of large vessels.
Previous researchers using similar pedicle models have demonstrated that biomaterials containing angiogenic growth factors, including VEGF, PDGF-BB and FGF-2, result in large amounts of adipose formation. [13, 15, 38] In studies by Vashi et al. biodegradable gelatin microspheres imbibed with FGF-2 were mixed with collagen, placed in a chamber models surrounding epigastric vessels and at time of harvest (6 weeks) significant adipose formation was found accompanied by angiogenesis.[15] In the epigastric model, extracellular matrices such as collagen 1, fibrin, and hyaluronan without exogenous angiogenic growth factors did neither produce adipose formation nor extensive vascularization. [39] These researchers concluded that the growth factors present in the surrounding materials were responsible for inducing angiogenesis which drives the formation of adipose tissue.
Our findings in the present study appear to contradict this hypothesis as increased angiogenesis was observed but with minimal adipogenesis. In the studies here the chamber was placed around the unligated femoral artery and vein rather that the epigastric vessels as used in many other studies.[13, 15, 40, 41] Differences between the two pedicles may help explain differences in adipose formation. The epigastric model uses pedicle directly connected to adipose tissue source which may provide easier migration of surrounding stromal precurosors and preadipocytes into the chamber. In these types of models migration of adipocytes is thought to mostly occur from the surrounding tissue.[40, 42] However with the epigastric model even when it is used as a sealed chamber (not in contact with the surrounding adipose tissue) some adipocytes are observed. These were attributed to either residual adipocytes left on the vascular pedicle or mesenchymal stems cells via the circulation.[40, 42] It is possible that this results from differences in surgical technique, however, we have previously shown that the femoral pedicle model supports extensive adipogenesis when an adipogenic biomaterial is used.[25, 43] Additional studies are needed to better understand how local environmental factors that help promote adipogenesis in the different pedicle models.
Another difference between this and previous studies is the use of FGF-1, which has not previously been applied in these pedicle models. Both FGF-1 and FGF-2 are thought to play a role in adipogenesis as mitogens. In one study, both proteins were able to stimulate preadipocyte proliferation; however, FGF-2 may have greater potency. [10] Another study, however found that human preadipocytes isolated from both subcutaneous and omental fat incubated with either FGF-1 or FGF-2 resulted in increased adipoctye differentiation and FGF-1 was found to be the more potent adipogenic agent.[44] These findings suggest that FGF-1 does not negatively affect fat formation. While many of the growth factors involved in adipogenesis also play a role in angiogenesis, further research is necessary for fully understanding the concomitant relationship between angiogenesis and adipogenesis, particularly in the rodent pedicle models frequently used for studying vascularized adipose formation. The potentially conflicting results in different models also suggest that a better in vivo test is needed. Potentially, the use of materials in orthotopic animal models would allow much better understanding of the results.
Conclusion
In summary, both in vitro and in vivo testing demonstrated that the sustained release of FGF-1 stimulated neovascularization. In vitro studies demonstrated the continued release of FGF-1 from the microbeads resulted in a significant and persistent vascular response. Results similar to in vitro were observed in vivo. Beads loaded with the higher amount of protein caused the greatest increase in microvascular density at 6 weeks compared to bolus and empty bead groups. Beads loaded with FGF-1 also induced a high number of SMA stained vessels. However no differences were observed in ratio of SMA coated vessels between the FGF-1 loaded group and any of the other groups. Adipose formation was not observed to accompany the increase in vessels formed in response to FGF-1 delivered from microbeads. These results suggest that FGF-1 beads can be used to increase the duration of the local vascular response and provide a compelling impetus for further experimental pursuit of FGF-loaded alginate microcapsules for therapeutic neovascularization.
Acknowledgements
The authors would like to thank Dr. Yu-Hsuan Hsieh, Dr. Chia-Hsuan Tsai and Dr. Chih-Wei Wu for their assistance with the animal studies. Also special thanks to Ms. Stephanie Lucas for her assistance in quantifying the adipose tissue formation. This research was supported by funding from the National Science Foundation (Grant Nos. 0852048 and 0731201), the National Institutes of Health (RO1 DK080897), the U.S. Department of Veterans Affairs, Chang Gung Memorial Hospital (CRPG371861) and the Taiwan National Science Council (NSC96-2314-B-182A-075-MY2). Dr. Moya received support from the Bill & Melinda Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patrick CW. Breast tissue engineering. Annu Rev Biomed Eng. 2004;6:109–130. doi: 10.1146/annurev.bioeng.6.040803.140032. [DOI] [PubMed] [Google Scholar]
- 2.Vacanti JP, Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354 Suppl 1:SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 3.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295(5557):1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 4.Morrison WA. Progress in tissue engineering of soft tissue and organs. Surgery. 2009;145(2):127–130. doi: 10.1016/j.surg.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Uriel S, Brey EM, Greisler HP. Sustained low levels of fibroblast growth factor-1 promote persistent microvascular network formation. Am J Surg. 2006;192(5):604–609. doi: 10.1016/j.amjsurg.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Peters MC, Isenberg BC, Rowley JA, Mooney DJ. Release from alginate enhances the biological activity of vascular endothelial growth factor. Journal of Biomaterials Science: Polymer Edition. 1998;9(12):1267–1278. doi: 10.1163/156856298x00389. [DOI] [PubMed] [Google Scholar]
- 7.Moya ML, Lucas S, Francis-Sedlak M, Liu X, Garfinkel MR, Huang JJ, et al. Sustained delivery of FGF-1 increases vascular density in comparison to bolus administration. Microvasc Res. 2009;78(2):142–147. doi: 10.1016/j.mvr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Lijnen HR. Angiogenesis and obesity. Cardiovasc Res. 2008;78:286–293. doi: 10.1093/cvr/cvm007. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117(9):2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutley L, Shurety W, Newell F, McGeary R, Pelton N, Grant J, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53(12):3097–3106. doi: 10.2337/diabetes.53.12.3097. [DOI] [PubMed] [Google Scholar]
- 11.Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1998;95(3):1062–1066. doi: 10.1073/pnas.95.3.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura Y, Ozeki M, Inamoto T, Tabata Y. Time course of de novo adipogenesis in matrigel by gelatin microspheres incorporating basic fibroblast growth factor. Tissue Eng. 2002;8(4):603–613. doi: 10.1089/107632702760240526. [DOI] [PubMed] [Google Scholar]
- 13.Rophael JA, Craft RO, Palmer JA, Hussey AJ, Thomas GP, Morrison WA, et al. Angiogenic growth factor synergism in a murine tissue engineering model of angiogenesis and adipogenesis. Am J Pathol. 2007;171(6):2048–2057. doi: 10.2353/ajpath.2007.070066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12(6):1475–1487. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 15.Vashi AV, Abberton KM, Thomas GP, Morrison WA, O'Connor AJ, Cooper-White JJ, et al. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Eng. 2006;12(11):3035–3043. doi: 10.1089/ten.2006.12.3035. [DOI] [PubMed] [Google Scholar]
- 16.D'Amore PA, Smith SR. Growth factor effects on cells of the vascular wall: a survey. Growth Factors. 1993;8(1):61–75. doi: 10.3109/08977199309029135. [DOI] [PubMed] [Google Scholar]
- 17.Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annual Review of Biochemistry. 1989;58(1):575–602. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- 18.Jaye M, Schlessinger J, Dionne CA. Fibroblast growth factor receptor tyrosine kinases: molecular analysis and signal transduction. Biochim Biophys Acta. 1992;1135(2):185–199. doi: 10.1016/0167-4889(92)90136-y. [DOI] [PubMed] [Google Scholar]
- 19.Monestano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proceeding of the National Academy of Sciences. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bjornsson TD, Dryjski M, Tluczek J, Mennie R, Ronan J, Mellin TN, et al. Acidic fibroblast growth factor promotes vascular repair. Proceeding of the National Academy of Sciences. 1991;88:8651–8655. doi: 10.1073/pnas.88.19.8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caron A, Michelet S, Caron A, Sordello S, Ivanov MA, Delaere P, et al. Human FGF-1 gene transfer promotes the formation of collateral vessels and arterioles in ischemic muscles of hypercholesterolemic hamsters. J Gene Med. 2004;6(9):1033–1045. doi: 10.1002/jgm.594. [DOI] [PubMed] [Google Scholar]
- 22.Moya ML, Garfinkel MR, Liu X, Lucas S, Opara EC, Greisler H, et al. Fibroblast growth factor-1 (FGF-1) loaded microbeads enhance local capillary neovascularization. Journal of Surgical Research. doi: 10.1016/j.jss.2009.06.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garfinkel MR, Harland RC, Opara EC. Optimization of the microencapsulated islet for transplantation. J Surg Res. 1998;76(1):7–10. doi: 10.1006/jsre.1997.5258. [DOI] [PubMed] [Google Scholar]
- 24.Korff T, Kimmina S, Martiny-Baron G, Augustin HG. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. The FASEB journal. 2001;15(2):447–457. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 25.Uriel S, Huang JJ, Moya ML, Francis ME, Wang R, Chang SY, et al. The role of adipose protein derived hydrogels in adipogenesis. Biomaterials. 2008;29(27):3712–3719. doi: 10.1016/j.biomaterials.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 26.Brey EM, McIntire LV, Johnston CM, Reece GP, Patrick CW., Jr Three-dimensional, quantitative analysis of desmin and smooth muscle alpha actin expression during angiogenesis. Ann Biomed Eng. 2004;32(8):1100–1107. doi: 10.1114/b:abme.0000036646.17362.c4. [DOI] [PubMed] [Google Scholar]
- 27.Laca A, García LA, Argüeso F, Díaz M. Protein diffusion in alginate beads monitored by confocal microscopy. The application of wavelets for data reconstruction and analysis. Journal of Industrial Microbiology and Biotechnology. 1999;23(3):155–165. [Google Scholar]
- 28.Pye DA, Vives RR, Turnbull JE, Hyde P, Gallagher JT. Heparan sulfate oligosaccharides require 6-O-sulfation for promotion of basic fibroblast growth factor mitogenic activity. J Biol Chem. 1998;273(36):22936–22942. doi: 10.1074/jbc.273.36.22936. [DOI] [PubMed] [Google Scholar]
- 29.Kang SS, Gosselin C, Ren D, Greisler HP. Selective stimulation of endothelial cell proliferation with inhibition of smooth muscle cell proliferation by fibroblast growth factor-1 plus heparin delivered from fibrin glue suspensions. Surgery. 1995;118(2):280–286. doi: 10.1016/s0039-6060(05)80335-6. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 30.Schroeder-Tefft JA, Bentz H, Estridge TD. Collagen and heparin matrices for growth factor delivery. Journal of Controlled Release. 1997;48(1):29–33. [Google Scholar]
- 31.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. Journal of Controlled Release. 2000;69(1):149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 32.Sakiyama-Elbert SE, Hubbell JA. Development of fibrin derivatives for controlled release of heparin-binding growth factors. J Control Release. 2000;65(3):389–402. doi: 10.1016/s0168-3659(99)00221-7. [DOI] [PubMed] [Google Scholar]
- 33.Ho Y-C, Mi F-L, Sung H-W, Kuo P-L. Heparin-functionalized chitosan-alginate scaffolds for controlled release of growth factor. International Journal of Pharmaceutics. 2009;376(1–2):69–75. doi: 10.1016/j.ijpharm.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 34.Abramsson A, Berlin O, Papayan H, Paulin D, Shani M, Betsholtz C. Analysis of mural cell recruitment to tumor vessels. Circulation. 2002;105(1):112–117. doi: 10.1161/hc0102.101437. [DOI] [PubMed] [Google Scholar]
- 35.Darland DC, D'Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103(2):157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaengel K, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 37.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 38.Walton RL, Beahm EK, Wu L. De novo adipose formation in a vascularized engineered construct. Microsurgery. 2004;24(5):378–384. doi: 10.1002/micr.20056. [DOI] [PubMed] [Google Scholar]
- 39.Cronin KJ, Messina A, Thompson EW, Morrison WA, Stevens GW, Knight KR. The role of biological extracellular matrix scaffolds in vascularized three-dimensional tissue growth in vivo. J Biomed Mater Res B Appl Biomater. 2007;82(1):122–128. doi: 10.1002/jbm.b.30713. [DOI] [PubMed] [Google Scholar]
- 40.Kelly JL, Findlay MW, Knight KR, Penington A, Thompson EW, Messina A, et al. Contact with existing adipose tissue is inductive for adipogenesis in matrigel. Tissue Eng. 2006;12(7):2041–2047. doi: 10.1089/ten.2006.12.2041. [DOI] [PubMed] [Google Scholar]
- 41.Cronin KJ, Messina A, Knight KR, Cooper-White JJ, Stevens GW, Penington AJ, et al. New murine model of spontaneous autologous tissue engineering, combining an arteriovenous pedicle with matrix materials. Plast Reconstr Surg. 2004;113(1):260–269. doi: 10.1097/01.PRS.0000095942.71618.9D. [DOI] [PubMed] [Google Scholar]
- 42.Stillaert FB, Di Bartolo C, Hunt JA, Rhodes NP, Tognana E, Monstrey S, et al. Human clinical experience with adipose precursor cells seeded on hyaluronic acid-based spongy scaffolds. Biomaterials. 2008;29(29):3953–3959. doi: 10.1016/j.biomaterials.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Cheng MH, Uriel S, Moya ML, Francis-Sedlak M, Wang R, Huang JJ, et al. Dermis-derived hydrogels support adipogenesis in vivo. J Biomed Mater Res A. doi: 10.1002/jbm.a.32410. In Press. [DOI] [PubMed] [Google Scholar]
- 44.Widberg CH, Newell FS, Bachmann AW, Ramnoruth SN, Spelta MC, Whitehead JP, et al. Fibroblast growth factor receptor 1 is a key regulator of early adipogenic events in human preadipocytes. Am J Physiol Endocrinol Metab. 2009;296(1):E121–E131. doi: 10.1152/ajpendo.90602.2008. [DOI] [PubMed] [Google Scholar]