Abstract
Type I collagen is the most abundant protein in human body, produced by folding of two α1(I) and one α2(I) polypeptides into the triple helix. A conserved stem-loop structure is found in the 5’ UTR of collagen mRNAs, encompassing the translation start codon. We cloned La ribonucleoprotein domain family, member 6 (LARP6) as the protein which binds the collagen 5’ stem-loop in the sequence specific manner. LARP6 has a distinctive bipartite RNA binding domain, not found in other members of the La superfamily. LARP6 interacts with the two single stranded regions of 5’ stem-loop. The Kd for binding of LARP6 to the 5’ stem-loop is 1.4 nM. LARP6 binds the 5’ stem-loop in both, the nucleus and cytoplasm. In the cytoplasm, LARP6 does not associate with polysomes, however, overexpression of LARP6 blocks ribosomal loading on collagen mRNAs. Knocking down LARP6 by siRNA also decreased polysomal loading of collagen mRNAs, suggesting that it regulates translation. Collagen protein is synthesized at discrete regions of the endoplasmic reticulum (ER). We could reproduce this focal pattern of synthesis using collagen/GFP reporter protein, but only when the reporter was encoded by the mRNA with the 5’ stem-loop and in the presence of LARP6. When the reporter was encoded by mRNA without the 5’ stem-loop, or in absence of LARP6, it accumulated diffusely throughout the ER. This indicates that LARP6 activity is needed for focal synthesis of collagen polypeptides. We postulate that LARP6 dependent mechanism increases local concentration of collagen polypeptides for more efficient folding of the collagen heterotrimer.
Keywords: type I collagen, translation, LARP6, RNA binding
INTRODUCTION
Type I collagen is the most abundant protein in human body and is highly expressed in skin, bone and tendon 1; 2. Fibroproliferative disorders are characterized by excessive and persistent production of type I collagen in parenchimal organs and 45% of all deaths in the USA are attributable to fibroproliferative disorders 3. There is no cure for uncontrolled collagen synthesis and molecular details of its regulation are incomplete. The regulation is complex, because type I collagen is a heterotrimeric protein requiring synthesis, modifications and folding of two α1(I) chains and one α2(I) chain 1. The rate of posttranslational modifications and the rate of folding are coupled, because the mutations which delay folding result in hypermodifications of the chains and severe forms of osteogenesis imperfecta 4; 5. Posttranscriptional regulation of collagen expression by stabilization of the α1(I) mRNA is an important mechanism regulating collagen expression in various cell types 6; 7; 8; 9; 10; 11; 12. Destabilization of collagen α1(I) mRNA is one of the features associated with quiescence of fibroblasts 8; 10; 13, while TGFβ, the major profibrotic cytokine, induces collagen synthesis by increasing the half-life of the transcripts several fold 11; 14; 15. α-CP, also known as PCBP or hnRNP-E, binds to the C-rich sequence located 23 nt 3’ to the stop codon of collagen α1(I) mRNA 16 and stabilizes collagen α1(I) mRNA 17. α-CP stabilizes several other long lived mRNAs, including α globin mRNA 18, 15-1ipoxygenase mRNA and tyrosine hydroxylase mRNA 19, by binding to similar C-rich sequences.
In the 5’ UTRs of the three collagen mRNAs, α1(I), α2(I) and α1(III), there is a stem-loop structure encompassing the translation initiation codon 20. The 5’ stem-loop structure is located 75–85 nt from the cap and has a stability of ΔG=25–30 kcal/M. The 5’ stem-loop is well conserved in evolution, differing by only two nucleotides in Xenopus and human collagen mRNAs 21. The sequence constraints required to maintain the 5' stem-loop dictate the sequence around the start codon of collagen mRNAs. Therefore, the start codon is not in the optimal sequence context for translation initiation 22. In addition, collagen α1(I), α2(I) and α1(III) mRNAs have two short upstream open reading frames (uORF) preceding the coding region, which are usually inhibitory for translation 23. Thus, it seems that the three collagen mRNAs are designed to be inefficiently translated.
Our previous work indicated that collagen polypeptides have to be encoded by the mRNA with 5’ stem-loop to be properly assembled into triple helix 24. This suggests that 5' stem-loop couples translational machinery to the collagen assembly pathway and is the first example of an RNA element that affects protein folding.
In this manuscript we describe cloning and characterization of the collagen 5’ stem-loop binding protein and its effects on translation of collagen mRNAs.
RESULTS
5’ stem-loop in the 5’ UTR of collagen mRNAs
Three collagen mRNAs, α1(I), α2(I) and α1(III), encoding collagens type I and III, have a conserved 5’ stem-loop structure in their 5’ UTRs 20. The sequence conservation of the 5’ stem-loop from three distant species is shown in Fig 1A. The translation initiation codon is buried in the stem 2. Being unique for collagen mRNAs, we hypothesized that 5’ stem-loop may regulate coordinated translation of collagen mRNAs by binding specific RNA binding protein(s).
Figure 1.
Protein binding to the 5’ stem-loop of collagen α1(I) mRNA. A. Conserved stem-loop in the 5’ UTR of collagen α1(I), α2(I) and α1(III) mRNAs from distantly related vertebrates. The sequence of α1(III) from fish is not available. S1; bottom stem, S2; top stem, B1; left side of the bulge, B2; right side of the bulge. Right panel; folding of α1(I) 5’ stem-loop. Translation start codon is boxed. B. Protein binding to the 5’ stem-loop RNA in nuclear extracts. Gel mobility shift assay; lane 1, probe alone; lane 2, extract without competitor; lanes 3 and 4, the indicated molar excess of specific competitor (SP) added; lanes 5 and 6, the indicated molar excess of the inverted 5’ stem-loop RNA as nonspecific competitor (NS) added. Migration of RNA probe and RNA/protein complex is indicated. C. Protein binding to the 5’ stem-loop RNA in cytosolic extracts. Experiment as in B except cytosolic extract was used. Lanes 7 and 8, gel mobility shift with inverted 5’ stem-loop as probe. D. 65 kD protein crosslinks to 5’ stem-loop RNA. Lane 1, sample without UV irradiation; lane 2, sample after UV irradiation; lanes 3 and 4, UV crosslinking to inverted 5’ stem-loop RNA. E. Gel mobility shift using cytosolic extract and various mutant probes; WT, wild-type probe; U, probe with a single U nucleotide changed into an A; B2, probe with all 4 nucleotides in the B2 segment of the bulge mutated; S1, probe with bottom stem reversed; S2, probe with top stem reversed. Lanes 1, 3, 5, 7 and 9, probes alone; lanes 2, 4, 6, 8 and 10, extract added. Migration of RNA probes and RNA/protein complex is indicated. Asterisk indicates a nonspecific complex.
Sequence specific binding of a protein to the collagen 5’ stem-loop
To identify binding activity to 5’ stem-loop RNA, nuclear and cytosolic extracts of human lung fibroblasts were used in gel mobility assays. In nuclear extracts an RNA/protein complex was detected (Fig 1B, lane 2), binding of which was effectively competed with 50-fold and 250-fold molar excess of the same unlabelled RNA (lanes 3 and 4). When the RNA with the inverted sequence of 5’ stem-loop (inverted 5’ stem-loop RNA) was used as competitor, only a weak competition was observed at a molar excess of 250-fold (lanes 6). In cytosolic extracts (Fig 1C) an RNA/protein complex with similar electrophoretic mobility was seen (lane 2). While the specific RNA effectively competed for its binding (lanes 3 and 4), the inverted 5’ stem-loop RNA did not compete at all (lanes 5 and 6). The inverted 5’ stem-loop RNA also did not bind any protein factors in the same extract (lanes 7 and 8). To verify that the complex formation on the 5’ stem-loop RNA is due to binding of proteins and not to binding of RNA, DNA or carbohydrates, the cytosolic extract was treated with SDS and proteinase K prior to loading on the gel. Both treatments completely abolished the complex formation (not shown). Similar, but weaker, 5’ stem-loop binding activity was also found in extracts of mouse embryonic fibroblasts, human skin fibroblasts and rat liver myofibroblasts (not shown). To obtain information about the molecular weight of the protein which directly binds to the 5’ stem-loop RNA, we performed UV crosslinking experiments using cytosolic extracts (Fig 1D). A crosslink of 65 kD was obtained with 5’ stem-loop RNA (lane 2) and no crosslinking was seen with the inverted 5’ stem-loop RNA. A similar crosslink was observed using nuclear extracts (not shown). Since this crosslink contains a protein and a small piece of RNA that resisted digestion with RNase T1, it represents only an approximation of the actual size of the protein.
Single stranded bulge as target for protein binding
Evolutionary conservation of the 5’ stem-loop suggests that, not only the structure, but also the sequence of both stems and the bulge may be important for protein binding. We made a series of mutant probes where we mutated a single U in the B1 segment of the bulge (U mut), 4 nt in the B2 segment (B2 mut) or we inverted the sequence of the stems S1 or S2, but maintained the base pairing (S1 mut and S2 mut, respectively). The sequences of these probes are shown in table 2. Each mutant probe was analyzed for binding using cytosolic extracts (Fig 1E). Changing a single U nucleotide into an A in the B1 segment completely abolished protein binding (compare lanes 4 and 2). Changing 4 nt in the B2 segment also completely abolished the binding (compare lanes 6 and 2). Inverting the sequence of the bottom stem diminished the binding (compare lanes 8 and 2), while the inversion of the top stem had a weaker effect (compare lanes 10 and 2). From this experiment we concluded that the single stranded regions of the bulge are important for binding of the detected protein to the 5’ stem-loop RNA.
Table 2.
Mutant 5’ stem-loop RNAs used as probes in gel mobility shift experiments. Wt probe is shown on the top and the changed nucleotides in each mutant RNA are underlined and indicated in bold letters.
| CCACAAAGAGUCUACAUGUCUAGGGUCUAGACAUGUUCAGCUUUGUGG | WT |
| CCACAAAGAGACUACAUGUCUAGGGUCUAGACAUGUUCAGCUUUGUGG | U MUT |
| CCACAAAGAGUCUACAUGUCUAGGGUCUAGACAUGUUGGACUUUGUGG | B2 MUT |
| AUCGUGCAAGUCUACAUGUCUAGGGUCUAGACAUGUUCAGUGCACGAU | S1 MUT |
| CCACAAAGAGUCUGCUAGACUUCGGUGAAGUCUAGCUCAGCUUUGUGG | S2 MUT |
Expression cloning of the 5’ stem-loop binding protein
We used a gel mobility shift assay as a read-out for expression screening of human fibroblast cDNA library. The library was amplified in pools containing 100 plasmids and each pool was transfected into HEK293 cells. Cell lysates were analyzed by gel mobility shift and one of the 130 pools screened yielded an increased binding to the 5’ stem-loop. This pool was divided into subpools containing 20 clones, which were identically screened until a single clone was isolated. This clone encoded a full sized open reading frame identical to human La ribonucleoprotein domain family member 6 (LARP6) protein 25. The predicted molecular weight of LARP6 is 55 kD. This is similar to the protein crosslinked to 5’ stem-loop (Fig 1D), taking into account the inaccuracy of UV crosslinking.
LARP6 as the 5’ stem-loop RNA binding protein
LARP6 is an uncharacterized RNA binding protein 25. To characterize its RNA binding properties, the binding to the 5’ stem-loop RNA was analyzed by gel mobility shift experiments (Fig 2). First, we verified that endogenous LARP6 is in the complex bound to the 5’ stem-loop RNA. To this goal we added anti-LARP6 antibody to the cytosolic extracts of lung fibroblasts and compared the mobility shift to that obtained with the control antibody (Fig 2A). LARP6 antibody reduced binding of the endogenous protein to the 5’ stem-loop (lane 3). Second, we overexpressed LARP6 in cells expressing very little of the endogenous LARP6 (HEK293 cells). Binding to the 5’ stem-loop was dramatically enhanced in HEK293 cells when LARP6 was overexpressed compared to control cells (Fig 2B). Two RNA/protein complexes were seen, probably representing monomer and dimer of LARP6 bound to the 5’ stem-loop RNA. A 50-fold excess of specific competitor RNA significantly reduced the binding, while a 250-fold excess completely abolished it (lanes 2 and 3). Nonspecific competitor (inverted RNA) had no effect (lanes 4 and 5).
Figure 2.
LARP6 as the sequence specific 5’ stem-loop RNA binding protein. A. Endogenous LARP6 binds 5’ stem-loop. Gel mobility shift analysis with cytosolic extracts of lung fibroblasts in presence of control antibody (CON, lane 1) and anti-LARP6 antibody (LARP6, lane 2). Lane 1 is probe alone. Migration of RNA probe and RNA/protein complex is indicated. B. Sequence specific binding of LARP6 to 5’ stem-loop RNA. Experiment as in Fig 1C, except cytosolic extracts from HEK293 cells overexpressing LARP6 were used (lanes 1–5). Lanes 6–10 are control HEK293 cells. C. LARP6 binds the bulge of 5’ stem-loop. LARP6 was overexpressed in HEK293 cells (lanes 1–5) and gel mobility shift assay was done with wt 5’ stem-loop RNA probe (lane 1), U mutant probe (lane 2), B2 mutant probe (lane 3), S1 mutant probe (lane 4) and S2 mutant probe (lane 5). The sequences of these probes are shown in table 2. D. Recombinant LARP6 binds 5’ stem-loop. Gel mobility shift with recombinant GST-LARP6. Lane 1, wt 5’ stem-loop probe; lane 2, U mutant stem-loop probe; lane 3, B2 mutant stem-loop probe. E. LARP6 binds collagen mRNAs in vivo. Lane 1, immunoprecipitation of collagen α1(I) and α2(I) mRNAs with HA-tagged LARP6. RT-PCR products specific for collagen α1(I) mRNA (COL1A1), collagen α2(I) mRNA (COL1A2) and fibronectin mRNA (FIB) are indicated. Lane 2, immunoprecipitation from cells expressing control protein, RBMS3.
To verify if LARP6 primarily binds the bulge of 5’ stem-loop, we analyzed its binding to the mutant 5’ stem-loop probes. Fig 2C shows that changing a single U nt into an A in the B1 segment of the bulge almost completely abolished the binding (lane 2). Likewise, changing 4 nt in the B2 segment of the bulge dramatically reduced the binding (lane 3). Inverting the top or the bottom stem, but preserving the structure, resulted in stronger formation of the monomer complex and attenuation of the putative dimer, but the overall binding was not significantly affected (lanes 4 and 5). From these experiments we concluded that LARP6 binds the collagen 5’ stem-loop bulge in a sequence specific manner and with specificity similar to the endogenous 5’ stem-loop binding activity detected in fibroblasts (Fig 1).
To verify that LARP6 is sufficient for binding 5’ stem-loop, we purified the bacterially expressed GST-LARP6 fusion protein. Recombinant LARP6 strongly bound wt 5’ stem-loop RNA (Fig 2D, lane 1), while the binding was almost completely abolished when the U mutation of B1 was used (lane 2). The B2 mutant showed a decreased, but significant binding (lane 3), what is in contrast to LARP6 expressed in mammalian cells, which does not bind this probe (Fig 1E and Fig 2C). Two RNA/LARP6 complexes were resolved indicating that the purified protein can form dimers. This experiment verified that LARP6 is sufficient for binding 5’ stem-loop and that posttranslational modification of the protein is not absolutely necessary for binding.
To analyze if LARP6 interacts in vivo with collagen α1(I) and α2(I) mRNAs we expressed HA-tagged LARP6 in HEK293 cells and performed immunoprecipitation from the cytosolic extracts. The immunoprecipitated material was analyzed by RT-PCR for pull-down of collagen α1(I) and α2(I) mRNAs. As shown in Fig 2E, collagen α1(I) and α2(I) mRNAs coimmunoprecipitated with LARP6, while fibronectin mRNA did not (lane 1). Control RNA binding protein, RBMS3 26 did not pull down any mRNA (lane 2). Therefore, we concluded that both collagen mRNAs associate with LARP6 in vivo.
High affinity of binding of LARP6 to the 5’ stem-loop
Purified recombinant RNA binding proteins are commonly used to assess the affinity of binding to their targets 17; 27; 28. These results may not reflect the binding in the cellular environment, where the proteins are complexed with their interacting factors and where other proteins limit the accessibility to the target. To provide an estimate for binding affinity of LARP6 to the 5’ stem-loop RNA, LARP6 was overexpressed in HEK293 cells and a fixed amount of cytosolic extract was added to the increasing amounts of radiolabeled 5’ stem-loop RNA probe (Fig 3A). The fractions of the bound and unbound RNA were resolved by gel mobility shift and Scatchard plot analysis was performed to determine the Kd (Fig 3B), as described 17; 29; 30. From three independent experiments we estimated the Kd to be 1.4 nM (SD = 0.7). This is comparable to that of other sequence specific RNA binding proteins 31; 32.
Figure 3.
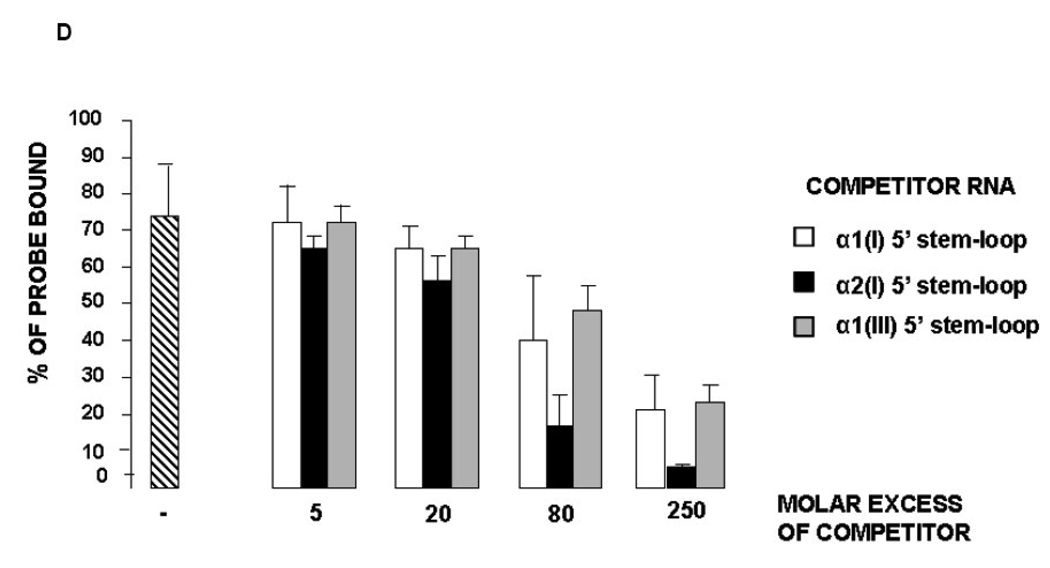
High affinity binding of LARP6 to collagen 5’ stem-loop. A. Gel mobility shift assay with increasing amounts of 5’ stem-loop RNA probe and a fixed amount of cellular extract expressing LARP6. Femtomoles of probe used in the binding reactions is indicated at the bottom and migration of the bound and unbound probe is indicated to the right. B. A representative Scatchard plot from the data shown in A. The fractions of the bound and unbound RNA were quantified by phosphoimaging and Scatchard plot is shown. Kd of binding was estimated from three independent experiments. C. Gel mobility shift assay in absence and presence of competitor RNA. WT α1(I) 5’ stem-loop RNA probe and cytosolic extract of HEK293 expressing LARP6 were used in all lanes. Lane 1, binding in absence of competitor. Lanes 2–5, binding in presence of indicated molar excess of α1(I) 5’ stem-loop RNA; lanes 6–9, binding in presence of molar excess of α2(I) 5’ stem-loop RNA; lanes 10–13, binding in presence of molar excess of α1(III) 5’ stem-loop RNA. D. Statistical evaluation of the degree of competition in three independent experiments. Bound and unbound probe from experiments in C was measured by phosphoimaging and the percent probe bound is shown for each molecular excess of competitor RNA added. Error bars: +- 1SD.
To assess if LARP6 binds the 5’ stem-loop of collagen α2(I) and α1(III) mRNAs with an affinity similar to that of α1(I) mRNA, we competed the binding of LARP6 to α1(I) 5’ stem-loop probe with an excess of unlabeled 5’ stem-loop RNA derived from α2(I) and α1(III) mRNAs. As seen in Fig 3C, the α2(I) 5’ stem-loop seemed to compete the binding of LARP6 to α1(I) 5’ stem-loop slightly better than the α1(I) 5’ stem-loop (compare lanes 6–9 to lanes 2–5). The competing effect of the α1(III) 5’stem-loop was similar to that of the α1(I) 5’ stem-loop (lanes 10–13). We repeated this experiment three times and plotted the percent of bound probe vs. the molar excess of competitor (Fig 3D). Although the competition by the α2(I) 5’ stem-loop was stronger than that of α1(I) and α1(III) 5’ stem-loops, the effect did not reach statistical significance at p<0.05. Therefore, we concluded that LARP6 recognizes collagen α1(I), α2(I) and α1(III) 5’ stem-loops with similar affinity.
Unique RNA binding domain of LARP6
Figure 4A shows the predicted domains of LARP6. Amino acids 85–183 have a similarity to the La domain found in the members of La-related protein super family (LARPs) 33; 34. Adjacent to the La domain there is a domain with a weak similarity to a generic RNA binding domain (RBD) (amino acids 183–296) 35. The other regions of LARP6 have no homology to known proteins. To assess which region of LARP6 is required for binding the collagen 5’ stem-loop RNA, we derived several deletion mutants. Fig 4A summarizes the mutants and their ability to bind 5’ stem-loop RNA and Fig 4B shows the binding. When the C-terminal 300–491 amino acids were deleted (mutant A) the protein still had the ability to bind the 5’ stem-loop RNA (Fig 4B, lane 3), however, deletion of most of the predicted RBD (mutant B) completely abolished the binding (lane 4). Deletion of the N-terminal 183 amino acids, including the La domain (mutant C), also completely abolished the binding (lane 5). To assess if reintroducing the La domain would restore the binding, we constructed a protein which had only the La domain and RBD (mutant D). This mutant was unable to bind the 5’ stem loop (lane 6), suggesting that amino acids N-terminal to the La domain are needed. When we added 40 amino acids N-terminal to the La domain, the binding was restored (mutant E, lane 7). Next, we made two internal deletions adjacent to the La domain (mutants F and G). While the mutant F, which contained 4 amino acids upstream of the boundary to the La domain was able to bind (lane 8), the mutant in which these amino acids were deleted (mutant G) lost the ability to interact with 5’ stem-loop (lane 9). From these experiments we concluded that LARP6 needs the RBD and the several amino acids adjacent to the N-terminus of the La domain to interact with the 5’ stem-loop. These amino acids are not present in the other La containing proteins.
Figure 4.
Bipartite RNA binding domain of LARP6. A. Schematic representations of the domains of LARP6. Predicted La domain is shown in grey, predicted RNA binding domain is shown in black, amino-acid numbering is on the top, the designation of the full size protein (FS) and mutants is to the left and their ability to bind 5’ stem-loop RNA is indicated to the right. B. Binding of LARP6 mutants to 5’ stem-loop RNA. Full size LARP6 (FS, lane 2) and the mutants shown in A (lanes 3–9) were expressed and cytosolic extract was used in gel mobility shift assay. Lane 1, probe alone. The level of expression of each protein analyzed by western blot is shown at the bottom. C. Subcellular accumulation of LARP6. Immunostaining of endogenous LARP6; confocal image using LARP6 antibody (upper left panel) and omitting LARP6 antibody (lower left panel). Right panels; overlay with nuclear staining (DAPI). D. Subcellular accumulation of LARP6 mutants. Full size LARP6 (FS) and mutants A and B were expressed and after fractionation into cytosolic (lane 1) and nuclear (lane 2) fractions the relative proportion of LARP6 was assessed by western blot. Expression of tubulin (TUB) is shown as control.
LARP6 is nuclear and cytosolic protein
Immunostaining of the endogenous protein showed that LARP6 accumulates in both, nucleus and cytoplasm (Fig 4C). This is consistent with the presence of the endogenous 5’ stem-loop binding activity in the nuclear and cytosolic extracts of fibroblasts (Fig 1). LARP6 has a predicted nuclear localization signal (NLS) between amino-acids 293 and 303 25. To assess if this signal is required for nuclear localization of LARP6, we expressed full size LARP6, mutant A, lacking the part of NLS, and mutant B, lacking the whole NLS and prepared cytosolic and nuclear fractions. The fractions were analyzed by Western blot for distribution of LARP6 (Fig 4D). Full size LARP6 was equally distributed between nuclear and cytosolic fractions, consistent with immunostaining of the endogenous protein, while mutants A and B were predominantly cytoplasmic. Tubulin, which is exclusively a cytosolic protein 36, was absent from the nuclear preparations indicating that there was no cross-contamination. From these experiments, we concluded that LARP6 accumulates in the nucleus and cytosol and that a functional NLS resides in the C-terminal domain.
Excess of LARP6 is inhibitory to translation of collagen mRNAs
To test the hypothesis that LARP6 may regulate translation of collagen mRNAs, we constructed adenoviruses expressing full size LARP6, mutant A (Fig 4A) and control protein LOX. Using adenoviruses, we could express these proteins in 100% of lung fibroblasts, allowing studies of their effects on the endogenous collagen mRNAs. When we expressed full size LARP6 and mutant A (both of these proteins can bind 5’ stem- loop, Fig 4B), there was a dramatic decrease in the cellular level of type I collagen protein (Fig 5A, lanes 1 and 2) and in secretion of procollagen into the cellular medium (lanes 4 and 5). The cellular level and secretion of fibronectin were not significantly affected, indicating that the effect of LARP6 was collagen specific. Since the steady state level of collagen mRNAs was unchanged (Fig 5B), we concluded that the excess of LARP6, as well as of mutant A, inhibited translation of collagen mRNAs.
Figure 5.
Excess of LARP6 is inhibitory to translation of collagen mRNAs. A. Effect of excess of LARP6 on collagen protein expression. Full size LARP6 (FS, lane 1), mutant A (lane 2) and control protein LOX (lane 3) were expressed in lung fibroblasts. Cellular level of collagen α1(I) polypeptide (COLL) was assessed by western blot. Expression of fibronectin (FIB) is shown as control. Lanes 4–6, medium from the same cells analyzed by western blot. B. Effect of excess of LARP6 on collagen mRNA expression. Total RNA from cells in A was analyzed by RT-PCR for expression of collagen α1(I) mRNA (COL1A1), α2(I) mRNA (COL1A2) and α1(III) mRNA (COL3A1). Actin is shown as loading control.
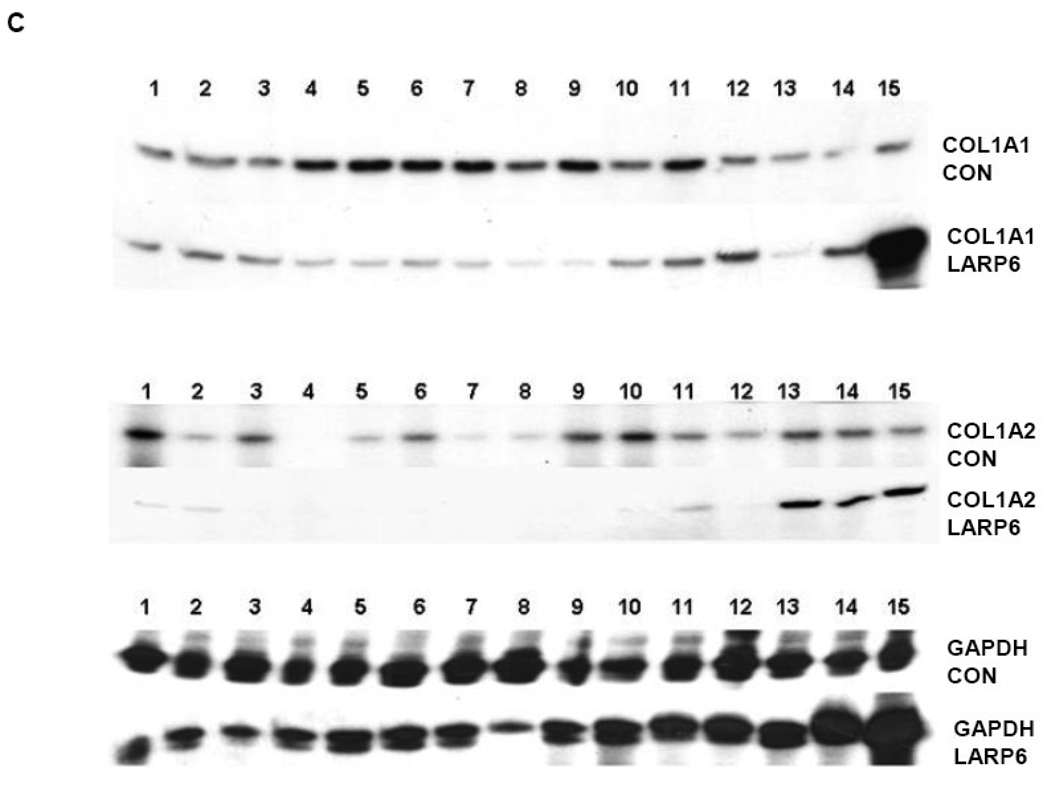
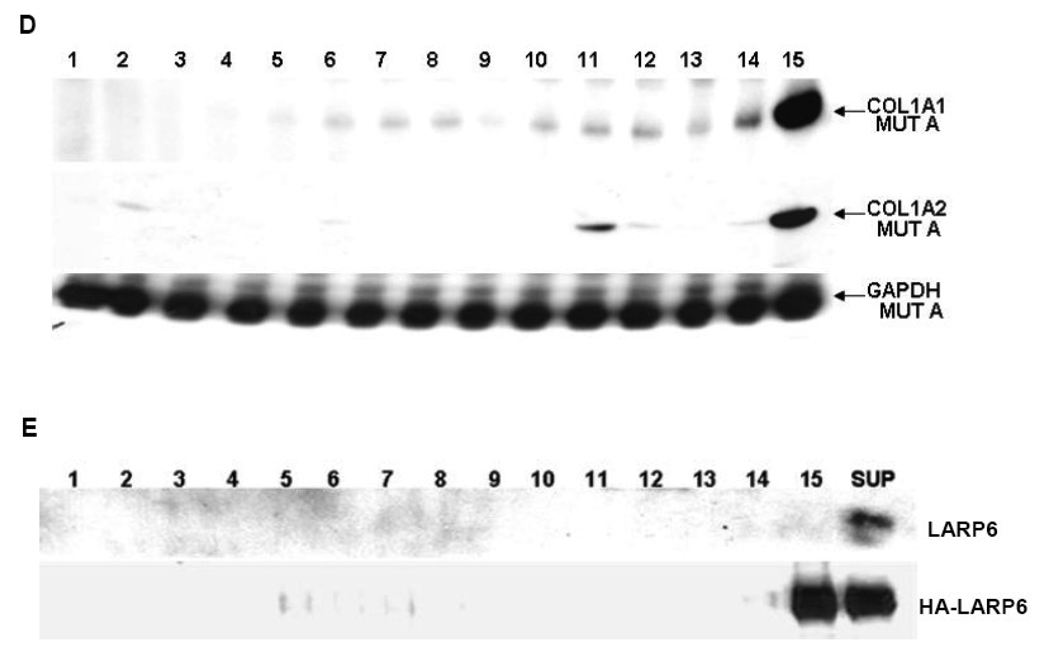
To verify this, we fractionated polysomes from LARP6 overexpressing cells and analyzed polysomal loading of collagen α1(I) and α2(I) mRNAs. Fig 6A shows distribution of ribosomal RNA in sucrose fractions of polysomes after treatment of cells with cycloheximide and puromycin, while Fig 6B shows OD260 profile of sucrose fractions from cycloheximide treated cells. Based on the sensitivity to puromycin and on fractionation of 80S ribosomes into fraction 13 and ribosomal subunits into fractions 14 and 15, we estimated that fractions 1–12 represent polysomes. Fig 6C shows that when control protein was overexpressed, collagen α1(I) mRNAs were found in all polysomal fractions (fractions 1–12) and to a lesser extent in fractions 13 to 15. However, when LARP6 was overexpressed, collagen α1(I) mRNA was reduced in polysomal fractions and accumulated predominantly in fraction 15 (Fig 6C, top panel). A similar result was obtained with collagen α2(I) mRNA. It was distributed throughout polysomal fractions in control cells and confined predominantly into fractions 13–15 in LARP6 overexpressing cells (Fig 6C middle panel). GAPDH mRNA was distributed in all fractions and not affected by LARP6 overexpression (Fig 6C, bottom panel). Similar polysomal distribution of GAPDH mRNA was described by other investigators 37 and allowed us to use this mRNA as a control for loading. We concluded that the redistribution of collagen mRNAs into fractions lighter than polysomes is responsible for the decrease of collagen protein synthesis in cells overexpressing LARP6 (Fig 5A).
Figure 6.
Overexpression of LARP6 precludes polysomal loading of collagen mRNAs. A. Analysis of ribosomal RNA. Polysomes were isolated from cells treated with cycloheximide (CHX, upper panel) or puromycin (PUR, lower panel) and distribution of ribosomal RNA in 45% (lane 1) to 15% (lane 15) linear sucrose gradient is shown. B. Polysomal profile of cells treated with cycloheximide. OD260 profile of sucrose fractions. Position of ribosomal subunits is indicated. C. Polysomal distribution of collagen mRNAs in cells overexpressing LARP6. Upper panel, distribution of collagen α1(I) mRNA in control cells (COL1A1 CON) and LARP6 overexpressing cells (COL1A1 LARP6). Middle panel, distribution of collagen α2(I) mRNA in control cells (COL1A2 CON) and LARP6 overexpressing cells (COL1A2 LARP6). Bottom panel, distribution of GAPDH mRNA. Polysomal fractions were analyzed by RT-PCR. D. Polysomal distribution of collagen mRNAs in cells overexpressing mutant A. Experiment as in B, except mutant A was overexpressed. E. Distribution of LARP6 in polysomal fractions. Upper panel, endogenous LARP6 was analyzed in sucrose fractions and postpolysomal supernatant (SUP) of control cells by western blot. Lower panel, analysis of overexpressed HA-tagged LARP6.
A similar result was obtained in the presence of excess of mutant A, which can bind the 5’ stem-loop, but is lacking the C-terminus (Fig 6D). Overexpression of this mutant showed even stronger exclusion of collagen mRNAs from the polysomes and their shift into the lightest fraction (fraction 15). This explained the lower level of collagen synthesis in the cells expressing mutant A than LARP6 (Fig 5A).
Presence of the endogenous LARP6 in polysomal fractions was assessed by Western blot. LARP6 was absent from all sucrose fractions and it was detected only in the supernatant above the sucrose gradient (Fig 6E, top panel), indicating that LARP6 does not associate with polysomes or ribosomal subunits. Similarly, the overexpressed LARP6 was found only in fraction 15 and in postpolysomal supernatant (bottom panel).
Knock down of LARP6 results in decreased collagen protein synthesis
To assess the effect of loss of function of LARP6 on collagen expression, we inactivated LARP6 by siRNA. We used adenoviruses expressing one effective siRNA gene targeting LARP6 (D2) and one scrambled siRNA gene (CON). The depletion of LARP6 protein was assessed by Western blot (Fig 7A) and by gel mobility shift assay (Fig 7B). Both assays revealed that LARP6 was depleted by about 70%. When LARP6 was knocked down there was no change in the steady state level of collagen α1(I) and α2(I) mRNAs (Fig 7C, lanes 1 and 4) and no difference in their decay rates after a transcription block with actinomycin D (lanes 2, 3, 5 and 6). However, when we measured collagen protein in LARP6 depleted cells, there was a reduction in the intracellular level (Fig 7D, top panel) and in the rate of secretion into the medium (bottom panel). The cellular level and secretion of fibronectin were not significantly altered. This suggested that the normal cellular level of LARP6 is needed for high steady state level of collagen protein in lung fibroblasts. A similar result was obtained for skin fibroblasts (not shown). Taken together, these results indicate that LARP6 can be a translational repressor if expressed at high levels, however, at physiological levels it acts to stimulate collagen synthesis.
Figure 7.
Inactivation of LARP6 decreases collagen synthesis. A. Depletion of LARP6 protein by siRNA. LARP6 expression was analyzed by western blot; lane 1, LARP6 specific siRNA (D2); lane 2, scrambled siRNA (CON). Control for loading, fibronectin (FIB). B. Depletion of LARP6 5’ stem-loop binding activity by siRNA analyzed by gel mobility shift assay. C. Steady state level and decay rate of collagen mRNAs in LARP6 depleted cells (lanes 1–3) and control cells (lanes 4–6). Steady state level of collagen α1(I) and α2(I) mRNAs (lanes 1 and 4) and their decay after transcription block with actinomycin D (lanes 2, 3, 5 and 6) were analyzed by RT-PCR. D. Depletion of LARP6 decreases collagen protein expression. Cellular extracts (top panel) and medium (bottom panel) were analyzed for collagen protein by western blot. Fibronectin is shown as loading control. Lane 1, LARP6 depleted cells; lane 2, control cells. E. Effect of proteosome inhibitor MG132. Cells expressing LARP6 specific siRNA (D2, lanes 1 and 3) or control siRNA (lanes 2 and 4) were treated with MG132 (lanes 1 and 2) or left untreated (lanes 3 and 4) and cellular level of collagen was analyzed by western blot.
The decreased level of collagen protein upon inactivation of LARP6 may be due to either increased protein turn-over or to decreased synthesis. To distinguish between these two possibilities we first determined collagen protein level after treatment of cells with proteosome inhibitor MG132 (Fig 7E). The cells expressing LARP6 specific siRNA (D2) or control siRNA were treated with MG132 and intracellular collagen level was compared to the untreated cells. D2 siRNA decreased collagen expression in control cells (compare lanes 3 and 4), as well as in MG132 treated cells (compare lanes 1 and 2). Thus, we concluded that increased protein degradation is not likely to be responsible for the decrease in collagen protein level, but, since protein degradation can also be by a nonproteosomal mechanism, we can not completely exclude this possibility.
LARP6 targets collagen synthesis to discrete regions in the cell
Collagen, as extracellular protein, is synthesized on the membrane of the endoplasmic reticulum (ER) and folded within the lumen. Immunostaining of lung fibroblasts with collagen specific antibody revealed that the collagen protein found intracellularly is not uniformly distributed throughout the ER. It colocalized with the ER marker calnexin and, while calnexin showed diffuse immunostaining, collagen was found at discrete foci (Fig 8A). This suggests that its synthesis or accumulation in the ER is not random. This prompt us to analyze if LARP6 regulates this focal synthesis. To this goal we made a collagen/GFP reporter gene, where we fused GFP to the C-terminus of collagen α1(I) open reading frame. The reporter protein was expressed from the mRNAs with or without the 5’ stem-loop (Fig 8B) in a HEK293 cell line stably expressing LARP6 and in control cells. These cells were chosen because they express very little endogenous LARP6. When LARP6 was expressed in HEK293 cells, collagen/GFP protein encoded by the mRNA with the 5’stem-loop accumulated in a granular pattern, showing discrete spots of increased accumulation (Fig 8C, upper left panel). The protein encoded by the mutant mRNA accumulated in a diffuse pattern resembling the ER and lacking the spots of concentration (upper right panel). The focal enrichment of collagen/GFP protein was also lost when the 5’ stem-loop mRNA was expressed in cells lacking LARP6 (bottom left panel), suggesting that the subcellular accumulation of the reporter protein is both, 5’ stem-loop and LARP6-dependent. To exclude the possibility that focal subcellular accumulation of the reporter protein is due to the transfected LARP6 we transfected the reporters into lung fibroblasts which express only endogenous LARP6 (Fig 8D). A similar focal accumulation of COLL/GFP protein was observed when it was encoded by mRNA with the 5’ stem-loop (upper panel), compared to diffuse accumulation of COLL/GFP protein encoded by an mRNA without the 5’ stem-loop (lower panel).
Figure 8.
LARP6 concentrates collagen synthesis at discrete subcellular regions. A. Focal accumulation of collagen in the ER. Immunostaining of calnexin as ER marker (CAL, left panel), type I collagen (COLL, middle panel) and their overlay with DAPI staining (MERGE, right panel). Image of one confocal plane is shown. B. Collagen/GFP fusion constructs. CMV promoter is shown as dotted line, collagen 5’ UTR as thin gray line and collagen coding region as thick black line. GFP in frame with collagen is shown as broken line. The genes have the identical open reading frame; AUG, start codon; UAA, stop codon. C. Confocal images of collagen/GFP expression. 5’SLCOLL/GFP gene (upper left panel) and ΔSLCOLL/GFP gene (upper right panel) were expressed in HEK293 cells stably expressing LARP6 and in control cells (lower left and lower right panels). Confocal images of GFP signal merged with DAPI staining are shown. D. 5’SLCOLL/GFP gene (upper panel) and ΔSLCOLL/GFP gene (lower panel) were expressed in human lung fibroblasts expressing only endogenous LARP6 and images were taken as in C.
From these experiments we concluded that LARP6 targets the synthesis of collagen reporter protein to discrete subcellular sites in 5’ stem-loop-dependent manner.
DISCUSSION
The 5’ stem-loop has emerged as the key cis-acting element regulating expression of type I collagen 13; 24; 38; 39, but the trans-acting factors have been unknown. In this manuscript we show that LARP6 is the protein which binds 5’ stem-loop of collagen α1(I) and α2(I) mRNAs. So far, LARP6 has been an uncharacterized RNA binding protein, belonging to the La-domain containing protein superfamily 40. The binding of LARP6 to the collagen 5’ stem-loop is strictly sequence specific, because changing of a single nucleotide completely abolishes the binding (Fig 1 and Fig 2). The binding affinity is in the nanomolar range and with similar affinity to collagen α1(I), α2(I) and α1(III) 5’ stem-loops (Fig 3). High affinity and specificity of binding indicate that the function of LARP6 is to regulate expression of type I (and possibly type III) collagen. LARP6 has a unique bipartite RNA binding domain, not found in the other members of La-superfamily (Fig 4). This domain recognizes the two regions of the 5’ stem-loop predicted to have single stranded conformation, while double stranded stems are dispensable for binding. However, the predicted single stranded regions may be folded into a complex tertiary structure.
Binding of LARP6 to collagen 5’ stem-loop is likely to occur in the nucleus, before the export of collagen mRNAs into the cytosol. LARP6 binding activity was detected in nuclear extracts (Fig 1) and the protein has a nuclear localization signal and accumulates in the nucleus (Fig 4). So, it is possible that LARP6 shuttles between the nucleus and the cytoplasm and participates in nuclear export of collagen mRNAs. However, we provide evidence that its cytoplasmic role is to regulate translation of collagen mRNAs. Excess of LARP6 can prevent formation of polysomes on collagen mRNAs, they co-fractionated with ribosomal subunits when LARP6 was overexpressed. The same result was obtained with the mutant of LARP6 lacking the C-terminus (Fig 6). Since both of these proteins bind 5’ stem-loop, it is possible that they can mask collagen mRNAs. This suggests that when collagen mRNAs emerge into the cytoplasm bound by LARP6 they may be inaccessible for translation until LARP6 dissociates. This repression is transient and we postulate that it is needed to prevent independent translation of collagen α1(I) and α2(I) mRNAs and random synthesis of collagen polypeptides. Assembly of the triple helix of type I collagen has a slow step of registration of α1(I) and α2(I) chains and a fast step of propagation of the triple helix 41; 42. The slow registration step is concentration dependent and a mechanism that can increase local concentration of the chains in vivo has been suggested 13; 43; 44. The coordination of synthesis of collagen α1(I) and α2(I) chains is also underscored by the fact that >99% of naturally synthesized type I collagen is a heterotrimer of two α1(I) chains and one α2(I) chain and not a homotrimer of α1(I) chains 45. Collagen α1(I) homotrimers readily form a triple helix in humans having a complete absence of α2(I) chain 46 and in knock-out mice in which the α2(I) gene had been inactivated 47. Therefore, α1(I) chains have the propensity for folding into a functional triple helix, but some coordination of translation of α1(I) mRNA and α2(I) mRNA must occur to assure almost 100% formation of the heterotrimer. Binding of LARP6 may serve this purpose. This can explain why collagen synthesis decreases when LARP6 is knocked down (Fig 7).
Using collagen/GFP reporter protein, we visualized the subcellular regions which may represent the sites of collagen synthesis or accumulation. In cells expressing LARP6 a focal or granular pattern of collagen/GFP localization was observed, but only when this reporter was encoded by the mRNA with the 5’ stemloop (Fig 8B). This is similar to the subcellular localization of endogenous collagen protein. Since COLL/GFP protein has a signal peptide, the granular pattern represents either regions on the ER membrane where the synthesis of the reporter protein takes place or the regions in the ER lumen where the protein accumulates after translocation. Since the formation of these granules was dependent on both, the presence of 5’stem-loop in the mRNA and LARP6 expression, and not on the protein sequence, we favor the hypothesis that these are the sites where translation of collagen mRNAs takes place. The LARP6-dependent aggregation of collagen/GFP protein synthesis is consistent with hypothesis that LARP6 prevents independent translation of collagen mRNAs and targets them to discrete regions on the ER membrane. This can increase the local concentration of chains for preferential folding of the heterotrimer. Inactivation of LARP6 resulted in a decrease of total collagen protein (Fig 7), suggesting that physiological levels of LARP6 are needed for efficient collagen synthesis. This implies that the level of LARP6 in the cell has to be tightly regulated; overexpression may overwhelm the mechanism needed to dissociate LARP6 from 5’ stem-loop. Since endogenous LARP6 is not found in polysomal fractions (Fig 6D), it must dissociate from the 5’ stem-loop once collagen mRNAs are committed for translation. Dissociation of LARP6 can be achieved either by posttranslational modifications, which would reduce its affinity for the 5’ stem-loop, or by the ubiquitin-dependent degradation. Treatment of cells with the proteosome inhibitors 48 increased the level of LARP6 protein (not shown), suggesting that it is regulated, at least in part, by proteosomal degradation. Our preliminary experiments also suggest that LARP6 is phosphorylated. Since bacterially expressed LARP6 has the ability to bind 5’ stem-loop (Fig 3D), phosphorylation is not absolutely required for binding, but may regulate the affinity.
The cloning and characterization of LARP6 as a collagen 5’ stem-loop RNA binding protein is a critical step towards understanding the synthesis of the most abundant protein in human body, type I collagen. The 5’ stem-loop is not absolutely required for collagen synthesis, because triple helical collagen is made, albeit at a reduced level, by the embryonic fibroblasts which have a mutated 5’ stem-loop in the endogenous α1(I) gene (in preparation). We propose that the 5’ stem-loop/LARP6 mechanism is activated when large amounts of type I collagen are produced, as in wound healing or in pathologic fibrosis 49; 50.
MATERIAL AND METHODS
Expression cloning of the 5’ stem-loop binding protein
Human fibroblast cDNA library cloned in the pSPORT6 plasmid was obtained from Invitrogen. The library was amplified in pools containing approximately 100 colonies and plasmid preparations were made from these pools. Each pool was transfected into HEK293 cells and cytosolic extracts were used in the gel mobility shift assay using 5’ stem-loop RNA as probe 13. The complexity of one positive pool was reduced by retransforming the plasmids from the pool and collecting 20 colonies into subpools. The subpools were screened as above until a single clone was isolated.
Construction of clones and adenoviruses
HA-tagged LARP6 was constructed by PCR amplification of the clone isolated from the library and cloning of the PCR product into EcoRV-XbaI sites of pCDNA3 vector (Stratagene). C-terminal deletion mutants were made by cutting with XcmI, and HindIII restriction sites and blunting, N-terminal deletions were made by removing KpnI-EcoRV or EcoRI-EcoRV fragments, while internal deletions were made with Bal31 nuclease. In frame deletions were confirmed by Western blot and sequencing. Collagen/GFP fusion protein was made by cloning human collagen cDNA 51 lacking 10 C-terminal amino acids in pCDNA3 vector (Stratagene). The GFP cassette was then cloned in frame at the C-terminal end. The 5’ stem-loop was deleted from the above clone by cutting with HindIII and XbaI, blunting and religating the plasmid.
Adenoviruses were constructed by recloning of LARP6 pCDNA3 constructs into the pAdCMVTRACK vector, followed by recombination with the pAdEasy vector, as described 52. Control adenoviruses expressed either a truncated version of lysyl oxidase (LOX) 53 or unrelated RNA binding protein, RBMS3 26. Adenoviruses were amplified in HEK293 cells and purified by a Virapure kit (Clontech). Expression of each construct was verified by Western blot.
SiRNA genes were made by cloning several small hairpin double-stranded (ds) oligonucleotides targeting human LARP6 into pSuper vector (Oligoengine). The siRNA genes were cotransfected with HA-tagged LARP6 into 293 cells and efficacy of LARP6 knock down was assessed by Western blot. One effective siRNA (D2) was found and the cassette from pSuper vector containing H1 promoter and D2 siRNA sequence was recloned into the pAdTRACK vector and adenovirus was constructed as above. Control adenovirus contained an siRNA gene with a scrambled sequence.
Cells and transfections
HEK293 cells and human lung fibroblasts immortalized by expression of telomerase reverse transcriptase 54 were grown under standard conditions. HEK293 cells were transfected with 1 µg of plasmid per 35 mm dish using 293TransIT reagent (Mirus). Stable cell lines expressing LARP6 were developed by transfecting the HEK293 cells as above and selecting with G418 for 3–4 weeks.
Transduction of lung fibroblasts with adenoviruses was done by adding adenoviruses at a multiplicity of infection (MOI) of 100. With this MOI, between 95–100% of the cells were transduced, as judged by expression of the viral marker, GFP. The cells were harvested for analysis 2–5 days after the viral delivery.
Proteosome inhibitor MG132 was used at concentration of 100 µM for 18h prior to analysis of collagen protein.
Purification of recombinant LARP6
The coding region of LARP6 cDNA was amplified by PCR and cloned into BamHI and XbaI of pGEX3Ti vector in frame with glutathione S-transferase. The protein was expressed in E. coli and purified on a glutathione Sepharose column, as described 55.
Cellular fractionation
Cytosolic extracts were prepared by hypotonic lysis of cells in 10 mM Tris-HCl pH 7.6, 5 mM MgCl2, 10 mM KCl, 0.4% NP-40 and after removal of nuclei by centrifugation, the supernatant was used as cytosolic extract. For nuclear extract the pelleted nuclei were washed several times in the above buffer and nuclear proteins were extracted by the method of Dignam 56. Protein concentration was measured by the method of Bradford using BSA as a standard 57.
Gel mobility shifts
Ds-oligonucleotides with the sequence of wt and various mutant 5’ stem-loops were cloned into SmaI site of pGEM3 vector (Promega). The RNA probes were prepared by in vitro transcription from these templates after linearization with BamHI 38. The sequence of the probes is shown in table 2.
40 µg of total protein from cytosolic or nuclear extracts were incubated with 20 fM of the probes in the presence of 5 mM MgCl2 and 10 µg of tRNA for 10 min on ice. The RNA protein complexes were resolved on a 6% native gel and visualized by autoradiography. For competition experiments the unlabeled RNA was transcribed from the 5’ stem-loop template cloned into the correct orientation (specific competitor) or cloned in an inverted orientation (nonspecific competitor). For supershift experiments 2 µL of LARP6 specific antibody or control antibody (anti-GFP) was added prior to addition of RNA probes.
For quantitative assessment of binding the amount of bound and unbound probe was measured by phosphoimaging. Scatchard plot was constructed as described (17).
UV croslinking
The RNA probe was mixed with cytosolic extracts as described for gel mobility shift. The samples were irradiated with UV light at 230 nm for 15 min, treated with 2 U of RNAse T1 for 10 min at room temperature and analyzed by SDS-PAGE and autoradiography.
RT-PCR
RT-PCR reactions were done with 100 ng of total RNA according to the published procedure 13; 38; 39; 58; 59; 60. The primers used are listed in table 1. Tth DNA polymerase and 3’gene specific primer were used for reverse transcription, while PCR amplification was done in the subsequent step by adding 5’ primer and alpha 32P-dATP. The radiolabeled PCR products were resolved on sequencing gels and visualized by autoradiography. Radiolabeling of PCR products allowed the use of a minimal number of cycles for detection of the products; for analysis of total RNA 16–18 cycles were used, while for analysis of polysomal fractions 25–30 cycles were used.
Table 1.
Primers used for RT-PCR and sequence of D2 siRNA. F, forward primer, R, reverse primer. The length of the PCR product is indicated in parenthesis.
| h-collagen α1(I) | (122 nt) | F: TGAGCCAGCAGATCGAGAAC R: TGATGGCATCCAGGTTGCAG |
| h-collagen α2(I) | (160 nt) | F: CAGCAGGAGGTTTCGGCTAA R: CAACAAAGTCCGCGTATCCA |
| h-collagen α1(III) | (120 nt) | F: ATCTTGGTCAGTCCTATGCGG R: GCAGTCTAATTCTTGATCGTCA |
| h-fibronectin | (220 nt) | F: ACCAACCTACGGATGACTCG R: GCTCATCATCTGGCCATTTT |
| h-LARP6 | (246 nt) | F: TTACACGGGACTGGAGAACC R: GTCCCAAAAAGCTTGAGCAG |
| h-GAPDH | (74 nt) | F: ACCGGTTCCAGTAGGTACTG R: CTCACCGTCACTACCGTACC |
| h-actin | (213 nt) | F: 5’-GTGCGTGACATTAAGGAGAAG R: 5’- GAAGGTAGTTTCGTGGATGCC |
| D2 siRNA: | 5’-UCCAACUCGTCCACGTCCU |
Fractionation of polysomes
Polysomes were fractionated from 3×107 cells on linear 15–45% sucrose gradients, as described 61. 0.5 ml fractions were collected and RNA was extracted with phenol/chlorophorm and precipitated with isopropanol. RNA samples were run on 1% agarose gel to estimate the distribution of ribosomal RNAs. Expression of collagen mRNAs and GAPDH mRNA was analyzed by RT-PCR. For protein analysis the proteins from sucrose fractions were precipitated with 0.05% deoxycholate and 6.5% trichloroacetic acid, washed with acetone and analyzed by western blot. When the cells were treated with puromycin, the treatment was for 1 h prior to isolation of polysomes.
Western blots
Western blots were done under reducing conditions, as described 13; 24; 39; 59; 62. Antibody against type I collagen was from Rockland and has been used before 13; 24; 39. Collagen α2(I) specific antibody (C19) was from Santa Cruz Biotechnology, anti-LARP6 antibody was from Abnova, anti-fibronectin antibody was from BD Transduction Laboratories, anti-tubulin antibody was from Zymed and anti-HA antibody was from Sigma.
To analyze collagen secretion into cellular medium, after expression of LARP6, its derivatives or siRNA, fresh serum free medium was added to the cells and incubation continued for 3 h. This allowed for accumulation of the secreted collagen in the fixed time and without contamination by collagen present in calf serum.
Immunostaining and confocal microscopy
Immunostaining was done by fixing the cells in 4% paraformaldehyde and, after blocking with 5% goat serum for 1h, anti-LARP6 antibody (Abnova) or anti-collagen antibody (Rockland) together with anti-calnexin antibody (Cell Signaling) were added at 1:10 dilution and incubated overnight at 4°C. After washing, Alexa-fluor or Cy2 labeled secondary antibody was added at 1:50 dilution for 1 h. For control cells the primary antibody was omitted. Immunostained cells mounted in Vectashield containing DAPI stain (Vector Laboratories) and the images were taken with Leica TCS SP2 AOBS laser confocal microscope under 110-fold magnification. Images through one of the focal planes are shown.
For detection of collagen/GFP fusion proteins, the cells were fixed in paraformaldehyde, mounted in Vectshield and images taken as above.
Acknowledgments
This work was supported by Scleroderma Research Foundation grant and NIH grant 5R01DK059466-08 to B.S and American Heart Association grant to L.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kivirikko KI. Collagen biosynthesis: a mini-review cluster. Matrix Biol. 1998;16:355–356. doi: 10.1016/s0945-053x(98)90008-7. [DOI] [PubMed] [Google Scholar]
- 2.Canty EG, Kadler KE. Collagen fibril biosynthesis in tendon: a review and recent insights. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:979–985. doi: 10.1016/s1095-6433(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 3.Bitterman PB, Henke CA. Fibroproliferative disorders. Chest. 1991;99:81S–84S. doi: 10.1378/chest.99.3_supplement.81s. [DOI] [PubMed] [Google Scholar]
- 4.Tajima S, Takehana M, Azuma N. Production of overmodified type I procollagen in a case of osteogenesis imperfecta. J Dermatol. 1994;21:219–222. doi: 10.1111/j.1346-8138.1994.tb01726.x. [DOI] [PubMed] [Google Scholar]
- 5.Lamande SR, Bateman JF. Procollagen folding and assembly: the role of endoplasmic reticulum enzymes and molecular chaperones. Semin Cell Dev Biol. 1999;10:455–464. doi: 10.1006/scdb.1999.0317. [DOI] [PubMed] [Google Scholar]
- 6.Focht RJ, Adams SL. Tissue specificity of type I collagen gene expression is determined at both transcriptional and post-transcriptional levels. Mol Cell Biol. 1984;4:1843–1852. doi: 10.1128/mcb.4.9.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eckes B, Mauch C, Huppe G, Krieg T. Differential regulation of transcription and transcript stability of pro-alpha 1(I) collagen and fibronectin in activated fibroblasts derived from patients with systemic scleroderma. Biochem J. 1996;315:549–554. doi: 10.1042/bj3150549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckes B, Mauch C, Huppe G, Krieg T. Downregulation of collagen synthesis in fibroblasts within three- dimensional collagen lattices involves transcriptional and posttranscriptional mechanisms. FEBS Lett. 1993;318:129–133. doi: 10.1016/0014-5793(93)80006-g. [DOI] [PubMed] [Google Scholar]
- 9.Mauch C, Hatamochi A, Scharffetter K, Krieg T. Regulation of collagen synthesis in fibroblasts within a three- dimensional collagen gel. Exp Cell Res. 1988;178:493–503. doi: 10.1016/0014-4827(88)90417-x. [DOI] [PubMed] [Google Scholar]
- 10.Mauch C, Kozlowska E, Eckes B, Krieg T. Altered regulation of collagen metabolism in scleroderma fibroblasts grown within three-dimensional collagen gels. Exp Dermatol. 1992;1:185–190. doi: 10.1111/j.1600-0625.1992.tb00187.x. [DOI] [PubMed] [Google Scholar]
- 11.Penttinen RP, Kobayashi S, Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988;85:1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maatta A, Ekholm E, Penttinen RP. Effect of the 3'-untranslated region on the expression levels and mRNA stability of alpha 1(I) collagen gene. Biochim Biophys Acta. 1995;1260:294–300. doi: 10.1016/0167-4781(94)00207-j. [DOI] [PubMed] [Google Scholar]
- 13.Stefanovic B, Lindquist J, Brenner DA. The 5' stem-loop regulates expression of collagen alpha1(I) mRNA in mouse fibroblasts cultured in a three-dimensional matrix. Nucleic Acids Res. 2000;28:641–647. doi: 10.1093/nar/28.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato M, Shegogue D, Hatamochi A, Yamazaki S, Trojanowska M. Lysophosphatidic acid inhibits TGF-beta-mediated stimulation of type I collagen mRNA stability via an ERK-dependent pathway in dermal fibroblasts. Matrix Biol. 2004;23:353–361. doi: 10.1016/j.matbio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Rishikof DC, Kuang PP, Poliks C, Goldstein RH. Regulation of type I collagen mRNA in lung fibroblasts by cystine availability. Biochem J. 1998;331(Pt 2):417–422. doi: 10.1042/bj3310417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanovic B, Hellerbrand C, Holcik M, Briendl M, Aliebhaber S, Brenner DA. Posttranscriptional regulation of collagen alpha1(I) mRNA in hepatic stellate cells. Mol Cell Biol. 1997;17:5201–5209. doi: 10.1128/mcb.17.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindquist JN, Kauschke SG, Stefanovic B, Burchardt ER, Brenner DA. Characterization of the interaction between alphaCP(2) and the 3'-untranslated region of collagen alpha1(I) mRNA. Nucleic Acids Res. 2000;28:4306–4316. doi: 10.1093/nar/28.21.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Kiledjian M, Weiss IM, Liebhaber SA. Detection and characterization of a 3' untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol Cell Biol. 1995;15:1769–1777. doi: 10.1128/mcb.15.3.1769. [published erratum appears in Mol Cell Biol 1995 Apr;15(4):2331] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holcik M, Liebhaber SA. Four highly stable eukaryotic mRNAs assemble 3' untranslated region RNA- protein complexes sharing cis and trans components. Proc Natl Acad Sci U S A. 1997;94:2410–2414. doi: 10.1073/pnas.94.6.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada Y, Mudryj M, de Crombrugghe B. A uniquely conserved regulatory signal is found around the translation initiation site in three different collagen genes. J Biol Chem. 1983;258:14914–14918. [PubMed] [Google Scholar]
- 21.Su MW, Suzuki HR, Bieker JJ, Solursh M, Ramirez F. Expression of two nonallelic type II procollagen genes during Xenopus laevis embryogenesis is characterized by stage-specific production of alternatively spliced transcripts. J Cell Biol. 1991;115:565–575. doi: 10.1083/jcb.115.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987;196:947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- 23.Kozak M. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene. 2005;361:13–37. doi: 10.1016/j.gene.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Stefanovic B, Brenner DA. 5' stem-loop of collagen alpha 1(I) mRNA inhibits translation in vitro but is required for triple helical collagen synthesis in vivo. J Biol Chem. 2003;278:927–933. doi: 10.1074/jbc.M209175200. [DOI] [PubMed] [Google Scholar]
- 25.Valavanis C, Wang Z, Sun D, Vaine M, Schwartz LM. Acheron, a novel member of the Lupus Antigen family, is induced during the programmed cell death of skeletal muscles in the moth Manduca sexta. Gene. 2007;393:101–109. doi: 10.1016/j.gene.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fritz D, Stefanovic B. RNA-binding protein RBMS3 is expressed in activated hepatic stellate cells and liver fibrosis and increases expression of transcription factor Prx1. J Mol Biol. 2007;371:585–595. doi: 10.1016/j.jmb.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsamba PS, Park S, Laird-Offringa IA. Kinetic studies of RNA-protein interactions using surface plasmon resonance. Methods. 2002;26:95–104. doi: 10.1016/S1046-2023(02)00012-9. [DOI] [PubMed] [Google Scholar]
- 28.Metzinger L, Hallier M, Felden B. The highest affinity binding site of small protein B on transfer messenger RNA is outside the tRNA domain. Rna. 2008 doi: 10.1261/rna.1185808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meisterernst M, Gander I, Rogge L, Winnacker EL. A quantitative analysis of nuclear factor I/DNA interactions. Nucleic Acids Res. 1988;16:4419–4435. doi: 10.1093/nar/16.10.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohman M, Kallman AM, Bass BL. In vitro analysis of the binding of ADAR2 to the pre-mRNA encoding the GluR-B R/G site. Rna. 2000;6:687–697. doi: 10.1017/s1355838200000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R, Valcarcel J. Building specificity with nonspecific RNA-binding proteins. Nat Struct Mol Biol. 2005;12:645–653. doi: 10.1038/nsmb961. [DOI] [PubMed] [Google Scholar]
- 33.Markert A, Grimm M, Martinez J, Wiesner J, Meyerhans A, Meyuhas O, Sickmann A, Fischer U. The La-related protein LARP7 is a component of the 7SK ribonucleoprotein and affects transcription of cellular and viral polymerase II genes. EMBO Rep. 2008;9:569–575. doi: 10.1038/embor.2008.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolin SL, Cedervall T. The La protein. Annu Rev Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 35.Nagai K, Oubridge C, Ito N, Avis J, Evans P. The RNP domain: a sequence-specific RNA-binding domain involved in processing and transport of RNA. Trends Biochem Sci. 1995;20:235–240. doi: 10.1016/s0968-0004(00)89024-6. [DOI] [PubMed] [Google Scholar]
- 36.McKean PG, Vaughan S, Gull K. The extended tubulin superfamily. J Cell Sci. 2001;114:2723–2733. doi: 10.1242/jcs.114.15.2723. [DOI] [PubMed] [Google Scholar]
- 37.Laurent AM, Madjar JJ, Greco A. Translational control of viral and host protein synthesis during the course of herpes simplex virus type 1 infection: evidence that initiation of translation is the limiting step. J Gen Virol. 1998;79(Pt 11):2765–2775. doi: 10.1099/0022-1317-79-11-2765. [DOI] [PubMed] [Google Scholar]
- 38.Stefanovic B, Hellerbrand C, Brenner DA. Regulatory role of the conserved stem-loop structure at the 5' end of collagen alpha1(I) mRNA. Mol Cell Biol. 1999;19:4334–4342. doi: 10.1128/mcb.19.6.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stefanovic B, Schnabl B, Brenner DA. Inhibition of collagen alpha 1(I) expression by the 5' stem-loop as a molecular decoy. J Biol Chem. 2002;277:18229–18237. doi: 10.1074/jbc.M108065200. [DOI] [PubMed] [Google Scholar]
- 40.Maraia RJ, Bayfield MA. The La protein-RNA complex surfaces. Mol Cell. 2006;21:149–152. doi: 10.1016/j.molcel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Bachmann A, Kiefhaber T, Boudko S, Engel J, Bachinger HP. Collagen triple-helix formation in all-trans chains proceeds by a nucleation/growth mechanism with a purely entropic barrier. Proc Natl Acad Sci U S A. 2005;102:13897–13902. doi: 10.1073/pnas.0505141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boudko S, Frank S, Kammerer RA, Stetefeld J, Schulthess T, Landwehr R, Lustig A, Bachinger HP, Engel J. Nucleation and propagation of the collagen triple helix in single-chain and trimerized peptides: transition from third to first order kinetics. J Mol Biol. 2002;317:459–470. doi: 10.1006/jmbi.2002.5439. [DOI] [PubMed] [Google Scholar]
- 43.Beck K, Boswell BA, Ridgway CC, Bachinger HP. Triple helix formation of procollagen type I can occur at the rough endoplasmic reticulum membrane. J Biol Chem. 1996;271:21566–21573. doi: 10.1074/jbc.271.35.21566. [DOI] [PubMed] [Google Scholar]
- 44.Gura T, Hu G, Veis A. Posttranscriptional aspects of the biosynthesis of type 1 collagen pro- alpha chains: the effects of posttranslational modifications on synthesis pauses during elongation of the pro alpha 1 (I) chain. J Cell Biochem. 1996;61:194–215. doi: 10.1002/(sici)1097-4644(19960501)61:2<194::aid-jcb4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 45.Uitto J. Collagen polymorphism: isolation and partial characterization of alpha 1(I)-trimer molecules in normal human skin. Arch Biochem Biophys. 1979;192:371–379. doi: 10.1016/0003-9861(79)90105-x. [DOI] [PubMed] [Google Scholar]
- 46.Malfait F, Symoens S, Coucke P, Nunes L, De Almeida S, De Paepe A. Total absence of the alpha2(I) chain of collagen type I causes a rare form of Ehlers-Danlos syndrome with hypermobility and propensity to cardiac valvular problems. J Med Genet. 2006;43:e36. doi: 10.1136/jmg.2005.038224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sims TJ, Miles CA, Bailey AJ, Camacho NP. Properties of collagen in OIM mouse tissues. Connect Tissue Res. 2003;44(Suppl 1):202–205. [PubMed] [Google Scholar]
- 48.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leask A, Denton CP, Abraham DJ. Insights into the molecular mechanism of chronic fibrosis: the role of connective tissue growth factor in scleroderma. J Invest Dermatol. 2004;122:1–6. doi: 10.1046/j.0022-202X.2003.22133.x. [DOI] [PubMed] [Google Scholar]
- 50.Abraham DJ, Eckes B, Rajkumar V, Krieg T. New developments in fibroblast and myofibroblast biology: implications for fibrosis and scleroderma. Curr Rheumatol Rep. 2007;9:136–143. doi: 10.1007/s11926-007-0008-z. [DOI] [PubMed] [Google Scholar]
- 51.Geddis AE, Prockop DJ. Expression of human COL1A1 gene in stably transfected HT1080 cells: the production of a thermostable homotrimer of type I collagen in a recombinant system. Matrix. 1993;13:399–405. doi: 10.1016/s0934-8832(11)80045-4. [DOI] [PubMed] [Google Scholar]
- 52.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 54.Yamada NA, Castro A, Farber RA. Variation in the extent of microsatellite instability in human cell lines with defects in different mismatch repair genes. Mutagenesis. 2003;18:277–282. doi: 10.1093/mutage/18.3.277. [DOI] [PubMed] [Google Scholar]
- 55.Hengen PN. Methods and reagents. Purification of GST fusion proteins. Trends Biochem Sci. 1996;21:400–401. [PubMed] [Google Scholar]
- 56.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 58.Stefanovic B, Hellerbrand C, Brenner DA. Post-transcriptional regulation of collagen alpha 1(I) mRNA in hepatic stellate cells. Nucleic Acids Symp Ser. 1995:212–214. [PubMed] [Google Scholar]
- 59.Stefanovic B, Stefanovic L, Schnabl B, Bataller R, Brenner DA. TRAM2 protein interacts with endoplasmic reticulum Ca2+ pump Serca2b and is necessary for collagen type I synthesis. Mol Cell Biol. 2004;24:1758–1768. doi: 10.1128/MCB.24.4.1758-1768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefanovic L, Stefanovic B. Mechanism of Direct Hepatotoxic Effect of KC Chemokine: Sequential Activation of Gene Expression and Progression from Inflammation to Necrosis. J. Interf. Cytokine Res. 2006;26:760–770. doi: 10.1089/jir.2006.26.760. [DOI] [PubMed] [Google Scholar]
- 61.Chen CY, Xu N, Shyu AB. mRNA decay mediated by two distinct AU-rich elements from c-fos and granulocyte-macrophage colony-stimulating factor transcripts: different deadenylation kinetics and uncoupling from translation. Mol Cell Biol. 1995;15:5777–5788. doi: 10.1128/mcb.15.10.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stefanovic L, Stephens CE, Boykin D, Stefanovic B. Inhibitory effect of dicationic diphenylfurans on production of type I collagen by human fibroblasts and activated hepatic stellate cells. Life Sci. 2005;76:2011–2026. doi: 10.1016/j.lfs.2004.09.043. [DOI] [PubMed] [Google Scholar]