Abstract
Chemokines are small secreted proteins that act as chemo-attractants, and their role as neuromodulators in the brain has recently been appreciated. CXCL12 is one of the few chemokines found in neurons and expressed constitutively in the central nervous system. Previous data from our laboratory demonstrate the ability of CXCL12 to modulate the behavioral effects of cocaine and this modulation is dependent on the central site of administration of CXCL12. The present study used single staining immunohistochemical and dual staining immunofluorescent methods to determine the localization of the CXCL12 receptor, CXCR4, in the caudate putamen and nucleus accumbens of the adult rat brain. Results demonstrated that individual neurons in both the caudate putamen and lateral shell of the nucleus accumbens express both CXCR4 and D1 dopamine receptors. Immunofluorescent studies showed that CXCR4 was co-expressed with ChAT, a marker for cholinergic neurons, and with GAD C38, a marker for GABAergic neurons, in the caudate putamen and lateral shell of the accumbens. No evidence of CXCR4 was found in the medial shell or core regions of the nucleus accumbens. These data demonstrate that CXCR4 is expressed by subpopulations of cholinergic and GABAergic neurons in the striatum and suggest that CXCR4 is well-positioned to modulate striatal function.
Keywords: Immunohistochemistry, Immunofluorescence, CXCL12, Striatum, Accumbens, Dopamine
1. Introduction
Chemotactic cytokines, also known as chemokines, are small secreted proteins that exert their effects through the activation of G protein-coupled receptors. Chemokines have been studied for their chemoattractant properties and their ability to control leukocyte migration, as well as their involvement in the neuropathology of multiple sclerosis, HIV/AIDS, Alzheimer’s and Parkinson’s disease (Cartier et al., 2005; Streit et al., 2001; Ubogu et al., 2006). Most chemokines are not constitutively expressed, rather their production and secretion can be induced by an inflammatory response (White et al., 2007). CXCL12, formerly known as stromal cell derived factor-1 (SDF-1), is one of the few chemokines that is expressed constitutively (Banisadr et al., 2003; Tham et al., 2001; Tissir et al., 2004). CXCL12 binds to and activates two receptors, CXCR4 and CXCR7, the former being the major receptor for CXCL12 in brain (Bleul et al., 1996). CXCR4 is vital for embryonic development as CXCL12, acting through CXCR4, regulates the migration of progenitor stem cells (Lu et al., 2002; Tachibana et al., 1998). Mice with genetic deletion of either CXCL12 or CXCR4 exhibit major defects in their vasculature and central nervous system (CNS) and die shortly after birth (Nagasawa et al., 1998; Stumm et al., 2003; Tachibana et al., 1998; Zou et al., 1998).
CXCR4 is expressed by microglia, astrocytes and neurons of the mature CNS (Bajetto et al., 2001b; Ohtani et al., 1998; Tanabe et al., 1997). Neuronal expression of CXCR4 has been shown via immunohistochemistry in several brain regions of the rat. CXCR4 is expressed by cholinergic interneurons in the substantia innominata and caudate putamen (Banisadr et al., 2002), arginine-vasopressinergic neurons of the supraoptic and paraventricular hypothalamic nuclei (Banisadr et al., 2003), neurons expressing melanin-concentrating hormone in the lateral hypothalamus (Guyon et al., 2005), dopaminergic neurons in the substantia nigra (Banisadr et al., 2002), GABAergic neurons of the substantia nigra pars reticulata (Guyon et al., 2006) and Purkinje neurons and granule cells of the cerebellum (Ragozzino, 2002). The expression of CXCR4 in various brain regions suggests it has wide-ranging effects in the CNS.
Recent studies support a role for CXCL12 and CXCR4 in the modulation of dopaminergic pathways in the brain. The mesolimbic dopamine pathway projects from the ventral tegmental area (VTA) to the nucleus accumbens and the nigrostriatal dopamine pathway projects from the substantia nigra to the caudate putamen. Injection of CXCL12 into the substantia nigra increases extracellular dopamine in the caudate putamen and results in an increase in locomotor activity (Skrzydelski et al., 2007). Previous results from our laboratory demonstrate that CXCL12 can modulate the behavioral effects of cocaine. CXCL12 administered into the VTA or caudate putamen prior to systemic administration of cocaine, potentiated cocaine-induced ambulatory and stereotypic activities, respectively. In contrast, injection of CXCL12 into the lateral shell of the nucleus accumbens reduced cocaine-induced ambulation (Trecki and Unterwald, 2009).
The mechanisms by which CXCR4 activation can modulate the behavioral effects of cocaine are not known. The present study utilized single staining immunohistochemistry to further evaluate the expression of CXCR4 in the forebrain of the adult rat. Dual labeling immunofluorescent studies were used to determine the localization of CXCR4 on cholinergic and GABAergic neurons and the potential co-expression of CXCR4 and D1 dopamine receptors on individual neurons in the striatum. The aim of this research was to determine the neurochemical phenotype of cells expressing CXCR4 in the caudate putamen and nucleus accumbens in order to further understand potential mechanisms by which this chemokine system can regulate dopamine-mediated behaviors.
2. Results
Immunohistochemistry
Coronal brain sections ranging from bregma +1.56 to +1.08 mm from four adult Sprague Dawley rats were examined for the presence of CXCR4-ir in the caudate putamen and nucleus accumbens. Fig. 1 shows a diagram of a coronal brain section (bregma +1.32 mm) indicating the regions of interest (Paxinos and Watson, 2007). As shown by diaminobenzidine staining in Figure 2, no CXCR4 labeling was observed in the medial shell or core of the nucleus accumbens (Fig. 2A). Results demonstrate that CXCR4-ir is expressed on cells and processes in the caudate putamen (Fig. 2B) and lateral shell of the nucleus accumbens (Fig. 2C, 2D).
Figure 1.

Immunohistochemical analysis was conducted in coronal sections of rat brain from bregma +1.56 to +1.08 mm. Areas of interest are labeled. Diagram is reproduced from Paxinos and Watson (2007) with permission. Abbreviations: CPu – caudate putamen, LAccSh – lateral accumbens shell, MAccSh – medial accumbens shell, AccC – accumbens core.
Figure 2.
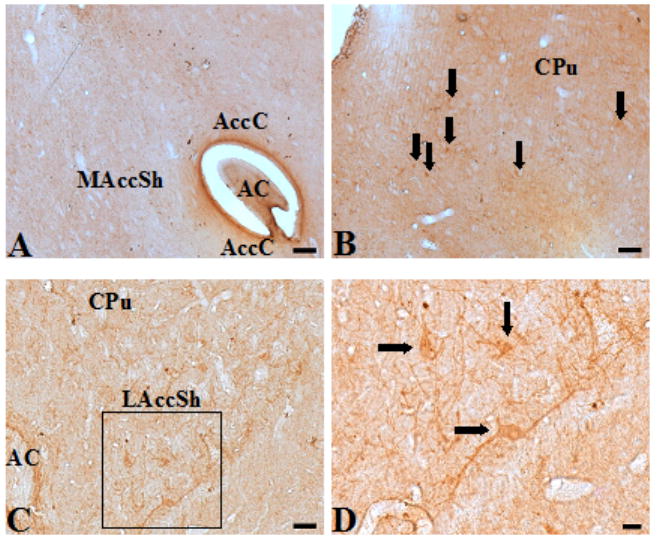
Sections of rat forebrain labeled with the CXCR4 antibody (diaminobenzidine staining). A. No CXCR4-ir was observed in the medial shell or the core of the nucleus accumbens. B. CXCR4-ir cell bodies and processes were stained throughout the caudate putamen. C. CXCR4-ir neurons in the lateral accumbens shell D. A higher magnification of the area outlined by the black box in C shows cell bodies (black arrows) and processes positive for CXCR4. Abbreviations: CPu – caudate putamen, LAccSh – lateral accumbens shell, MAccSh – medial accumbens shell, AccC – accumbens core, AC – anterior commisure, LV – lateral ventricle. Scale bar, A-B, 100 μm; C, 50 μm; D, 20 μm
Separate sections were incubated with antibodies to one of the following proteins in order to characterize their localization in the striatum and to optimize antibody concentrations for dual-labeling immunofluorescence: the dopamine D1 receptor (D1R); choline acetyl transferase (ChAT), a marker for cholinergic neurons; glial fibrillary acidic protein (GFAP), a marker for astrocytes; and glutamic acid decarboxylase (GAD C38), a marker for GABAergic neurons. Antibody studies were completed using single staining immunohistochemistry for D1R, ChAT, GFAP, GAD C38 and GABA to determine the appropriate concentrations for immunofluorescence. Control studies were conducted in the absence of primary antibody for each protein of interest to establish the lack of non-specific staining (data not shown). A description of the distribution of CXCR4-ir neurons and associated markers in the caudate putamen and nucleus accumbens are presented below.
Caudate Putamen
Cells positively stained with the antibody to CXCR4 were found in the caudate putamen (Fig. 2B). Visual analysis revealed a homogenous pattern of CXCR4-positive cells throughout the rostral and caudal portion of the caudate putamen within the limits examined. No differences were observed between medial and lateral aspects, or between dorsal and ventral areas. Control studies, conducted in the absence of either primary (CXCR4) or secondary (biotinylated) antibody, resulted in no immunoreactivity in any of the sections examined (data not shown). Sections incubated with the anti-D1R antibody revealed staining of cell bodies throughout the caudate putamen (data not shown). Sections incubated with antibody to either GAD C38 (data not shown) or GFAP (data not shown) demonstrated staining of cell bodies and processes in the caudate putamen. Sections incubated with the anti-ChAT antibody contained a low density of processes and cell bodies that were heavily stained for ChAT (data not shown).
Nucleus Accumbens
CXCR4-positive cells were identified within the lateral shell of the nucleus accumbens (Fig. 2C, 2D). Both cell bodies and processes positive for CXCR4 were observed throughout the rostral and caudal portions of the lateral accumbens shell. No CXCR4-positive cells were observed in the medial shell or the core of the nucleus accumbens. Control studies, conducted in the absence of either primary (CXCR4) or secondary (biotinylated) antibody, resulted in no immunoreactivity in any of the sections examined (data not shown). Sections incubated with antibody against the D1R revealed staining on cell bodies throughout the nucleus accumbens (Fig. 3A). Sections labeled with either anti-GAD C38 (Fig. 3B), anti-GFAP (Fig. 3C), or anti-ChAT (Fig. 3D) antibodies stained both cell bodies and processes throughout the nucleus accumbens.
Figure 3.
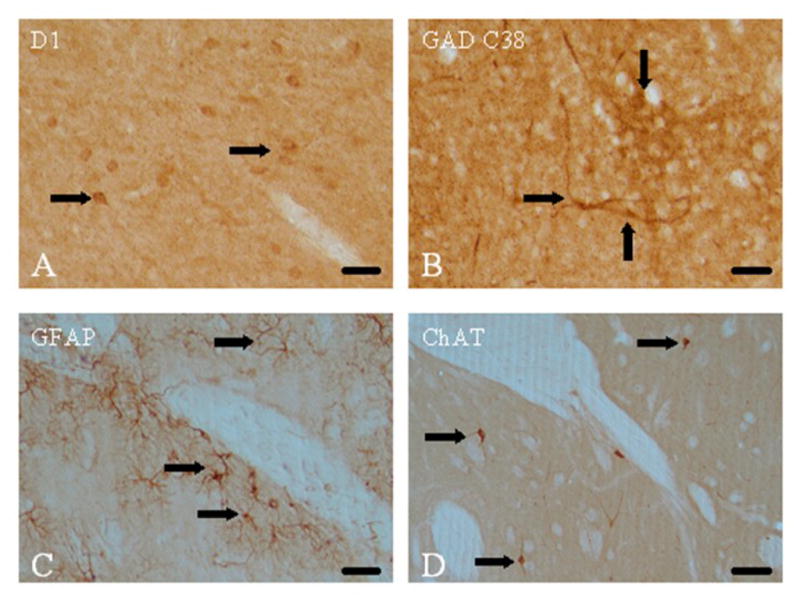
Single labeling in the nucleus accumbens for D1 receptors, GAD C38, GFAP, and ChAT. A. The D1 dopamine receptor (D1R) antibody labeled small cells resembling medium spiny neurons (black arrows). B. Labeling with the GAD C38 antibody demonstrated positive GABAergic cell bodies (black arrows) and processes. C. Astrocytes are shown as labeled with GFAP antibody (black arrows) D. Sparse ChAT-positive cholinergic neurons were noted (black arrows) Scale bar, A-D 100 μm.
Dual Labeling Immunofluorescence
Results from dual immunofluorescence experiments demonstrate that cholinergic and GABAergic cells of the caudate putamen and lateral shell of the accumbens express CXCR4. Double labeling of striatal sections with CXCR4 and D1R antiserum revealed that a population of D1R-positive neurons in the caudate putamen and lateral shell of the accumbens express CXCR4 (Fig. 4C, 5C). Sections double-labeled with CXCR4 and GAD C38 antibodies revealed cells positive for CXCR4 only, cells positive for both CXCR4 and GAD C38, and cells positive for GAD C38 only (Fig. 4F, 5F). Examination of sections double-stained with anti-CXCR4 and anti-GFAP antibodies showed no profiles positive for both markers, demonstrating that CXCR4 is not expressed on naïve astrocytes within the caudate putamen or lateral shell of the nucleus accumbens (Fig. 4J, 5J).
Figure 4.

Confocal images of rat caudate putamen sections double labeled with CXCR4 and D1R, GAD C38, GFAP or ChAT antiserum. A. CXCR4-ir cells. B. D1R-positive cells. C. A merged image of A and B: some cells are positive only for CXCR4 (white arrow) whereas others express both CXCR4 and D1 (yellow arrow). D. CXCR4-ir cells. E. GAD C38-ir cells. F. A merged image of D and E showing examples of cells which are only CXCR4-positive (white arrow), only GAD C38-positive (pink arrow) or co-express CXCR4 and GAD C38 (yellow arrow). G. Cell nuclei identified with DAPI staining. H. CXCR4-ir cells and fibers. I. GFAP-positive cells. J. A merged image of G, H and I showing cells that are only CXCR4-positive (white arrow) or only GFAP positive (pink arrow) demonstrating the lack of expression of CXCR4 by naïve astrocytes. K. DAPI staining for nuclei. L. CXCR4-ir cells. M. ChAT-positive cells. N. A merged image of K, L and M showing cells that are positive only for CXCR4 (white arrow) and others that co-express CXCR4 and ChAT (yellow arrow). O. ChAT-ir cell. P. GAD C38-positive cells. Q. A merged image of O and P showing no overlap between staining with the ChAT (white arrow) and GAD C38 (pink arrow). Scale bar, A-F, O-Q, 25 μm; G-N, 50 μm.
Figure 5.
Confocal images of sections from the lateral accumbens shell that were double-labeled with antisera to CXCR4 and D1R, GAD C38, GFAP or ChAT. A. CXCR4-ir cells. B. D1R-positive cells. C. A merged image showing cells that are only positive for CXCR4 (white arrow), only positive for D1 receptors (pink arrow) or co-express CXCR4 and D1R (yellow arrow). D. CXCR4-ir cells. E. GAD C38-ir cells. F. A merged image of D and E showing examples of cells that are only CXCR4-positive (white arrow), only GAD C38-positive (pink arrow) or positive for both CXCR4 and GAD C38 (yellow arrow). G. Cell nuclei identified with DAPI staining. H. CXCR4-ir cell. I. GFAP-positive cells. J. A merged image of G, H and I showing no colocalization of CXCR4 and GFAP. Processes of adjacent astrocytes are shown extending towards a CXCR4 positive cell (white arrow). K. DAPI staining for nuclei. L. CXCR4-ir cells. M. ChAT-positive cells. N. A merged image of K, L and M showing cells which are only CXCR4-positive (white arrows) and others that co-express CXCR4 and ChAT (yellow arrows). O. ChAT-ir cell. P. GAD C38-positive cells. Q. A merged image of O and P showing no overlap between labeling for ChAT (white arrows) and GAD C38 (pink arrow). Scale bar, A-J, O-Q, 25 μm; K-N, 50 μm.
To determine if CXCR4 is expressed by cholinergic interneurons of the caudate putamen and accumbens, sections were double-stained with CXCR4 and ChAT antisera. Sections representing the rostral, medial and caudal caudate putamen were examined from four rats. Results demonstrated that all of the cholinergic interneurons in the caudate putamen and lateral shell of the nucleus accumbens expressed CXCR4 on the cell surface and processes (Fig. 4N, 5N). Some CXCR4-positive cells did not stain for ChAT (Fig. 4N, 5N). Choline acetyltransferase exists in both a membrane bound and a soluble form. Previous immunohistochemical staining of ChAT demonstrates staining of the entire cell body as well as processes (Wang et al., 2009), similar to results shown in these studies. CXCR4 staining, as shown by this study, is primarily localized to the cell membrane. Lastly, as another control, adjacent sections were incubated with ChAT and GAD (Fig. 4Q, 5Q). No cells were positive for both ChAT-ir and GAD C38-ir.
3. Discussion
The results of this study demonstrate that CXCR4 receptors are expressed by cholinergic interneurons and by a sub-population of GABAergic neurons in the caudate putamen and lateral shell of the nucleus accumbens. Results also show that some cells co-express CXCR4 and D1 dopamine receptors, which are found on GABAergic medium spiny neurons in the striatum (Yung and Bolam, 2000). CXCR4-ir was not observed on astrocytes in brain sections from naïve rats (i.e., in the absence of an inflammatory condition), similar to an earlier report (Stumm et al., 2002).
Few studies have demonstrated the localization of CXCR4 in the adult rat brain using immunohistochemical techniques. Banisadr et al. reported the localization of CXCR4 in the substantia nigra, VTA and caudate putamen of the adult rat brain. Their results did not identify the CXCR4 receptor in the nucleus accumbens (Banisadr et al., 2002). Similarly, a subsequent study by the same group identified CXCL12 in similar areas reporting the absence of detectable levels of CXCL12 in the nucleus accumbens (Banisadr et al., 2003). Neither of these studies reported on subregions of the accumbens. Our results confirmed the absence of CXCR4 in both the medial shell and core of the nucleus accumbens. Upon closer inspection of the subregions of the nucleus accumbens, the present study clearly identified CXCR4-immunopositive neurons in the lateral aspects of the shell region of the nucleus accumbens. Both GABAergic and cholinergic neurons in the lateral shell expressed CXCR4.
The cell bodies of dopaminergic neurons in the mesolimbic dopamine system originate in the VTA and project primarily to the nucleus accumbens (Koob, 1992). The nucleus accumbens contains populations of GABAergic and cholinergic interneurons, as well as a large number of GABAergic medium spiny neurons (Meredith et al., 1993). The activity of the medium spiny neurons is regulated partially by glutamatergic afferents arising from the VTA and the cortex. Medium spiny neurons have been shown to project to specific brain regions; to the substantia nigra and dorsolateral ventral pallidum from the core of the nucleus accumbens and the caudate putamen, and to the VTA, medial ventral pallidum, hypothalamus and amygdala from the shell of the nucleus accumbens (Heimer et al., 1991; Pennartz et al., 1992).
Previous research has demonstrated that administration of CXCL12 into the substantia nigra increases extracellular dopamine in the caudate putamen and stimulates locomotor activity by a CXCR4-dependent mechanism (Skrzydelski et al., 2007). Studies from our laboratory have demonstrated that CXCL12 via activation of CXCR4 can modulate the hyperactivity produced by cocaine, and its effects are dependent upon the brain region where CXCL12 is administered. Injection of CXCL12 into either the VTA or the caudate putamen, respectively, potentiated locomotor and stereotypic activity produced by cocaine, whereas CXCL12 administered into the lateral shell of the accumbens inhibited locomotor activity (Trecki and Unterwald, 2009). These results prompted an investigation into the localization of CXCR4 within the forebrain in an attempt to further understand its cellular mechanisms.
The anatomical data presented herein can be used to further hypothesize the mechanism by which CXCR4 might modulate the mesolimbic and nigrostriatal dopamine pathways. In the mid-brain, CXCR4 has been shown to be expressed by dopaminergic (Banisadr et al., 2002) and GABAergic (Guyon et al., 2006) neurons in the substantia nigra. One mechanism by which CXCL12 administration in the VTA or substantia nigra could be causing dopamine release in the striatum and increasing locomotion (Skrzydelski et al., 2007; Trecki and Unterwald, 2009) is through disinhibition of dopaminergic neurons. Activation of CXCR4 expressed on GABAergic interneurons in the substantia nigra could inhibit GABA release, thus disinhibiting dopaminergic cell firing. Alternatively, CXCL12 has been shown to stimulate a CXCR4-mediated increase in intracellular calcium via N-type calcium currents (Guyon and Nahon, 2007; Zheng et al., 1999). This suggests that CXCR4 activation on dopaminergic cell bodies in the midbrain could be partially responsible for increased dopamine release in the striatum. Intracellular signaling activated by CXCL12 involves pertussis toxin-sensitive Gαi and Gq components which give rise to two distinct signaling pathways. One pathway, involving phosphatidylinositol-3 (PI-3) kinase and extracellular signal regulated kinase (ERK 1/2), has been shown to be activated by CXCL12 in rodent astrocytes, neuronal progenitors and cortical neurons (Bacon and Harrison, 2000; Bajetto et al., 2001a; Bonavia et al., 2003; Lazarini et al., 2000). IP3 binds its specific receptor in the endoplasmic reticulum and releases calcium from intracellular stores which acts as a second messenger (Boutet et al., 2001; Gillard et al., 2002). A second pathway involves phospholipase Cβ whose activation leads to an increase in intracellular calcium in astrocytes, neurons, microglia and cerebellar granule cells (Bajetto et al., 1999a; Klein et al., 1999; Zheng et al., 1999). CXCR4 stimulation has been shown to modulate high threshold calcium channels (Guyon and Nahon, 2007; Zheng et al., 1999) which can stimulate intracellular calcium release and tyrosine kinase activation (Lazarini et al., 2003). In addition, CXCR4 can inhibit cAMP production through Gi-coupling (Liu et al., 2003).
The caudate putamen and the nucleus accumbens have been shown to share the same neuronal cell types (Onn et al., 1994), as was confirmed by immunofluorescent staining in this study. GABAergic medium spiny neurons represent 95% of striatal neurons. Medium spiny neurons can be divided into two groups depending on their projection site: the striatonigral neurons which express D1 receptors and substance P, and the striatopallidal neurons which express D2 receptors and enkephalin (David et al., 2005; Le Moine and Bloch, 1995; Yung et al., 1995). Some medium spiny neurons have been shown to express both the D1 and D2 receptors (Surmeier et al., 2007). The remaining striatal cells include cholinergic and GABAergic interneurons (Kawaguchi, 1997). A reduction of GABAergic tone in the VTA or substantia nigra, via either the GABAergic interneurons or the GABAergic projections from the striatum, could lead to an increase of the firing of dopaminergic VTA or substantia nigra neurons (Szabo et al., 2002) and subsequent enhancement of dopamine release in the caudate putamen and nucleus accumbens.
In summary, this study demonstrates that CXCR4 is expressed by GABAergic and cholinergic neurons in the caudate putamen and the lateral shell of the nucleus accumbens. Furthermore, CXCR4 and the D1R are co-expressed by a sub-population of striatal neurons which opens the possibility of receptor cross-talk in these neurons. The results presented herein provide insights into potential functions of CXCL12 and CXCR4 in the forebrain of the rat and their interactions with classical neurotransmitter systems.
4. Experimental Procedure
Animals
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 325–350g were used in this study. Animals were housed in groups of four per cage on a 12 h light-dark cycle (7 AM – 7 PM) with ad libitum access to food and water. Animal care and experimental procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). Experimental protocols were approved by the Institutional Animal Care and Use Committee of Temple University.
Immunohistochemistry
Animals were anesthetized with Telazol (tiletamine/zolazepam, 40mg/kg, i.p., Fort Dodge Animal Health, Overland Park, KS) and intracardially perfused with 0.1M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were removed, post-fixed in 4% paraformaldehyde for two hours, and stored in 30% sucrose/PBS solution overnight. Single antibody labeling immunohistochemistry studies and dual labeling immunofluorescent studies were conducted using eight adult male Sprague Dawley rats.
Tissues were processed for CXCR4-immunoreactivity (CXCR4-ir) by the avidin-biotin complex procedure (Brailoiu et al., 2007; Dun et al., 1993b). Brains were embedded in agar and coronal sections of 50 μm thickness were cut on a vibratome. Sections were taken 1.56 to 1.08 mm anterior to bregma (Fig. 1) (Paxinos and Watson, 2007). Tissue sections were treated with 3% H202 to quench endogenous peroxidase, washed several times, blocked with normal horse serum (1:30 dilution), and incubated in anti-CXCR4 antibody (1:100 dilution), which was an affinity purified goat polyclonal antibody raised against a peptide mapping to the C-terminus of CXCR4 of human origin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). This antibody has been previously characterized for use in immunohistochemical studies of rat brain (Banisadr et al., 2002; Guyon et al., 2005; Skrzydelski et al., 2007). After rinsing, sections were incubated in biotinylated antigoat IgG (1:150 dilution; Vector Laboratories, Burlingame, CA) for two hours, rinsed with PBS and incubated in avidin-biotin complex solution for 1.5 hours (1:100 dilution; Vector Laboratories, Burlingame, CA). After several washes in Tris-buffered saline, sections were developed in 0.05% diaminobenzidine/0.001% H202 solution and washed with Tris-buffered saline. Sections were mounted on slides with 0.25% gel alcohol, air dried, dehydrated with absolute alcohol followed by xylene and cover-slipped with Permount.
A similar avidin-biotin complex procedure was used to detect the dopamine D1 receptor-ir (D1R), choline acetyltransferase-ir (ChAT), glial fibrillary acidic protein-ir (GFAP) and glutamic acid decarboxylase C38-ir (GAD C38). For D1R, ChAT and GAD C38 immunolabeling, sections were blocked with normal goat serum (1:100) and incubated in either anti-D1R antibody (1:500; rabbit polyclonal, Millipore/Chemicon International, Billerica, MA) (Zhou et al., 1992), anti-ChAT antibody (1:300; rabbit polyclonal, Millipore/Chemicon International, Billerica, MA) (Dun et al., 1993a) or anti-GAD C38 (1:500; a rabbit polyclonal directed against soluble and membrane bound GAD generously provided by Dr. JY Wu (Wu et al., 1986) to Dr. NJ Dun). Glutamic acid decarboxylase (GAD) is an enzyme which catalyzes the reaction of glutamate to GABA and CO2. Two isoforms of GAD exist in mammals, GAD65 and GAD67, with their nomenclature being derived from their molecular weights of 65 and 67 kDa. GAD 65 is an amphiphilic membrane bound protein while GAD67 is a cytoplasmic protein. Another isoform, GADC38, has been described and an antibody against it was chosen due to its cross reactivity with both membrane and soluble forms of GAD (Bao et al., 1994; Bao et al., 1995; Hsu et al., 1999) For identification of astrocytes, sections were blocked with normal horse serum (1:30) and incubated with anti-GFAP antibody (1:300; mouse monoclonal antibody, Millipore/Chemicon International, Billerica, MA) (Roth et al., 2007). After rinsing, sections were incubated in biotinylated antigoat IgG (1:50 dilution; Vector Laboratories, Burlingame, CA) for D1R, ChAT and GAD C38, or biotinylated antimouse IgG (1:150 dilution; Vector Laboratories, Burlingame, CA) for GFAP. The antibodies used to measure D1R, ChAT, GFAP and GAD C38 have been previously characterized for antigen specificity (Banisadr et al., 2002; Dun et al., 1993a; Roth et al., 2007; Wu et al., 1986; Zhou et al., 2002).
Double labeling immunofluorescent studies were conducted according to the methods of Brailoiu et al. (2007). Sections were blocked with normal horse serum (1:30 dilution in PBS, 0.5% BSA, 0.4% Triton X-100) and then incubated with anti-CXCR4 antibody (1:50) for 48 hours. Following several washes with PBS, sections were incubated with biotinylated antigoat IgG (1:50, Vector Laboratories), washed with PBS, and incubated with fluoroscein avidin D. After rinsing with PBS, sections were blocked with normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and incubated with anti-D1R antibody (1:250 dilution), anti-ChAT antibody (1:150 dilution), anti-GAD C38 antibody (1:250 dilution) or anti-GFAP antibody (1:150 dilution) for 48 hours. Following rinsing, tissues were incubated in appropriate IgG Texas Red antibodies. After rinsing with PBS, select sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI; 1:5000 dilution) for 10 minutes, washed with PBS, and mounted in Citifluor and cover-slipped. Sections were examined under a confocal scanning laser microscope (TCS SP5; Leica Microsystems Inc., Exton, PA) with excitation/emission wavelengths set to 488/520 nm for fluorescein isothiocyanate, 561/620 nm for Texas Red and 405/458 nm for DAPI in the sequential mode.
Acknowledgments
We would like to thank Mrs. Mary Dun and Dr. Nae Dun for their extensive help on this project. This work was supported by F31 DA024516 (JT), T32 DA07237 (EMU), P30 DA13429 (MW Adler/EMU) and PA Dept. of Health (EMU).
Abbreviations Used
- ChAT
choline acetyltransferase
- GABA
gamma amino butyric acid
- GFAP
glial fibrillary acidic protein
- D1R
D1 dopamine receptor
- GAD C38
glutamic acid decarboxylase
- DAPI
4′,6-diamidino-2-phenylindole
- VTA
ventral tegmental area
- CNS
central nervous system
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bacon KB, Harrison JK. Chemokines and their receptors in neurobiology: perspectives in physiology and homeostasis. J Neuroimmunol. 2000;104:92–7. doi: 10.1016/s0165-5728(99)00266-0. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Costa A, Schettini G. Expression of chemokine receptors in the rat brain. Ann N Y Acad Sci. 1999a;876:201–9. doi: 10.1111/j.1749-6632.1999.tb07640.x. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Barbero S, Bonavia R, Piccioli P, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001a;77:1226–36. doi: 10.1046/j.1471-4159.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol. 2001b;22:147–84. doi: 10.1006/frne.2001.0214. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Fontanges P, Haour F, Kitabgi P, Rostene W, Melik Parsadaniantz S. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–71. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- Banisadr G, Skrzydelski D, Kitabgi P, Rostene W, Parsadaniantz SM. Highly regionalized distribution of stromal cell-derived factor-1/CXCL12 in adult rat brain: constitutive expression in cholinergic, dopaminergic and vasopressinergic neurons. Eur J Neurosci. 2003;18:1593–606. doi: 10.1046/j.1460-9568.2003.02893.x. [DOI] [PubMed] [Google Scholar]
- Bao J, Nathan B, Hsu CC, Zhang Y, Wu R, Wu JY. Role of Protein Phosphorylation in Regulation of Brain L-Glutamate Decarboxylase Activity. J Biomed Sci. 1994;1:237–244. doi: 10.1007/BF02253308. [DOI] [PubMed] [Google Scholar]
- Bao J, Cheung WY, Wu JY. Brain L-glutamate decarboxylase. Inhibition by phosphorylation and activation by dephosphorylation. J Biol Chem. 1995;270:6464–7. doi: 10.1074/jbc.270.12.6464. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–33. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Bonavia R, Bajetto A, Barbero S, Pirani P, Florio T, Schettini G. Chemokines and their receptors in the CNS: expression of CXCL12/SDF-1 and CXCR4 and their role in astrocyte proliferation. Toxicol Lett. 2003;139:181–9. doi: 10.1016/s0378-4274(02)00432-0. [DOI] [PubMed] [Google Scholar]
- Boutet A, Salim H, Leclerc P, Tardieu M. Cellular expression of functional chemokine receptor CCR5 and CXCR4 in human embryonic neurons. Neurosci Lett. 2001;311:105–8. doi: 10.1016/s0304-3940(01)02149-8. [DOI] [PubMed] [Google Scholar]
- Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–94. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- David HN, Ansseau M, Abraini JH. Dopamine-glutamate reciprocal modulation of release and motor responses in the rat caudate-putamen and nucleus accumbens of “intact” animals. Brain Res Brain Res Rev. 2005;50:336–60. doi: 10.1016/j.brainresrev.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Chiaia NL. Hemorrhage induces Fos immunoreactivity in rat medullary catecholaminergic neurons. Brain Res. 1993a;608:223–32. doi: 10.1016/0006-8993(93)91462-2. [DOI] [PubMed] [Google Scholar]
- Dun NJ, Dun SL, Wu SY, Forstermann U, Schmidt HH, Tseng LF. Nitric oxide synthase immunoreactivity in the rat, mouse, cat and squirrel monkey spinal cord. Neuroscience. 1993b;54:845–57. doi: 10.1016/0306-4522(93)90579-5. [DOI] [PubMed] [Google Scholar]
- Gillard SE, Lu M, Mastracci RM, Miller RJ. Expression of functional chemokine receptors by rat cerebellar neurons. J Neuroimmunol. 2002;124:16–28. doi: 10.1016/s0165-5728(02)00005-x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Banisadr G, Rovere C, Cervantes A, Kitabgi P, Melik-Parsadaniantz S, Nahon JL. Complex effects of stromal cell-derived factor-1 alpha on melanin-concentrating hormone neuron excitability. Eur J Neurosci. 2005;21:701–10. doi: 10.1111/j.1460-9568.2005.03890.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Skrzydelsi D, Rovere C, Rostene W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem. 2006;96:1540–50. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- Guyon A, Nahon JL. Multiple actions of the chemokine stromal cell-derived factor-1alpha on neuronal activity. J Mol Endocrinol. 2007;38:365–76. doi: 10.1677/JME-06-0013. [DOI] [PubMed] [Google Scholar]
- Heimer L, Zahm DS, Churchill L, Kalivas PW, Wohltmann C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Thomas C, Chen W, Davis KM, Foos T, Chen JL, Wu E, Floor E, Schloss JV, Wu JY. Role of synaptic vesicle proton gradient and protein phosphorylation on ATP-mediated activation of membrane-associated brain glutamate decarboxylase. J Biol Chem. 1999;274:24366–71. doi: 10.1074/jbc.274.34.24366. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Neostriatal cell subtypes and their functional roles. Neurosci Res. 1997;27:1–8. doi: 10.1016/s0168-0102(96)01134-0. [DOI] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–46. [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–84. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–25. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–48. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–26. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Liu Z, Geng L, Li R, He X, Zheng JQ, Xie Z. Frequency modulation of synchronized Ca2+ spikes in cultured hippocampal networks through G-protein-coupled receptors. J Neurosci. 2003;23:4156–63. doi: 10.1523/JNEUROSCI.23-10-04156.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci U S A. 2002;99:7090–5. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Pennartz CM, Groenewegen HJ. The cellular framework for chemical signalling in the nucleus accumbens. Prog Brain Res. 1993;99:3–24. doi: 10.1016/s0079-6123(08)61335-7. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–85. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- Ohtani Y, Minami M, Kawaguchi N, Nishiyori A, Yamamoto J, Takami S, Satoh M. Expression of stromal cell-derived factor-1 and CXCR4 chemokine receptor mRNAs in cultured rat glial and neuronal cells. Neurosci Lett. 1998;249:163–6. doi: 10.1016/s0304-3940(98)00425-x. [DOI] [PubMed] [Google Scholar]
- Onn SP, Berger TW, Grace AA. Identification and characterization of striatal cell subtypes using in vivo intracellular recording in rats: I. Basic physiology and response to corticostriatal fiber stimulation. Synapse. 1994;16:161–80. doi: 10.1002/syn.890160302. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; Amsterdam ; Boston: 2007. [Google Scholar]
- Pennartz CM, Dolleman-Van der Weel MJ, Kitai ST, Lopes da Silva FH. Presynaptic dopamine D1 receptors attenuate excitatory and inhibitory limbic inputs to the shell region of the rat nucleus accumbens studied in vitro. J Neurophysiol. 1992;67:1325–34. doi: 10.1152/jn.1992.67.5.1325. [DOI] [PubMed] [Google Scholar]
- Ragozzino D. CXC chemokine receptors in the central nervous system: Role in cerebellar neuromodulation and development. J Neurovirol. 2002;8:559–72. doi: 10.1080/13550280290100932. [DOI] [PubMed] [Google Scholar]
- Roth TM, Ramamurthy P, Ebisu F, Lisak RP, Bealmear BM, Barald KF. A mouse embryonic stem cell model of Schwann cell differentiation for studies of the role of neurofibromatosis type 1 in Schwann cell development and tumor formation. Glia. 2007;55:1123–33. doi: 10.1002/glia.20534. [DOI] [PubMed] [Google Scholar]
- Skrzydelski D, Guyon A, Dauge V, Rovere C, Apartis E, Kitabgi P, Nahon JL, Rostene W, Parsadaniantz SM. The chemokine stromal cell-derived factor-1/CXCL12 activates the nigrostriatal dopamine system. J Neurochem. 2007;102:1175–83. doi: 10.1111/j.1471-4159.2007.04639.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Harrison JK. Chemokines and Alzheimer’s disease. Neurobiol Aging. 2001;22:909–13. doi: 10.1016/s0197-4580(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–78. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Hollt V, Schulz S. CXCR4 regulates interneuron migration in the developing neocortex. J Neurosci. 2003;23:5123–30. doi: 10.1523/JNEUROSCI.23-12-05123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–35. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–61. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Heesen M, Yoshizawa I, Berman MA, Luo Y, Bleul CC, Springer TA, Okuda K, Gerard N, Dorf ME. Functional expression of the CXC-chemokine receptor-4/fusin on mouse microglial cells and astrocytes. J Immunol. 1997;159:905–11. [PubMed] [Google Scholar]
- Tham TN, Lazarini F, Franceschini IA, Lachapelle F, Amara A, Dubois-Dalcq M. Developmental pattern of expression of the alpha chemokine stromal cell-derived factor 1 in the rat central nervous system. Eur J Neurosci. 2001;13:845–56. doi: 10.1046/j.0953-816x.2000.01451.x. [DOI] [PubMed] [Google Scholar]
- Tissir F, Wang CE, Goffinet AM. Expression of the chemokine receptor Cxcr4 mRNA during mouse brain development. Brain Res Dev Brain Res. 2004;149:63–71. doi: 10.1016/j.devbrainres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Trecki J, Unterwald EM. Modulation of cocaine-induced activity by intracerebral administration of CXCL12. Neuroscience. 2009;161:13–22. doi: 10.1016/j.neuroscience.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang R, Zhao Z, Ji Z, Xu S, Holscher C, Sheng S. Coexistences of insulin signaling-related proteins and choline acetyltransferase in neurons. Brain Res. 2009;1249:237–43. doi: 10.1016/j.brainres.2008.10.046. [DOI] [PubMed] [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci U S A. 2007;104:20151–8. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Denner LA, Wei SC, Lin CT, Song GX, Xu YF, Liu JW, Lin HS. Production and characterization of polyclonal and monoclonal antibodies to rat brain L-glutamate decarboxylase. Brain Res. 1986;373:1–14. doi: 10.1016/0006-8993(86)90309-4. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–30. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Yung KK, Bolam JP. Localization of dopamine D1 and D2 receptors in the rat neostriatum: synaptic interaction with glutamate- and GABA-containing axonal terminals. Synapse. 2000;38:413–20. doi: 10.1002/1098-2396(20001215)38:4<413::AID-SYN6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li C, Civelli O. Characterization of gene organization and promoter region of the rat dopamine D1 receptor gene. J Neurochem. 1992;59:1875–83. doi: 10.1111/j.1471-4159.1992.tb11023.x. [DOI] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–9. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]



