Abstract
Ecto-5′-nucleotidase (NT5E, CD73) is a membrane-anchored protein that hydrolyzes extracellular adenosine 5′-monophosphate (AMP) to adenosine in diverse tissues but has not been directly studied in nociceptive neurons. We found that NT5E was located on peptidergic and nonpeptidergic nociceptive neurons in dorsal root ganglia (DRG) and on axon terminals in lamina II (the substantia gelatinosa) of spinal cord. NT5E was also located on epidermal keratinocytes, cells of the dermis, and on nociceptive axon terminals in the epidermis. Following nerve injury, NT5E protein and AMP histochemical staining were coordinately reduced in lamina II. In addition, AMP hydrolytic activity was reduced in DRG neurons and spinal cord of Nt5e−/− mice. The antinociceptive effects of AMP, when combined with the adenosine kinase inhibitor 5-iodotubericidin, were reduced by ∼50% in Nt5e−/− mice and were eliminated in Adenosine A1 receptor (A1R, Adora1) knock-out mice. Additionally, Nt5e−/− mice displayed enhanced sensitivity in the tail immersion assay, in the complete Freund's adjuvant model of inflammatory pain and in the spared nerve injury model of neuropathic pain. Collectively, our data indicate that the ectonucleotidase NT5E regulates nociception by hydrolyzing AMP to adenosine in nociceptive circuits and represents a new molecular target for the treatment of chronic pain. Moreover, our data suggest NT5E is well localized to regulate nucleotide signaling between skin cells and sensory axons.
Introduction
Nucleotides play fundamental roles in pain mechanisms (Tsuda et al., 2005; Burnstock, 2007; Sawynok, 2007; Dussor et al., 2009). Nucleotides are released extracellularly by sensory neurons, skin keratinocytes, and other cell types. Nucleotides then activate purinergic receptors and cause pain, in part, by exciting and sensitizing nociceptive (pain-sensing) neurons in DRG. The excitatory effects of nucleotides can be terminated by membrane-bound and secreted ectonucleotidases (Zimmermann, 2006). Ectonucleotidases dephosphorylate extracellular ATP, ADP, and AMP to adenosine. Adenosine has well studied inhibitory and antinociceptive effects that are dependent on A1R activation (Sawynok, 2007). Ectonucleotidases thus have the capacity to convert pronociceptive nucleotides into antinociceptive adenosine.
For >40 years, it was known that DRG neurons and their axon terminals in dorsal spinal cord contained one or more ectonucleotidases that could generate adenosine by hydrolyzing the 5′-phosphate from extracellular AMP (Scott, 1967; Suran, 1974; Nagy and Daddona, 1985). Initially, this ectonucleotidase activity was visualized using enzyme histochemistry—a technique that entails incubating tissue sections with AMP and detecting a lead phosphate precipitate. Subsequently, the existence of ectonucleotidases in nociceptive neurons was supported by electrophysiological and pharmacological studies. Salter and Henry (1985) found that iontophoretic application of AMP inhibited noxious heat-evoked activity in most dorsal horn neurons and this inhibition was dependent on adenosine receptor activation. Likewise, Li and Perl (1995) found that extracellularly applied AMP evoked an adenosine receptor-dependent inhibitory current in lamina II neurons. Together, these data suggest the inhibitory effects of AMP might be due to extracellular hydrolysis to adenosine. In support of this, Patterson et al. (2001) used a push–pull microprobe to sample extracellular adenosine concentrations in rat dorsal spinal cord while delivering AMP. They found that AMP was hydrolyzed to adenosine and that this hydrolysis was partially blocked by αβ-methylene-ADP, a relatively selective inhibitor of an enzyme called ecto-5′-nucleotidase (NT5E, also know as CD73). This study provided indirect pharmacological evidence that NT5E might contribute to AMP hydrolysis in the spinal cord. Last, Patterson et al. (2001) found that spinally administered AMP reversed capsaicin-mediated allodynia (an antinociceptive effect) that was dependent on A1R activation.
Recently, we found that prostatic acid phosphatase (PAP, also known as ACPP or fluoride-resistant acid phosphatase) was expressed in nociceptive neurons and hydrolyzed AMP to adenosine (Zylka et al., 2008; Sowa et al., 2009). Interestingly, this AMP hydrolytic activity was reduced but not eliminated in tissues from Pap−/− mice, suggesting additional AMP ectonucleotidases were present in nociceptive neurons. Here, using rigorous histochemical, genetic, and behavioral experiments we found that NT5E also contributes to AMP hydrolysis in nociceptive neurons.
Materials and Methods
Animals.
All procedures and behavioral experiments involving vertebrate animals were approved by the Institutional Animal Care and Use Committee at the University of North Carolina at Chapel Hill. C57BL/6 mice were purchased from Jackson Laboratories. Nt5e−/− (Thompson et al., 2004), A1R−/− (Johansson et al., 2001; Hua et al., 2007), and MrgprdΔEGFPf (Zylka et al., 2005) mice were backcrossed to C57BL/6 mice (Jackson) for 14, 12, and 10 generations, respectively.
Tissue preparation.
Adult male mice, 6–12 weeks of age, were killed by decapitation. Lumbar spinal cord, DRG (L3–L5), and hindpaw skin were dissected and immersion fixed (8 h, 4 h, and 3 h respectively) in 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4. Tissues were cryoprotected in 30% sucrose, 0.1 m phosphate buffer, pH 7.3 at 4°C for at least 24 h, and frozen in OCT. DRG and skin were sectioned on a cryostat at 20 μm and collected on Superfrost Plus slides. Slides were stored at −20°C until use. Free-floating spinal cord sections were sectioned at 30 μm and immediately stained.
DRG neurons were cultured following a previously published procedure (Campagnola et al., 2008). Briefly, DRG from all rostral-caudal levels were collected and pooled from adult male mice and dissociated using collagenase (1 mg/ml; Worthington, CLS1) and dispase (5 mg/ml; Invitrogen, 17105-041) in Hanks balanced salt solution (HBSS). Neurons were cultured on poly-d-lysine and laminin-coated glass coverslips in DH10 media (1:1 Hams F12/DMEM, 10% fetal bovine serum, 1% penicillin/streptomycin) with 25 ng/ml glial cell line-derived neurotrophic factor (GDNF; Millipore Bioscience Research Reagents, GF030) at 37°C. GDNF was added to support the survival of small diameter nonpeptidergic DRG neurons (Molliver et al., 1997). Enzyme histochemistry was performed 72 h after plating.
Histology.
Enzyme histochemistry was performed using 3 or 6 mm AMP as substrate and Tris-maleate buffer at pH 5.6 or 7.0, in the absence of detergent (Zylka et al., 2008). Immunofluorescence was performed as previously described (Zylka et al., 2008). Mrgprd-expressing neurons and axons were visualized by staining tissues from MrgprdΔEGFPf knock-in mice with antibodies to GFP (Zylka et al., 2005). Antibodies used included anti-NT5E (AF4488, R&D Systems; 1:50 for DRG and skin and 1:100 for spinal cord), rabbit anti-CGRP (T-4032; Peninsula; 1:750 for DRG and spinal cord; 1:500 for skin), mouse anti-NeuN (MAB377, Millipore Bioscience Research Reagents; 1:250), rabbit anti-P2X3 (RA10109, Neuromics; 1:750), rabbit anti-TRPV1 (RA14113, Neuromics; 1:750), chicken anti-GFP (GFP-1020, Aves Labs; 1:600 for DRG and 1:750 for spinal cord and skin), rabbit anti-NF200 (AB1982, Millipore Bioscience Research Reagents; 1:500), and rabbit anti-PGP 9.5 (Ultraclone, 1:500). PAP was detected using chicken anti-mouse PAP (Taylor-Blake and Zylka, 2010; 1:4000) with amplification as described previously (Zylka et al., 2008).
Secondary antibodies and reagents included donkey antibodies coupled to Alexa fluorophores (Invitrogen; 1:200), rat anti-mouse IgG1-FITC (Zymed/Invitrogen; 1:25), donkey anti-chicken IgY-Biotin (Jackson; 1:200), donkey anti-chicken IgY-FITC (Jackson; 1:200), and isolectin B4 (IB4)-Alexa 568 (Invitrogen; 1:100). DRAQ5 (Axxora; 1:10,000) was added to the secondary antibody mixtures when staining skin. Images were obtained using a Zeiss LSM 510 confocal microscope and collected as maximal projections.
Intrathecal injections.
Adenosine 5′-monophosphate (AMP, 80 mm stock, Fluka, 01930) and 5-iodotubericidin (25 mm stock, Biomol, EI-293) were dissolved in 0.9% saline and DMSO, respectively, and were then diluted in 0.9% saline before use. All drugs were intrathecally injected (5 μl) into unanesthetized mice using the direct lumbar puncture method (Fairbanks, 2003).
Behavior.
Male mice, 2–4 months old, were acclimated to the testing room, equipment, and experimenter for 1–3 d before behavioral testing. To further reduce variability, mice were almost exclusively tested when in the resting or light sleep behavioral state (Callahan et al., 2008). The experimenter was blind to genotype during behavioral testing. Noxious thermal sensitivity was measured by heating one hindpaw with a Plantar Test apparatus (IITC) following the Hargreaves method (Hargreaves et al., 1988). The radiant heat source intensity was calibrated so that a paw-withdrawal reflex was evoked in ∼10 s, on average, in wild-type C57BL/6 mice. Cutoff time was 20 s. One measurement was taken from each paw per day to determine paw withdrawal latency, with the exception of the AMP ± ITU experiments, where measurements were made at the indicated time points after injection. In the tail-immersion assay, mice were gently restrained in a towel and the distal two-thirds of the tail was immersed in 46.5 or 49°C water. Latency to flick or withdrawal the tail was measured once per mouse. For the hot plate test, mice were placed on a metal surface heated at 52°C and latency to lick a hindpaw or jump was measured. Mechanical sensitivity was measured using semiflexible tips attached to an electronic von Frey apparatus (IITC) as described previously (Cunha et al., 2004; Inoue et al., 2004). The force values obtained with this apparatus are higher than the force values obtained using calibrated von Frey filaments (Inoue et al., 2004). Three measurements were taken from each paw (separated at 10 min intervals) then averaged to determine paw withdrawal threshold in grams. To sensitize mice [complete Freund's adjuvant (CFA) inflammatory pain model], 20 μl of complete Freund's adjuvant (from MP Biomedicals) was injected into one hindpaw, centrally beneath glabrous skin, with a 30G needle. The spared nerve injury (SNI) model of neuropathic pain was performed as described previously (Shields et al., 2003).
Results
NT5E is found on membranes of nociceptive neurons and axon terminals in lamina II of dorsal spinal cord
NT5E is a glycophosphatidylinositol-anchored membrane protein that generates adenosine by dephosphorylating AMP extracellularly (Zimmermann, 1992). While NT5E regulates diverse physiological processes that are modulated by adenosine (Colgan et al., 2006), NT5E has never been directly studied in nociceptive neurons. To determine whether NT5E was expressed in nociceptive neurons, we performed double-label immunofluorescence with a commercially available anti-NT5E antibody and antibodies to various sensory neuron markers. We found that NT5E was primarily expressed in small- to medium-diameter DRG neurons, with particular enrichment on the plasma membrane and intracellular membranes (Fig. 1). High levels of NT5E were also found on the epineurium that surrounds dorsal root ganglia and peripheral nerves (data not shown). Nearly all (95.5 ± 1.1%) NT5E+ neurons expressed PAP (Fig. 1A–C; overlap quantified in Table 1), highlighting coincident expression of two molecularly distinct AMP ectonucleotidases. In addition, NT5E was found on almost all nonpeptidergic nociceptive neurons, as evidenced by extensive cellular overlap with the nonpeptidergic markers IB4, Mrgprd, and the nucleotide-gated ion channel P2X3 (Fig. 1D–L, Table 1). NT5E was also expressed in 38.0 ± 2.4% of all peptidergic calcitonin-gene related peptide+ (CGRP+) neurons and 18.9 ± 2.4% of all TRPV1+ neurons (Fig. 1M–R, Table 1). TRPV1 is an ion channel that can be activated by capsaicin or noxious heat (Caterina et al., 1997). Few (4.5 ± 0.9%) NT5E+ neurons expressed Neurofilament-200 (NF200), a marker of myelinated neurons (Fig. 1S–U, Table 1).
Figure 1.

NT5E is found on most nonpeptidergic and some peptidergic nociceptive neurons. A–U, Mouse L3–L5 DRG neurons were stained for sensory neuron markers (green) and with antibodies against NT5E (red). Arrowheads mark examples of double-labeled cells. Images were acquired by confocal microscopy. Scale bar: (in U) A–U, 50 μm.
Table 1.
Quantitative analysis of NT5E and sensory neuron marker colocalization within wild-type mouse L3–L5 DRG neurons
| Marker | Percentage of NT5E+ neurons expressing indicated marker | Percentage of marker+ neurons expressing NT5E |
|---|---|---|
| PAP | 95.5 ± 1.1 | 82.7 ± 1.5 |
| IB4 | 83.6 ± 2.6 | 87.4 ± 2.5 |
| Mrgprd | 74.9 ± 1.4 | 98.5 ± 0.3 |
| P2X3 | 89.3 ± 1.0 | 82.3 ± 1.9 |
| CGRP | 44.6 ± 3.6 | 38.0 ± 2.4 |
| TRPV1 | 18.9 ± 2.4 | 26.1 ± 3.0 |
| NF200 | 4.5 ± 0.9 | 4.4 ± 0.9 |
| NeuN | 100 | 40.2 ± 1.3 |
Ganglia from three adult mice and n > 900 cells were counted per marker combination. Data are means ± SEM.
In the spinal cord, NT5E+ axons terminated in lamina II (Fig. 2). Staining overlapped extensively with PAP, IB4, and Mrgprd (Fig. 2A–I) and partially overlapped with CGRP (Fig. 2J–L). Together, these studies suggest that NT5E is well localized to hydrolyze extracellular AMP in a subset of peptidergic and the majority of all nonpeptidergic nociceptive circuits.
Figure 2.

NT5E is colocalized with peptidergic and nonpeptidergic markers on axon terminals in dorsal spinal cord. Lumbar spinal cord sections were stained for selected axonal markers (A, D, G, J; green) and NT5E (B, E, H, K; red). C, F, I, L, Merged images. IB4, MrgprdΔEGFPf and PAP mark nonpeptidergic endings in lamina II. CGRP marks peptidergic endings in laminas I, II, and V. Images were acquired by confocal microscopy. Scale bar: (in L) A–L, 100 μm.
NT5E is found on free nerve endings in the epidermis and in cells of the skin
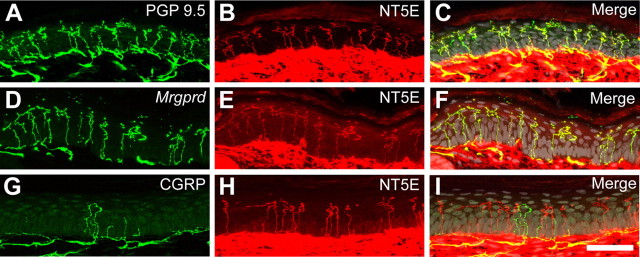
Many peptidergic (CGRP+) and nonpeptidergic (IB4+/Mrgprd+/P2X3+) nociceptive neurons terminate as free nerve endings in the epidermis, ideally positioning these neurons to sense noxious thermal and mechanical stimuli near the surface of the skin (Bennett et al., 1996; Bradbury et al., 1998; Wang et al., 1998; Lawson et al., 2002; Zylka et al., 2005; Cavanaugh et al., 2009; Rau et al., 2009; Taylor et al., 2009). Moreover, there is growing evidence that cells of the skin communicate with sensory neurons by releasing nucleotides extracellularly (Lumpkin and Caterina, 2007; Dussor et al., 2009). Since NT5E was coexpressed in nociceptive neurons that are known to project to skin, we next used immunostaining to determine whether NT5E was found in nerve endings or skin cells in glabrous and hairy skin. NT5E was extensively colocalized with PGP 9.5, a pan-neuronal marker of peripheral nerve fibers (Rice et al., 1997), and with MrgprdΔEGFPf, a genetically encoded axonal tracer that marks nonpeptidergic endings (Fig. 3A–F; supplemental Figs. S1, S2, available at www.jneurosci.org as supplemental material) (Zylka et al., 2005). Although NT5E and CGRP were colocalized to some extent in DRG neurons, we rarely found NT5E colocalized with CGRP in epidermal free nerve endings (Fig. 3G–I). Instead, NT5E+/CGRP+ fibers were found around and coursing along blood vessels in the deeper layers of skin (data not shown), suggesting NT5E+/CGRP+ neurons target other end organs. In addition, high levels of NT5E were found on the plasma membrane of cells throughout the dermis, with dense cellular labeling in the superficial dermis (Fig. 3; supplemental Figs. S1, S2, available at www.jneurosci.org as supplemental material). Low levels of NT5E were present on keratinocytes throughout the epidermis, with staining most prominent on keratinocytes in the stratum basalis (supplemental Fig. S1B,C, available at www.jneurosci.org as supplemental material). NT5E was not uniformly distributed in the epidermis but instead was patchy, with strongest staining on keratinocytes of weight-bearing volar foot pads (data not shown). NT5E expression is upregulated under hypoxic conditions (Synnestvedt et al., 2002; Ledoux et al., 2003), so this patchy distribution could reflect different metabolic demands in weight-bearing and non-weight-bearing regions of skin. Last, NT5E was also found at high levels on cells that make up the hair follicles, cells in the sweat gland, cells lining the sweat duct, and the epineurium of peripheral nerves that course through the skin (supplemental Fig. S2, available at www.jneurosci.org as supplemental material; data not shown).
Figure 3.
NT5E is primarily found in nonpeptidergic nerve terminals in the epidermis. A–I, Confocal images of mouse glabrous skin immunostained for NT5E (red) and the indicated markers (green). C, F, I, Nuclei were pseudocolored gray to highlight stratification of the epidermis. Scale bar: (in I) A–I, 50 μm.
For all experiments described above, NT5E antibody specificity was confirmed by the absence of staining when primary antibody was excluded and by the absence of staining in tissue sections (DRG, spinal cord, skin) from Nt5e−/− mice (supplemental Fig. S3, available at www.jneurosci.org as supplemental material).
NT5E protein and AMP hydrolytic activity are reduced in lamina II of spinal cord following nerve injury
Nerve injury can cause neuropathic pain and can dramatically alter protein levels in nociceptive neurons and spinal axon terminals (Campbell and Meyer, 2006). Indeed, expression and activity of PAP was reduced in DRG and spinal axon terminals following nerve injury (Colmant, 1959; Csillik and Knyihar-Csillik, 1986; Costigan et al., 2002; Davis-Taber, 2006). To determine whether NT5E protein and activity levels were similarly reduced following nerve injury, we unilaterally injured the peroneal and sural branches of the sciatic nerve, using the SNI model of neuropathic pain (Shields et al., 2003), then stained serial adjacent lumbar spinal cord sections using AMP histochemistry and immunofluorescence. Following nerve injury, AMP hydrolytic activity was reduced and NT5E immunoreactivity was absent in the regions of lamina II that received inputs from the transected peroneal and sural nerves (Fig. 4A,B) (Shields et al., 2003). IB4 binding was also abolished in these regions (Fig. 4C,D), consistent with previous studies (Casals-Díaz et al., 2009). No changes were evident on the contralateral, uninjured side (Fig. 4).
Figure 4.

NT5E protein and AMP hydrolytic activity are reduced in axon terminals following peripheral nerve injury. A–D, Sections of lumbar spinal cord from a mouse killed 14 d after ligation and transection of the sural and common peroneal branches of the sciatic nerve (the SNI model). A, Stained using AMP histochemistry (3 mm AMP; buffer pH was 7.0). B–D, An adjacent section labeled for the indicated markers. Arrowheads delimit the region of dorsal spinal cord where staining was reduced ipsilateral (ipsi) to the injured nerves. Contralateral (contra), uninjured side. Similar results were seen in n = 2 mice. Scale bar: (in D) A–D, 200 μm.
NT5E hydrolyzes AMP in nociceptive circuits
Next, to directly test whether NT5E hydrolyzes AMP in nociceptive circuits, we stained DRG and spinal cord sections from wild-type (WT) and Nt5e−/− mice using AMP histochemistry. Nt5e−/− mice lack NT5E catalytic activity but otherwise appear normal and produce average sized litters (Thompson et al., 2004). In WT DRG, the membrane covering the DRG (epineurium), small- to medium-diameter neurons and their axons were intensely stained at pH 7.0 while large-diameter neurons were weakly stained (Fig. 5A). Strikingly, staining of small- to medium-diameter DRG neurons and axons was reduced in Nt5e−/− mice while staining of large-diameter neurons was not altered (Fig. 5B). These reductions in staining were not due to developmental loss of small- to medium-diameter DRG neurons, as wild-type and Nt5e−/− mice had equivalent percentages of P2X3+ neurons and CGRP+ neurons relative to all NeuN+ neurons in lumbar ganglia (supplemental Table S1, available at www.jneurosci.org as supplemental material). Staining of the epineurium was also eliminated in Nt5e−/− mice (Fig. 5B), consistent with expression of NT5E in this tissue (described above).
Figure 5.

AMP hydrolytic activity is reduced in nociceptive circuits of Nt5e−/− mice. A–H, Lumbar DRG (A, B), lumbar spinal cord (C–F) and cultured DRG neurons (G, H) from WT and Nt5e−/− adult mice stained using AMP histochemistry. Arrows point to epineurium (en). C, D, Arrowheads mark the location of axon terminals in dorsal spinal cord (lamina II). E, F, Higher magnification of C, D. G, H, The plasma membrane was not permeabilized so that extracellular AMP hydrolytic activity could be assayed. Arrowheads point to neurites emanating from cultured neurons. AMP (6 mm in A, B, G, H and 3 mm in C–F) was used as substrate, and buffer was pH 7.0. Scale bars: (in B, H) A, B, G, H, 50 μm; (in D) C, D, 500 μm; (in F) E, F, 200 μm.
In the spinal cord, AMP histochemical staining at pH 7.0 was noticeably reduced in lamina II of Nt5e−/− mice (Fig. 5C–F), precisely where NT5E+ axons terminate (Fig. 2B). This reduction in staining was not due to loss of axon terminals in the dorsal horn of Nt5e−/− mice, as IB4-binding in lamina II and other dorsal horn markers did not differ between WT and Nt5e−/− mice (supplemental Fig. S4, available at www.jneurosci.org as supplemental material). In addition, staining was reduced throughout the gray matter of spinal cord, suggesting NT5E is expressed at low levels and contributes to AMP hydrolysis in neurons throughout the spinal cord. Last, minimal to moderate reductions in AMP histochemical staining were seen in DRG and spinal cord sections when histochemical staining was performed at pH 5.6 (supplemental Fig. S5A–F, available at www.jneurosci.org as supplemental material), the pH at which obvious reductions in AMP hydrolytic activity were seen in Pap−/− mice (Zylka et al., 2008).
To further demonstrate that NT5E hydrolyzed AMP extracellularly on sensory neurons and their axons, we cultured DRG neurons for 72 h to allow neurites to develop then histochemically stained these cultures in the absence of detergent (to maintain the integrity of the plasma membrane). In cultures from WT mice, AMP histochemical staining was predominantly on the soma and neurites of small- to medium-diameter DRG neurons (Fig. 5G). Staining of the soma was reduced and neurite staining was eliminated in cultures from Nt5e−/− mice (Fig. 5H). Together, these histochemical studies with gene knock-out mice reveal that NT5E contributes to extracellular AMP hydrolysis on nociceptive neurons and axon terminals. Since staining was reduced but not eliminated in Nt5e−/− mice, these data confirm that nociceptive circuits contain additional AMP ectonucleotidases that generate adenosine, one of which we previously identified as PAP (Zylka et al., 2008).
Inflammation- and nerve injury-induced thermal hyperalgesia and mechanical allodynia are enhanced in Nt5e−/− mice
Pap−/− mice and A1R−/− mice display enhanced nociceptive responses in animal models of chronic pain (Johansson et al., 2001; Wu et al., 2005; Zylka et al., 2008), suggesting deficiencies in adenosine production or adenosine signaling can be detected at the behavioral level. To determine whether Nt5e−/− mice similarly show enhanced nociceptive responses, we tested age-matched WT and Nt5e−/− male mice using behavioral models of acute and chronic pain. We found no significant differences between genotypes using a measure of acute mechanical sensitivity (electronic von Frey) or two different measures of noxious thermal sensitivity (Hargreaves test and hotplate test at 52°C) (Table 2). However, Nt5e−/− mice had significantly shorter tail flick latencies in the tail immersion assay at both temperatures studied (46.5°C and 49°C) (Table 2). In addition, Nt5e−/− mice had significantly enhanced thermal hyperalgesia and mechanical allodynia following inflammation of one hindpaw with CFA (Fig. 6A,B). Moreover, Nt5e−/− mice had significantly enhanced thermal hyperalgesia in the SNI model of neuropathic pain (Fig. 6C,D). In contrast, no significant differences were seen between genotypes in the control (non-inflamed/non-injured) paw using either chronic pain model (Fig. 6). Collectively, these studies reveal that Nt5e−/− mice have enhanced nociceptive responses that are phenotypically similar to A1R−/− and Pap−/− mice (Johansson et al., 2001; Wu et al., 2005; Zylka et al., 2008); summarized in supplemental Table S2, available at www.jneurosci.org as supplemental material.
Table 2.
Acute mechanical and thermal sensitivity in WT and Nt5e−/− mice
| Behavioral assay | Withdrawal threshold (g) or latency (s) |
|
|---|---|---|
| Wild-type | Nt5e−/− | |
| Electronic von Frey | 8.1 ± 0.2 g | 7.7 ± 0.2 g |
| Radiant heating of hindpaw (Hargreaves method) | 10.6 ± 0.3 s | 10.3 ± 0.4 s |
| Tail immersion at 46.5°C | 13.8 ± 1.1 s | 9.5 ± 0.5 s** |
| Tail immersion at 49.0°C | 5.6 ± 0.3 s | 4.2 ± 0.2 s** |
| Hot plate at 52°C | 29.4 ± 2.1 s | 31.4 ± 2.7 s |
n = 10 male mice tested per genotype. Data are expressed as means ± SEM. Paired t test was used to compare genotypes for each test,
**p < 0.005.
Figure 6.
Nt5e−/− mice show enhanced nociceptive responses following inflammation and nerve injury. A, B, CFA inflammatory pain model. WT and Nt5e−/− mice were tested for (A) thermal and (B) mechanical sensitivity before (BL) and following injection of CFA (arrow) into one hindpaw. The contralateral hindpaw served as control. C, D, SNI neuropathic pain model. WT and Nt5e−/− were tested for (C) thermal and (D) mechanical sensitivity before (BL) and after ligation and transaction of the sural and common peroneal branches of the sciatic nerve. A–D, Paired t tests were used to compare responses at each time point between WT and Nt5e−/− mice (n = 10 per genotype); same paw comparisons. *p < 0.05, **p < 0.005, ***p < 0.0005. All data are presented as means ± SEM.
NT5E inhibits nociception by hydrolyzing AMP and activating A1R
Patterson et al. (2001) found that AMP was rapidly converted to adenosine when perfused in vivo over the dorsal spinal cord and reduced hyperalgesia caused by intradermal injection of capsaicin. This antinociceptive effect of AMP was dependent on A1R activation. Moreover, they found that hydrolysis of AMP to adenosine could be inhibited ∼50% by αβ-methylene-ADP, a relatively selective inhibitor of NT5E. Considering that this pharmacological inhibitor could have off-target effects in vivo (such as inhibition of other ectonucleotidases like PAP), or that the concentration used might not completely inhibit NT5E, we instead eliminated NT5E activity in a genetically precise manner, using Nt5e−/− mice.
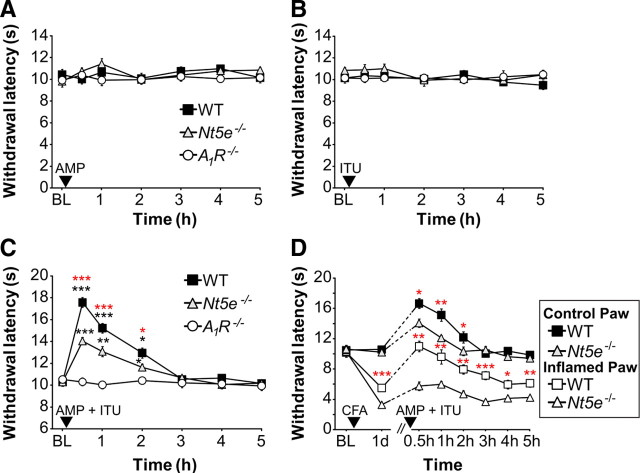
The push–pull microprobe used by Patterson et al. (2001) was optimized for use with rats, so we used the alternative approach of acute intrathecal injection of AMP to deliver AMP to DRG and spinal cord of mice. To our initial surprise, AMP alone (200 nmol) had no effect on noxious thermal sensitivity in WT mice (Fig. 7A), and higher doses were similarly without effect (data not shown). Moreover, AMP alone had no effect on noxious thermal sensitivity in Nt5e−/− or A1R−/− mice (Fig. 7A). We hypothesized this lack of an antinociceptive effect might be due to rapid degradation of ectonucleotidase-generated adenosine, resulting in insufficient levels of adenosine to activate A1R. In an effort to increase the half-life of adenosine, we coinjected AMP with the adenosine kinase inhibitor 5-iodotubericidin (ITU). This inhibitor blocks the phosphorylation of adenosine to AMP and prolongs the half-life of extracellular adenosine (Kowaluk and Jarvis, 2000). When injected alone, ITU had no effect on noxious thermal sensitivity in any of the genotypes tested (Fig. 7B). However, paw withdrawal latency significantly increased in WT mice when AMP was combined with ITU, revealing a thermal antinociceptive effect (Fig. 7C). Strikingly, this thermal antinociceptive effect of AMP combined with ITU was reduced by 45 ± 13.5% in Nt5e−/− mice (based on quantifying the area under the curve relative to WT mice) and was eliminated in A1R−/− mice. Together, these data indicate that the thermal antinociceptive effect of AMP combined with ITU was due to in vivo dephosphorylation of AMP to adenosine followed by activation of A1R, with NT5E accounting for ∼50% of all AMP hydrolytic activity in DRG and spinal cord.
Figure 7.
The antinociceptive effects of AMP + ITU are reduced in Nt5e−/− mice and are dependent on A1R activation. A, B, Noxious thermal sensitivity was measured before (baseline, BL) and after intrathecal injection of AMP (200 nmol) (A), ITU (5 nmol) (B), or AMP (200 nmol) + ITU (5 nmol) (C). Paired t tests were used to compare responses at each time point to BL values (black asterisks) within a given genotype (n = 8 mice per genotype). Paired t tests were also used to compare responses at each time point between WT and Nt5e−/− mice (red asterisks). D, Mice were tested for noxious thermal sensitivity before (BL) and after injection of CFA (arrowhead) into one hindpaw. The uninjected hindpaw served as control. One day later, all mice were injected intrathecally with AMP (200 nmol) + ITU (5 nmol) and thermal sensitivity was measured for several hours after injection. Paw withdrawal latencies were significantly elevated in both genotypes for up to 2 h following AMP + ITU injection, relative to the response 1 d after CFA injection (same paw comparisons). Paired t tests were used to compare responses at each time point between WT and Nt5e−/− mice (red asterisks; same paw comparisons). n = 8 mice per genotype. *p < 0.05, **p < 0.005, ***p < 0.0005. All data are presented as means ± SEM.
To further study the contribution of NT5E to AMP hydrolysis in vivo, we coinjected AMP with ITU in the setting of ongoing inflammation. After measuring baseline thermal sensitivity, we injected one hindpaw of WT and Nt5e−/− mice with CFA to induce thermal hyperalgesia (Fig. 7D). One day later, the inflamed hindpaw of Nt5e−/− mice displayed enhanced thermal hyperalgesia, reproducing our results above. We next coinjected (intrathecally) AMP with ITU then measured thermal sensitivity in the inflamed and non-inflamed (control) hindpaws. We found that AMP combined with ITU significantly reduced thermal hyperalgesia in the inflamed paw for 3 h in WT mice. In fact, withdrawal latency transiently returned to pre-CFA levels 30 min. after injection of AMP with ITU. In contrast, the thermal antinociceptive effects of AMP combined with ITU were significantly blunted in duration and magnitude in Nt5e−/− mice, both in the inflamed and non-inflamed paws (Fig. 7D). As controls, AMP alone (200 nmol, i.t.) and ITU alone (5 nmol, i.t.) had no effect on thermal sensitivity in the inflamed paws of WT and Nt5e−/− mice (data not shown). Together, these data further show that NT5E reduces thermal nociception in vivo by hydrolyzing AMP to adenosine.
Discussion
Our previous studies with Pap−/− mice suggested that nociceptive neurons contained additional ectonucleotidases that could hydrolyze AMP to adenosine (Zylka et al., 2008). Here, we found that NT5E is one of these additional enzymes. NT5E was found on DRG neurons, and primarily on the subset that expressed nociceptive neuron markers (IB4-binding, Mrgprd, CGRP, and TRPV1). In addition, NT5E was extensively coexpressed with PAP, suggesting that these two ectonucleotidases might coordinately metabolize AMP in nociceptive neurons under normal and pathological conditions, including inflammation where nucleotides and protons are released as part of the “inflammatory soup” (Julius and Basbaum, 2001). NT5E has a neutral pH optimum (Zimmermann, 1992), whereas PAP is catalytically active over a broader pH range (pH 3–8) (Van Etten, 1982). Tissue acidosis could thus alter the relative contribution of PAP and NT5E to AMP hydrolysis. Indeed, AMP hydrolytic activity was clearly reduced in nociceptive neurons and spinal axon terminals of Nt5e−/− mice at neutral pH but was less prominently reduced at an acidic pH. In contrast, PAP has a more prominent effect on nucleotide hydrolysis at acidic pH (Vihko, 1978; Zylka et al., 2008; Sowa et al., 2009). NT5E and PAP were colocalized on axon terminals in lamina II and both proteins were downregulated following nerve injury (Colmant, 1959; Csillik and Knyihar-Csillik, 1986; Costigan et al., 2002; Davis-Taber, 2006). This coordinate downregulation of enzymatically redundant proteins correlated with a loss of AMP hydrolytic activity in lamina II and could explain why nucleotide metabolism and A1R activity were altered in DRG and spinal cord following nerve injury (Bantel et al., 2002; Matsuoka and Ohkubo, 2004).
Nt5e−/−, Pap−/−, and A1R−/− mice displayed enhanced thermal sensitivity and, in some genotypes, enhanced mechanical sensitivity in animal models of inflammatory and neuropathic pain (Wu et al., 2005; Zylka et al., 2008) (supplemental Table S2, available at www.jneurosci.org as supplemental material). Interestingly, the magnitudes of these enhanced sensory responses were similar in all three knock-out lines. A priori, one might predict that the magnitude of these enhanced sensory responses would be greater in A1R−/− mice when compared with Nt5e−/− or Pap−/− animals since AMP hydrolytic activity (and hence adenosine production) was reduced but not eliminated in Nt5e−/− or Pap−/− animals. The similar phenotype in all three genotypes suggests either that these behavioral models lack the resolution to “report” intermediate levels of A1R activation or that reduced adenosine production and A1R signaling is as effective at enhancing nociceptive responses as complete elimination of A1R signaling.
Our findings also raise the question of why Nt5e−/−, Pap−/−, and A1R−/− mice display enhanced nociceptive responses when sensitized. The most straightforward possibility is that these enhanced responses are due to reductions in adenosine production or A1R signaling. Transient A1R activation acutely inhibits neurotransmitter release from nociceptive neurons and inhibits postsynaptic neurons (Li and Perl, 1994; Lao et al., 2001; Patel et al., 2001). In contrast, ectonucleotidases like PAP hydrolyze nucleotides extracellularly and inhibit nociception by activating A1R over a sustained time period (Zylka et al., 2008). Sustained, ectonucleotidase-dependent activation of A1R could contribute to the underlying “adenosine tone” that regulates the level of excitability throughout the nervous system (Boison, 2008). Reductions or loss of this tonic A1R activation could contribute to enhanced nociceptive responses. Currently, it is unknown how sustained, ectonucleotidase-dependent activation of A1R inhibits nociception at a mechanistic level.
We also observed enhanced nociceptive responses in Nt5e−/− mice to noxious thermal stimuli using the tail immersion assay but not using other assays of thermal sensitivity. This could reflect differences in heat intensities between the assays, the surface area of tissue heated, the type of tissue heated, or the relative contribution of spinal versus supraspinal influences to the scored behaviors. Indeed, mice missing the thermosensor TRPV1 also had thermosensory phenotypes that were dependent on the behavioral test and temperature used (Caterina et al., 2000). For example, Trpv1−/− mice had a significant deficit in the tail immersion assay at 50°C but no phenotype in the hot plate assay at 50°C or the Hargreaves assay at most intensity levels tested.
Curiously, we observed enhanced thermal and mechanical sensitivity following CFA inflammation but only enhanced thermal sensitivity following nerve injury in Nt5e−/− mice and in Pap−/− mice (Zylka et al., 2008). This differential effect on mechanical sensitization could reflect differences in how CFA and nerve injury promote mechanical sensitization, or this could reflect a “floor” effect. For example, for technical or physiological reasons, mechanical sensitization may be at maximal levels following nerve injury in all genotypes.
Adenosine is rapidly removed from the extracellular space by nucleoside transporters and metabolic enzymes, including adenosine kinase and adenosine deaminase. Indeed, the extracellular concentration of adenosine and the antinociceptive effects of adenosine can be increased by pharmacologically inhibiting these transporters or metabolic enzymes (Keil and DeLander, 1992; Poon and Sawynok, 1998; Lavand'homme and Eisenach, 1999; Kowaluk and Jarvis, 2000; Jarvis et al., 2002). In addition, a metabolically stable analog of adenosine, but not adenosine itself, had acute thermal antinociceptive effects in mice (Post, 1984). Other groups found that stable analogs of adenosine or high doses of adenosine had additional antinociceptive effects in rodents and humans (for review, see Sawynok, 2007) (Lee and Yaksh, 1996; Belfrage et al., 1999; Gomes et al., 1999; Eisenach et al., 2002; Hayashida et al., 2005). The use of stable adenosine analogs or high doses of adenosine provides an empirical means to bypass or overwhelm, respectively, the enzymes that inactivate adenosine.
We hypothesized that spinally injected AMP might be hydrolyzed by endogenous NT5E and the liberated adenosine would reduce thermal sensitivity. However, we found that intrathecal injection of AMP alone had no effect on thermal nociception. This finding, combined with observations by others described above, suggested that ectonucleotidase-generated adenosine might be metabolized before it could detectably affect thermal nociception. Indeed, by coinjecting AMP with the adenosine kinase inhibitor ITU, we were able to “unmask” an acute thermal antinociceptive effect of AMP. This antinociceptive effect was blunted by ∼50% in Nt5e−/− mice and eliminated in A1R−/− mice, indicating a partial dependence on NT5E activity and complete dependence on A1R activation. Similarly, we found that coinjection of AMP with the adenosine deaminase inhibitor 2-deoxycoformycin reduced noxious thermal sensitivity when acutely injected (intrathecally), whereas 2-deoxycoformycin alone was ineffective (data not shown). Together, our in vivo studies demonstrate that NT5E accounts for some, but not all, AMP hydrolytic activity in DRG and spinal cord. Future studies with pharmacological inhibitors or, more rigorously, genetic elimination of both NT5E and PAP will be needed to determine if these are the only two enzymes with ecto-5′-nucleotidase activity in nociceptive circuits. In addition, it might be possible to harness NT5E to make adenosine for the treatment of chronic pain.
In the skin, NT5E is found on microvessels and is used as a cell-surface marker of skin stem cells (Hoogduijn et al., 2006; Niemelä et al., 2008). Aside from reports such as these, the spatial distribution of NT5E in the skin has not been well studied. We found NT5E on nociceptive axon terminals in the epidermis, on cells of the superficial dermis, and on keratinocytes throughout the epidermis, including cells in the stratum basalis. Keratinocytes release nucleotides, particularly ATP, when stimulated mechanically or thermally. In turn, nucleotides activate purinergic receptors on sensory endings in the skin and regulate thermal selection behavior (Dixon et al., 1999; Koizumi et al., 2004; Moqrich et al., 2005; Shimizu et al., 2005; Mandadi et al., 2009). Nucleotide signaling can be terminated through receptor desensitization or nucleotide metabolism. ENTPD1 (CD39, NTPDase1) is found on epidermal Langerhans cells and hydrolyzes keratinocyte-derived ATP and ADP to AMP (Mizumoto et al., 2002). Enzymes with AMP hydrolytic activity are present on epidermal keratinocytes (Klaushofer and Böck, 1974). Our studies suggest that NT5E is well localized to catalyze this final step of nucleotide hydrolysis in the skin, namely hydrolysis of AMP to adenosine.
At present, it is unclear how NT5E-generated adenosine might regulate physiological processes in the skin. Adenosine could activate all four adenosine receptors (A1R, A2AR, A2BR, A3R), as all four receptors are found in skin keratinocytes (Braun et al., 2006), and some are found on skin microvessels. Confusingly, some studies suggest adenosine has proliferative effects on keratinocytes while others describe antiproliferative effects (Cook et al., 1995; Braun et al., 2006). These discrepancies could be due to the concentration of adenosine used or to the receptor subtypes that are activated. For example, adenosine produces cutaneous hyperalgesia when injected peripherally via activation of A2 receptors (Taiwo and Levine, 1990), whereas selective activation of A1R in the periphery is antinociceptive (Aley et al., 1995). Interestingly, at low concentrations, adenosine causes vasodilation in skin microcirculation and this response is A1R- and temperature-dependent (Stojanov and Proctor, 1989, 1990). In contrast, at high concentrations, adenosine produces temperature-independent vasoconstriction through A2R (Stojanov and Proctor, 1989, 1990). Considering the role of nucleotides in mediating thermal selection behavior and the importance of the peripheral vasculature in thermoregulation, NT5E could provide a molecular and biochemical link between nucleotide-dependent temperature sensation in the skin and adenosine-dependent vascular responses.
Footnotes
This work was supported by grants to M.J.Z. from The Searle Scholars Program, Rita Allen Foundation, and National Institute of Neurological Disorders and Stroke (NINDS) (R01NS060725, R01NS067688). N.A.S. was supported by NINDS (F30NS063507) and a Medical Scientist Training Program grant (T32GM008719). Confocal imaging was performed at the University of North Carolina at Chapel Hill Confocal Imaging Facility, which is cofunded by NINDS and National Institute of Child Health and Human Development (P30NS045892). M.J.Z. is a Rita Allen Foundation Milton E. Cassel Scholar. We thank Linda Thompson for providing congenic Nt5e−/− mice, Steven Tilley for providing congenic A1R−/− mice, David J. Anderson for providing congenic MrgprdΔEGFPf knock-in mice, and Yvette Chuang and Hong Wang for technical assistance.
References
- Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15:8031–8038. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bantel C, Tobin JR, Li X, Childers SR, Chen SR, Eisenach JC. Intrathecal adenosine following spinal nerve ligation in rat: short residence time in cerebrospinal fluid and no change in A(1) receptor binding. Anesthesiology. 2002;96:103–108. doi: 10.1097/00000542-200201000-00022. [DOI] [PubMed] [Google Scholar]
- Belfrage M, Segerdahl M, Arnér S, Sollevi A. The safety and efficacy of intrathecal adenosine in patients with chronic neuropathic pain. Anesth Analg. 1999;89:136–142. doi: 10.1097/00000539-199907000-00023. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Braun M, Lelieur K, Kietzmann M. Purinergic substances promote murine keratinocyte proliferation and enhance impaired wound healing in mice. Wound Repair Regen. 2006;14:152–161. doi: 10.1111/j.1743-6109.2006.00105.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Callahan BL, Gil AS, Levesque A, Mogil JS. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain. 2008;9:174–184. doi: 10.1016/j.jpain.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Campagnola L, Wang H, Zylka MJ. Fiber-coupled light-emitting diode for localized photostimulation of neurons expressing channelrhodopsin-2. J Neurosci Methods. 2008;169:27–33. doi: 10.1016/j.jneumeth.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casals-Díaz L, Vivó M, Navarro X. Nociceptive responses and spinal plastic changes of afferent C-fibers in three neuropathic pain models induced by sciatic nerve injury in the rat. Exp Neurol. 2009;217:84–95. doi: 10.1016/j.expneurol.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan SP, Eltzschig HK, Eckle T, Thompson LF. Physiological roles for ecto-5′-nucleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmant HJ. Aktivitatsschwankungen der sauren Phosphatase im Ruckenmark und den Spinalganglien der Ratte nach Durchschneidung des Nervus ischiadicus. Arch Psychiat Nervnekr. 1959;199:60–71. doi: 10.1007/BF00361100. [DOI] [PubMed] [Google Scholar]
- Cook PW, Ashton NM, Pittelkow MR. Adenosine and adenine nucleotides inhibit the autonomous and epidermal growth factor-mediated proliferation of cultured human keratinocytes. J Invest Dermatol. 1995;104:976–981. doi: 10.1111/1523-1747.ep12606228. [DOI] [PubMed] [Google Scholar]
- Costigan M, Befort K, Karchewski L, Griffin RS, D'Urso D, Allchorne A, Sitarski J, Mannion JW, Pratt RE, Woolf CJ. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci. 2002;3:16. doi: 10.1186/1471-2202-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csillik B, Knyihar-Csillik E. The protean gate: structure and plasticity of the primary nociceptive analyzer. Budapest: Akademiai Kiado; 1986. [Google Scholar]
- Cunha TM, Verri WA, Jr, Vivancos GG, Moreira IF, Reis S, Parada CA, Cunha FQ, Ferreira SH. An electronic pressure-meter nociception paw test for mice. Braz J Med Biol Res. 2004;37:401–407. doi: 10.1590/s0100-879x2004000300018. [DOI] [PubMed] [Google Scholar]
- Davis-Taber RA. Transcriptional profiling of dorsal root ganglia in a neuropathic pain model using microarray and laser capture microdissection. Drug Dev Res. 2006;67:308–330. [Google Scholar]
- Dixon CJ, Bowler WB, Littlewood-Evans A, Dillon JP, Bilbe G, Sharpe GR, Gallagher JA. Regulation of epidermal homeostasis through P2Y2 receptors. Br J Pharmacol. 1999;127:1680–1686. doi: 10.1038/sj.bjp.0702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussor G, Koerber HR, Oaklander AL, Rice FL, Molliver DC. Nucleotide signaling and cutaneous mechanisms of pain transduction. Brain Res Rev. 2009;60:24–35. doi: 10.1016/j.brainresrev.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach JC, Hood DD, Curry R. Preliminary efficacy assessment of intrathecal injection of an American formulation of adenosine in humans. Anesthesiology. 2002;96:29–34. doi: 10.1097/00000542-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA. Spinal delivery of analgesics in experimental models of pain and analgesia. Adv Drug Deliv Rev. 2003;55:1007–1041. doi: 10.1016/s0169-409x(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Gomes JA, Li X, Pan HL, Eisenach JC. Intrathecal adenosine interacts with a spinal noradrenergic system to produce antinociception in nerve-injured rats. Anesthesiology. 1999;91:1072–1079. doi: 10.1097/00000542-199910000-00028. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hayashida M, Fukuda K, Fukunaga A. Clinical application of adenosine and ATP for pain control. J Anesth. 2005;19:225–235. doi: 10.1007/s00540-005-0310-8. [DOI] [PubMed] [Google Scholar]
- Hoogduijn MJ, Gorjup E, Genever PG. Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 2006;15:49–60. doi: 10.1089/scd.2006.15.49. [DOI] [PubMed] [Google Scholar]
- Hua X, Erikson CJ, Chason KD, Rosebrock CN, Deshpande DA, Penn RB, Tilley SL. Involvement of A1 adenosine receptors and neural pathways in adenosine-induced bronchoconstriction in mice. Am J Physiol Lung Cell Mol Physiol. 2007;293:L25–32. doi: 10.1152/ajplung.00058.2007. [DOI] [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Mikusa J, Chu KL, Wismer CT, Honore P, Kowaluk EA, McGaraughty S. Comparison of the ability of adenosine kinase inhibitors and adenosine receptor agonists to attenuate thermal hyperalgesia and reduce motor performance in rats. Pharmacol Biochem Behav. 2002;73:573–581. doi: 10.1016/s0091-3057(02)00840-7. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Giménez-Llort L, Escorihuela RM, Fernández-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hårdemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Keil GJ, 2nd, DeLander GE. Spinally-mediated antinociception is induced in mice by an adenosine kinase-, but not by an adenosine deaminase-, inhibitor. Life Sci. 1992;51:PL171–176. doi: 10.1016/0024-3205(92)90566-8. [DOI] [PubMed] [Google Scholar]
- Klaushofer K, Böck P. Studies on 5′-nucleotidase histochemistry. II. Differences in 5′-nucleotidase activity in stratified squamous epithelia and skin appendages of mouse, rat and guinea pig. Histochemistry. 1974;40:39–49. doi: 10.1007/BF00490272. [DOI] [PubMed] [Google Scholar]
- Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J. 2004;380:329–338. doi: 10.1042/BJ20031089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowaluk EA, Jarvis MF. Therapeutic potential of adenosine kinase inhibitors. Expert Opin Investig Drugs. 2000;9:551–564. doi: 10.1517/13543784.9.3.551. [DOI] [PubMed] [Google Scholar]
- Lao LJ, Kumamoto E, Luo C, Furue H, Yoshimura M. Adenosine inhibits excitatory transmission to substantia gelatinosa neurons of the adult rat spinal cord through the activation of presynaptic A(1) adenosine receptor. Pain. 2001;94:315–324. doi: 10.1016/S0304-3959(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Lavand'homme PM, Eisenach JC. Exogenous and endogenous adenosine enhance the spinal antiallodynic effects of morphine in a rat model of neuropathic pain. Pain. 1999;80:31–36. doi: 10.1016/s0304-3959(98)00193-6. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Crepps B, Perl ER. Calcitonin gene-related peptide immunoreactivity and afferent receptive properties of dorsal root ganglion neurones in guinea-pigs. J Physiol. 2002;540:989–1002. doi: 10.1113/jphysiol.2001.013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledoux S, Runembert I, Koumanov K, Michel JB, Trugnan G, Friedlander G. Hypoxia enhances Ecto-5′-Nucleotidase activity and cell surface expression in endothelial cells: role of membrane lipids. Circ Res. 2003;92:848–855. doi: 10.1161/01.RES.0000069022.95401.FE. [DOI] [PubMed] [Google Scholar]
- Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996;277:1642–1648. [PubMed] [Google Scholar]
- Li J, Perl ER. Adenosine inhibition of synaptic transmission in the substantia gelatinosa. J Neurophysiol. 1994;72:1611–1621. doi: 10.1152/jn.1994.72.4.1611. [DOI] [PubMed] [Google Scholar]
- Li J, Perl ER. ATP modulation of synaptic transmission in the spinal substantia gelatinosa. J Neurosci. 1995;15:3357–3365. doi: 10.1523/JNEUROSCI.15-05-03357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin EA, Caterina MJ. Mechanisms of sensory transduction in the skin. Nature. 2007;445:858–865. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- Mandadi S, Sokabe T, Shibasaki K, Katanosaka K, Mizuno A, Moqrich A, Patapoutian A, Fukumi-Tominaga T, Mizumura K, Tominaga M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflugers Arch. 2009;458:1093–1102. doi: 10.1007/s00424-009-0703-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka I, Ohkubo S. ATP- and adenosine-mediated signaling in the central nervous system: adenosine receptor activation by ATP through rapid and localized generation of adenosine by ecto-nucleotidases. J Pharmacol Sci. 2004;94:95–99. doi: 10.1254/jphs.94.95. [DOI] [PubMed] [Google Scholar]
- Mizumoto N, Kumamoto T, Robson SC, Sévigny J, Matsue H, Enjyoji K, Takashima A. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KS, Andahazy M, Story GM, Patapoutian A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Daddona PE. Anatomical and cytochemical relationships of adenosine deaminase-containing primary afferent neurons in the rat. Neuroscience. 1985;15:799–813. doi: 10.1016/0306-4522(85)90079-x. [DOI] [PubMed] [Google Scholar]
- Niemelä J, Ifergan I, Yegutkin GG, Jalkanen S, Prat A, Airas L. IFN-beta regulates CD73 and adenosine expression at the blood-brain barrier. Eur J Immunol. 2008;38:2718–2726. doi: 10.1002/eji.200838437. [DOI] [PubMed] [Google Scholar]
- Patel MK, Pinnock RD, Lee K. Adenosine exerts multiple effects in dorsal horn neurones of the adult rat spinal cord. Brain Res. 2001;920:19–26. doi: 10.1016/s0006-8993(01)02844-x. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Sluka KA, Arnold MA. A novel transverse push-pull microprobe: in vitro characterization and in vivo demonstration of the enzymatic production of adenosine in the spinal cord dorsal horn. J Neurochem. 2001;76:234–246. doi: 10.1046/j.1471-4159.2001.00016.x. [DOI] [PubMed] [Google Scholar]
- Poon A, Sawynok J. Antinociception by adenosine analogs and inhibitors of adenosine metabolism in an inflammatory thermal hyperalgesia model in the rat. Pain. 1998;74:235–245. doi: 10.1016/s0304-3959(97)00186-3. [DOI] [PubMed] [Google Scholar]
- Post C. Antinociceptive effects in mice after intrathecal injection of 5′-N-ethylcarboxamide adenosine. Neurosci Lett. 1984;51:325–330. doi: 10.1016/0304-3940(84)90397-5. [DOI] [PubMed] [Google Scholar]
- Rau KK, McIlwrath SL, Wang H, Lawson JJ, Jankowski MP, Zylka MJ, Anderson DJ, Koerber HR. Mrgprd enhances excitability in specific populations of cutaneous murine polymodal nociceptors. J Neurosci. 2009;29:8612–8619. doi: 10.1523/JNEUROSCI.1057-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice FL, Fundin BT, Arvidsson J, Aldskogius H, Johansson O. Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J Comp Neurol. 1997;385:149–184. [PubMed] [Google Scholar]
- Salter MW, Henry JL. Effects of adenosine 5′-monophosphate and adenosine 5′-triphosphate on functionally identified units in the cat spinal dorsal horn. Evidence for a differential effect of adenosine 5′-triphosphate on nociceptive vs non-nociceptive units. Neuroscience. 1985;15:815–825. doi: 10.1016/0306-4522(85)90080-6. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Adenosine and ATP receptors. Handb Exp Pharmacol. 2007;177:309–328. doi: 10.1007/978-3-540-33823-9_11. [DOI] [PubMed] [Google Scholar]
- Scott TG. The distribution of 5′-nucleotidase in the brain of the mouse. J Comp Neurol. 1967;129:97–114. [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Iida T, Guan Y, Zhao C, Raja SN, Jarvis MF, Cockayne DA, Caterina MJ. Enhanced thermal avoidance in mice lacking the ATP receptor P2X3. Pain. 2005;116:96–108. doi: 10.1016/j.pain.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Sowa NA, Vadakkan KI, Zylka MJ. Recombinant mouse PAP has pH-dependent ectonucleotidase activity and acts through A(1)-adenosine receptors to mediate antinociception. PLoS ONE. 2009;4:e4248. doi: 10.1371/journal.pone.0004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanov I, Proctor KG. Pharmacological evidence for A1 and A2 adenosine receptors in the skin microcirculation. Circ Res. 1989;65:176–184. doi: 10.1161/01.res.65.1.176. [DOI] [PubMed] [Google Scholar]
- Stojanov I, Proctor KG. Temperature-sensitive adenosine-mediated vasoconstriction in the skin microcirculation. J Pharmacol Exp Ther. 1990;253:1083–1089. [PubMed] [Google Scholar]
- Suran AA. 5′-Nucleotidase and an acid phosphatase of spinal cord. Comparative histochemistry and specificity of the enzymes in mouse and cat spinal cords. Cytologic localization in mouse substantia gelatinosa. J Histochem Cytochem. 1974;22:802–811. doi: 10.1177/22.8.802. [DOI] [PubMed] [Google Scholar]
- Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Direct cutaneous hyperalgesia induced by adenosine. Neuroscience. 1990;38:757–762. doi: 10.1016/0306-4522(90)90068-f. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Peleshok JC, Ribeiro-da-Silva A. Distribution of P2X(3)-immunoreactive fibers in hairy and glabrous skin of the rat. J Comp Neurol. 2009;514:555–566. doi: 10.1002/cne.22048. [DOI] [PubMed] [Google Scholar]
- Taylor-Blake B, Zylka MJ. Prostatic acid phosphatase is expressed in peptidergic and nonpeptidergic nociceptive neurons of mice and rats. PLoS One. 2010;5:e8674. doi: 10.1371/journal.pone.0008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Van Etten RL. Human prostatic acid phosphatase: a histidine phosphatase. Ann N Y Acad Sci. 1982;390:27–51. doi: 10.1111/j.1749-6632.1982.tb40302.x. [DOI] [PubMed] [Google Scholar]
- Vihko P. Characterization of the principal human prostatic acid phosphatase isoenzyme, purified by affinity chromatography and isoelectric focusing. Part II. Clin Chem. 1978;24:1783–1787. [PubMed] [Google Scholar]
- Wang HF, Robertson B, Grant G. Anterograde transport of horseradish-peroxidase conjugated isolectin B4 from Griffonia simplicifolia I in spinal primary sensory neurons of the rat. Brain Res. 1998;811:34–39. doi: 10.1016/s0006-8993(98)00916-0. [DOI] [PubMed] [Google Scholar]
- Wu WP, Hao JX, Halldner L, Lövdahl C, DeLander GE, Wiesenfeld-Hallin Z, Fredholm BB, Xu XJ. Increased nociceptive response in mice lacking the adenosine A1 receptor. Pain. 2005;113:395–404. doi: 10.1016/j.pain.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. 5′-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H. Ectonucleotidases in the nervous system. Novartis Found Symp. 2006;276:113–128. discussion 128–130, 233–237, 275–281. [PubMed] [Google Scholar]
- Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P. Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron. 2008;60:111–122. doi: 10.1016/j.neuron.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]