Abstract
Background
Becker muscular dystrophy (BMD) and X-linked dilated cardiomyopathy (XLDCM) often result from deletion mutations in the dystrophin gene that may lead to expression of an altered dystrophin protein in cardiac muscle. Cardiac involvement is present in about 70% of BMD and all XLDCM cases. To date, the timing of cardiomyopathy development remains unpredictable. We analyzed 78 BMD and XLDCM patients with common deletion mutations predicted to alter the dystrophin protein and correlated their mutations to cardiomyopathy age of onset. This approach was chosen to connect dystrophin structure with function in the heart.
Methods and Results
Detailed cardiac information was collected for BMD and XLDCM patients with defined dystrophin gene deletion mutations. Patients were grouped based on the dystrophin protein domain affected by the deletion. Deletions affecting the amino-terminal domain are associated with early-onset DCM (mid-20’s), while deletions removing part of the rod domain and hinge 3 have a later onset DCM (mid-40’s). Further, we modeled the effects of the most common mutations occurring in the rod domain on the overall structure of the dystrophin protein. By combining genetic and protein information, this analysis revealed a strong correlation between specific protein structural modifications and DCM age of onset.
Conclusions
We identified specific regions of the dystrophin gene that when mutated predispose BMD patients to early-onset DCM. In addition, we propose that some mutations lead to early-onset DCM via specific alterations in protein folding. These findings have potential implications for early intervention in the cardiac care of BMD patients and for therapeutic approaches that target the heart in dystrophinopathies.
Keywords: Cardiomyopathy, genetics, risk factors, muscular dystrophy, dystrophin
Introduction
The dystrophin gene, located on the X-chromosome, is the largest known human gene (2.4 Mb, 79 exons) resulting in a high rate of spontaneous disease-causing mutations (30% of cases) with deletions forming the majority (about 60%). Dystrophin plays an essential structural role in both cardiac and skeletal muscle, protecting the sarcolemma from mechanical stresses of muscle contraction. Complete loss of dystrophin leads to Duchenne muscular dystrophy (DMD), the most common severe form of childhood muscular dystrophy, complicated by skeletal muscle degeneration and dilated cardiomyopathy (DCM).
In contrast to the well-defined clinical course of DMD, mutations that do not disrupt the reading frame can result in expression of an altered dystrophin protein, leading to a more variable clinical presentation. This includes Becker muscular dystrophy (BMD) that presents primarily with progressive skeletal muscle degeneration with variable age of onset and severity, and X-linked DCM (XLDCM) that typically has no detectable skeletal muscle signs accompanying the cardiac involvement. While BMD is historically diagnosed based on skeletal muscle manifestations, the primary cause of death is heart failure 1. Indeed, about 70% of BMD patients develop DCM 1–4, and a recent longitudinal study demonstrated an onset of cardiac involvement in the early teens in some BMD patients 5. Similar to DMD and XLDCM, the severity and age of onset of cardiac involvement in BMD show no correlation to skeletal muscle involvement 2, 3. Furthermore, DCM is often diagnosed after cardiac symptoms manifest, diminishing the efficacy of cardio-protective drugs. Therefore, the identification of parameters for cardiac risk assessment prior to symptom manifestation bears undeniable relevance for the clinical care of BMD patients and would assist in patient stratification in clinical trials testing the efficacy of cardiac treatments. It is with this in mind that we extensively examined a large cohort of BMD and XLDCM patients with deletion mutations to explore the hypothesis that loss of specific domains of the dystrophin protein predispose to early onset cardiomyopathy by potentially affecting protein expression levels, function, and/or structure. This hypothesis was born from previous studies exploring correlation between genotype and the presence of DCM in BMD patients 2–4 as well as reports on the association of deletions in specific dystrophin domains with severity of skeletal muscle symptoms 6–8.
Materials and Methods
Patient Sources and Data Collection
This is a cross-sectional retrospective study of patient information obtained from three major sources: 1) a database from the Muscular Dystrophy Association (MDA) clinics at Nationwide Children’s Hospital (NCH) and The Ohio State University Medical Center; 2) the United Dystrophinopathy Project (UDP) database (PI, Kevin Flanigan, Co-PIs Jerry R Mendell and Alan Pestronk); and 3) published studies. Information was obtained related to: age of onset and severity of skeletal and cardiac muscle manifestations, description of cardiac evaluation and skeletal muscle biopsy, the gene mutation, family history and serum creatine kinase. Echocardiograms at NCH were interpreted by the same cardiologist (author H.D.A.). The Institutional Review Board at Nationwide Children’s Hospital and The Ohio State University approved all protocols.
Inclusion and Exclusion Criteria
Inclusion criteria included: (1) a diagnosis of BMD and/or XLDCM; (2) cardiac evaluation; and (3) a confirmed exon deletion mutation spanning up to 11 exons. The eleven exon limit was set to exclude large deletions potentially affecting multiple functional domains of dystrophin, while including the vast majority of patients which were found to have deletions affecting 1 to 8 exons. Patients with exon 45–55 deletions known to be associated with mild, late-onset skeletal muscle involvement 9, 10 were also included. More stringent criteria potentially compromised statistical power. Exclusion criteria included: 1) reported or suspected cardiac viral infections; 2) cardiac biopsy without dystrophin expression; 3) deletions restricted to non-coding regions of the dystrophin gene potentially containing ill defined regulatory elements; 4) subjects younger than age 12 without proven family history of BMD; 5) wheelchair-dependent patients by age 12 carrying a diagnosis of BMD (preferably considered a severe form of dystrophinopathy 11). Deletions selectively affecting myocardial expression of dystrophin have been excluded from this study primarily because of low patient numbers, precluding meaningful statistical analysis. Supplemental Table 1 lists subjects excluded based on these criteria. Of 320 subjects initially screened for inclusion in this study, 118 satisfied the selection criteria.
Definition of Cardiomyopathy and Disease Onset
Cardiomyopathy was defined as follows: ejection fraction (EF) ≤ 55% and/or shortening fraction ≤ 32%. The EF cutoff agrees with previous studies based on the natural history of the disease 5, 12, and with timing of cardiac drug intervention common in clinical practice for BMD patients. When available, additional parameters were considered to support cardiac dilation: E-point septal separation above 5mm, left ventricular end diastolic diameter above 58 mm or above 2 z scores when indexed to body surface area, or cardiomegaly consistent with cardiomyopathy by chest radiograph. Electrocardiograms were not used to define cardiomyopathy 13. In this cross-sectional study, the time at which abnormal cardiac findings were first reported defines the “onset” of cardiomyopathy. The age of onset of DCM represents the youngest reported age at which cardiac parameters met the definition of cardiomyopathy.
For analyses involving non-cardiomyopathic patients, age corresponds to the oldest reported age at which cardiac findings were normal.
Echocardiography
For UDP and MDA clinic patients, images from two-dimensional and Doppler ultrasound studies were evaluated by standard techniques. Measurements included left ventricular (LV) diameter, shortening fraction (LV diastolic diameter minus LV systolic diameter divided by LV diastolic diameter), and EF (Simpsons’ formula applied to plamiterized diastolic and systolic LV cavity images derived from the apex view). For published cases, deviations from this methodology can be found in the original articles (Supplemental Tables 2 and 3).
Patient grouping
Patients were categorized into three groups based on the affected functional domain of the dystrophin protein. Group 1: subjects with deletions affecting any portion of the actin-binding amino-terminal domain of dystrophin (exons 2 to 9). Hinge 3 (a specific protein sequence joining two segments of dystrophin that allows flexible movement accounting for intrinsic protein folding) has been implicated in skeletal muscle involvement 6 and thus served to divide BMD subjects into two additional groups. Group 2: subjects with deletions preserving hinge 3 and affecting exons 45 to 49 (spectrin repeats 17 up to 19). Group 3: subjects with deletions affecting exons 50 and/or 51, removing or disrupting hinge 3.
Dystrophin protein modeling
Rod-region spectrin repeats were modeled based on the published structure of repeats 15 and 16 of chicken brain α-spectrin (PDB 1U5P) 14. The structure was manually extended by replication and RMS alignment of corresponding terminal residues, using PyMol (http://www.pymol.org/). The structure was briefly minimized using VMD/NAMD (http://www.ks.uiuc.edu/Research/namd/) to remove significant bad contacts. Hinge region and out-of-phase deletion mutation structures were constructed by manual deletion of structurally equivalent residues from the extended spectrin repeat and structural re-alignment of the resulting fragments. A brief minimization using VMD/NAMD was employed to correct any significant misplacement of fragment ends.
Statistical Analysis
Non-parametric Kruskal-Wallis test was performed for cross-group age comparisons (a priori p<0.05), followed by Mann-Whitney U test post hoc comparisons among groups (Bonferroni adjustment was utilized to achieve overall significance of p<0.05). Mann-Whitney U test was used to compare age of cardiomyopathy onset between in-phase and out-of-phase mutations in Group 2 patients. For blood relatives only one patient was randomly selected for inclusion in statistical analyses. Three sibling pairs were identified within cardiomyopathic patients: #224 and #225, #251 and #3, #AH11 and #MJ13 (Supplemental Table 2). Concordance in the age of cardiomyopathy onset was observed among siblings. All data were analyzed in SPSS (v15).
Results
Patient selection and description
A total of 118 BMD and XLDCM patients (Supplemental Tables 2 and 3) were enrolled. Table 1 shows the breakdown of patients based on diagnosis, source, and whether they were categorized as cardiomyopathic or non-cardiomyopathic. Only subjects with cardiomyopathy (n=78) were required to test our hypothesis that the age of DCM manifestation is associated with deletion of specific dystrophin protein domains. However, we did analyze non-cardiomyopathic patients for evidence of a cardio-protective effect of some deletion mutations. We found that the non-cardiomyopathic BMD patients were significantly younger than cardiomyopathic BMD patients (p<0.001) and that their deletion mutations overlap with those of cardiomyopathic patients (Supplemental Figure 1). This suggests that non-cardiomyopathic patients were too young to manifest cardiac involvement and will require follow-up studies to further test the hypothesis under consideration.
Table 1. Patient distribution according to diagnosis, source of information and categorization as affected or not with DCM.
The median age in years of the patients in each category is indicated as well as the number of patients (N).
| Cardiomyopathic | Non-Cardiomyopathic | |||||
|---|---|---|---|---|---|---|
| Diagnosis | Source | N | Median Age* | N | Median Age† | N |
| XLDCM | Publication | 17 | 30 | 17 | - | - |
| BMD | Publication | 59 | 29.5 | 38 | 18 | 21 |
| BMD | UDP | 42 | 33 | 23 | 13 | 19 |
Youngest age when fulfilling criteria for a diagnosis of cardiomyopathy.
Oldest age at which cardiac function was found to be within normal parameters.
Distribution of mutations relative to dystrophin protein structure and diagnosis
To determine whether all cardiomyopathic patients can be combined for maximum statistical power, we first tested whether the source of patient information (published versus UDP) or the diagnosis (BMD versus XLDCM) influences the age of cardiomyopathy onset. No significant effect was found (p>0.9). Therefore the UDP patient population is comparable to published case reports with respect to age, and XLDCM patients did not differ from cardiomyopathic BMD patients in their median age of cardiac involvement.
Next, we mapped the location of deletion mutations of cardiomyopathic patients to determine whether BMD and XLDCM patients differ in the affected dystrophin protein domains. The deletion mutations found in these patients clustered around two dystrophin protein regions : the amino-terminal domain corresponding to exons 2 to 7, and a region in the rod domain centered around hinge 3, corresponding to exons 45 to 55 (Figure 1). This distribution is in agreement with previous reports on mutation hotspots for BMD patients 15, 16. Deletions found in XLDCM patients overlapped or in some cases were identical to those reported for BMD patients. Thus, XLDCM and BMD patients do not segregate into separate groups based on deletion mutation site or age of DCM manifestations. Taken together, these results indicate that patients can be combined for statistical analyses regardless of diagnosis or source of information.
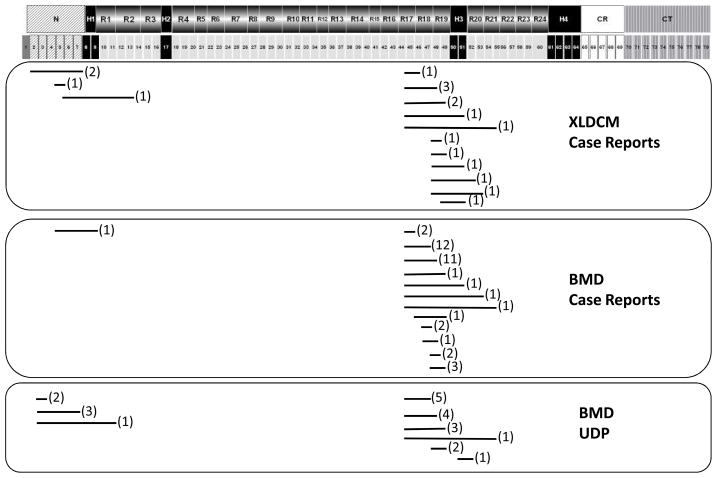
Figure 1. Mapping of deletions mutations in cardiomyopathic patients.
Dystrophin protein domains and corresponding exons are schematically represented at the top. Deleted regions are indicated below by a line. The number of patients with any given deletion mutation is indicated in parenthesis. Patients are grouped based on diagnosis and source. Dystrophin domains: N= amino terminus (diagonal stripes); R= spectrin repeats 1 through 24 (grey); H=hinges 1 through 4 (black); CR=cysteine rich domain (white); CT=carboxyl terminus (vertical stripes).
Description of patient groups
Group 1 (Figure 2A) includes 11 patients with deletions affecting exons 2 to 9 coding for the actin-binding amino-terminal domain of dystrophin. No information on dystrophin expression in the myocardium of these patients is available.
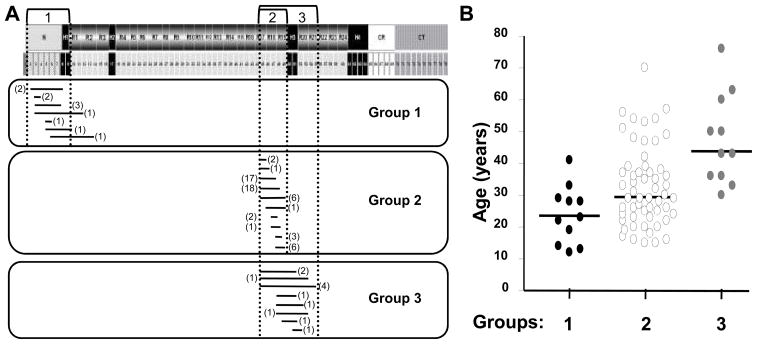
Figure 2. Grouping of deletion mutations based on the affected protein domain.
A. Top: Schematic representation of dystrophin protein domains and corresponding exons. Three patient groups are indicated in relation to dystrophin. Bottom: Mapping of deletion mutations for cardiomyopathic patients falling within each group. Number of patients is shown in parenthesis.
B. Dot plot of the age distribution of cardiomyopathic patients in each group. Bars indicate median ages. Median age of DCM onset is significantly different between each group (P<0.016, Mann–Whitney U test), except groups 1 and 2.
Group 2 represents the majority of patients (67%) and involves deletions affecting exons 45 to 49 (spectrin repeats 17 to 19) that preserve hinge 3 of the dystrophin protein (Figure 2A). This group comprises 56 cardiomyopathic patients and shows the broadest age range of all three groups, with most patients falling between 15 and 55 years of age. A single outlier (#161, Supplemental Table 2) was diagnosed at age 70 with an ejection fraction of 27% suggestive of advanced disease. Cardiac biopsy information was available for eight patients from published case reports (Supplemental Table 2). Dystrophin staining could be detected in the myocardium but was often fainter than in control tissues and was discontinuous along the cardiomyocyte membrane. Group 3 includes 11 patients with deletions between exons 45 and 55 that remove or disrupt hinge 3 (Figure 2A). Of note, none of the patients was younger than 30 years. Cardiac biopsies were available for four patients (Supplemental Table 2) and showed reduced levels of dystrophin expression with a discontinuous pattern along the cardiomyocyte membrane.
Association of deletion mutations with DCM
Group 1 patients had the earliest age of DCM manifestations (median: 23 years) followed by Group 2 patients (median: 29.5 years) (Figure 2B). Group 3 patients developed DCM later in the course of the disease (median: 43 years) (Figure 2B). A significant difference was detected among the groups (p<0.001, Kruskal-Wallis test) and for all post-hoc pair-wise comparisons (p<0.016) except between Groups 1 and 2 (p=0.03). Thus, BMD patients with deletions that lie within exons 45 to 55 resulting in a dystrophin protein lacking hinge 3 have a significantly later DCM onset compared to patients with overlapping deletions that preserve hinge 3 or with mutations affecting the amino-terminal region of dystrophin. By contrast, patients with deletion mutations affecting exons 2 to 9 or exons 45 to 49 are at risk of developing DCM in their second and third decades of life, respectively.
Disruption of spectrin repeat phasing results in early DCM
Since Group 2 patients showed the widest age range, we further investigated whether a second factor could be responsible for this heterogeneity. Prior studies focusing on this region have suggested a potential association of deletions of exons 48 and/or 49 with a more severe cardiomyopathy 2, 4. Sub-dividing Group 2 patients based on the presence/absence of exons 48 and/or 49 did not distinguish two sub-populations with significantly different ages of DCM onset (p>0.2).
One mechanism by which genotype can influence the age of DCM manifestations is by causing protein structure re-arrangements that are more or less compatible with the cellular functions of dystrophin. Prior evidence in mice has shown that the phasing of the dystrophin spectrin repeats affects function in skeletal muscle 17. Exons 45 to 49 code for spectrin repeats 17 (partial) to 19. Since exon boundaries do not correlate with the physical boundaries of individual spectrin repeats at the protein level, different combinations of exon deletions could affect spectrin repeat phasing. For each Group 2 mutation, the amino acid sequence of dystrophin was analyzed to assess whether the deleted sequence would disrupt (out-of-phase) or preserve (in-phase) the known spectrin repeat pattern 18 (Supplemental Figure 2). Sub-dividing Group 2 patients based on phasing pattern revealed that disruption of spectrin repeat phasing is associated with significantly earlier onset of DCM (26 versus 36 years, Figure 3A).
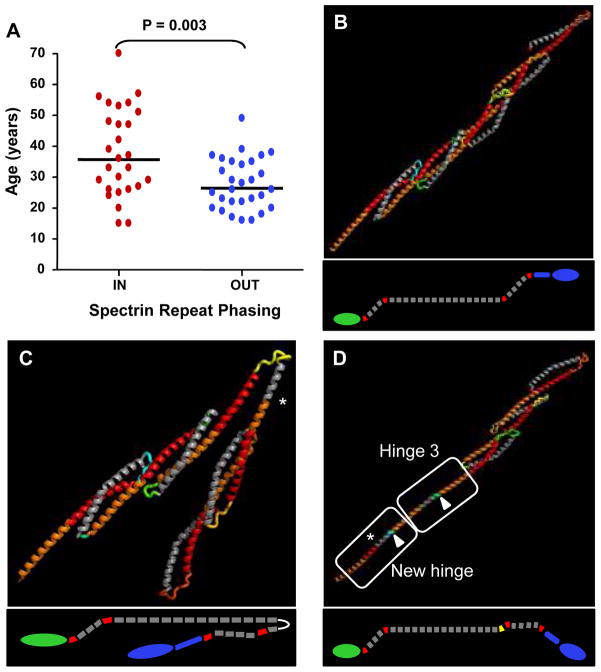
Figure 3. Disruption of spectrin repeat phasing is associated with an earlier DCM onset and is predicted to alter overall dystrophin structure.
A. Dot plot distribution of cardiomyopathic patient age versus spectrin repeat phasing. Out-of-phase mutations in Group 2 patients lead to a significantly earlier age of cardiac manifestations (26 years) compared to in-phase mutations (36 years; p=0.003). Bar indicates median age in years.
B. Modeling of a five-repeat segment of the rod domain of dystrophin highlighting interlocking of adjacent Helix 1 regions (orange-red) stabilized by a Helix 2 (grey). This arrangement results in a nested repeat structure and provides the rod domain with a relatively rigid backbone. The structure is oriented with the amino-terminus to the lower left, and the carboxyl-terminus to the upper right. Inset shows a diagram of the entire dystrophin protein. Grey: rod domain; red: hinges; green: amino terminus; blue: cysteine rich region and carboxyl terminus.
C. Model of the out-of-phase exon 45–47 deletion mutation. The structural alteration reverses the direction of extension of the Helix 1 and defines a new axis for the latter portion of the rod. The structure is shown in the folded position that may be energetically favored. Asterisk: deleted sequence site.
D. Model of the out-of-phase exon 48 deletion. This structural alteration recapitulates the sequence pattern of hinge 3, leading to an extended rod configuration. Highlighted in green (arrow) is a short stretch of amino acids that could adopt a range of structures, varying from an induced helix as shown through an unstructured loop to a structured turn which reverses the axis of the rod. Asterisk: deleted sequence site.
We further compared the age of DCM manifestations between all patient groups taking phasing into account (Table 2). No significant difference was detected in the age of DCM manifestations between Group 1 and Group 2 out-of-phase mutations. Thus, out-of-phase mutations in the rod domain and deletions in the actin-binding amino-terminal domain are both associated with early age cardiomyopathy. By contrast, Group 2 in-phase mutations led to a significantly later age of DCM manifestations compared to Group 1 patients but did not differ from Group 3 patients (Table 2). These results indicate that the effect of deletion mutations on the phasing pattern of spectrin repeats 17 to 19 is a strong determinant of DCM age of onset. Based on available information from cardiac biopsies, both in-phase and out-of-phase mutations result in expression of a mutant dystrophin protein in cardiomyocytes (Supplemental Table 2)19–23 suggesting that the observed difference in age of DCM is not due to obvious differences in the level of cardiac dystrophin expression.
Table 2. Pair-wise comparison of cardiomyopathic patient groups based on the location of the deletion mutation and the effects on spectrin repeat phasing.
The Mann-Whitney U test was used for post-hoc statistical comparisons among all 4 groups. Significance was set a priori at p=0.0125 (Bonferroni adjustment for overall significance of p=0.05). Significant p values are bolded and italicized. The median age of cardiac manifestations is indicated for each group.
| Groups | n | Median Age(years) | P value |
|---|---|---|---|
| Group 2 (in-phase) versus: | 27 | 36 | |
| Group 2 (out-of-phase) | 29 | 26 | 0.003 |
| Group 1 | 11 | 23 | 0.003 |
| Group 3 | 11 | 43 | 0.107 |
| Group 2 (out-of-phase) versus: | 29 | 26 | |
| Group 1 | 11 | 23 | 0.24 |
| Group 3 | 11 | 43 | <0.001 |
| Group 1 versus: | 11 | 23 | |
| Group 3 | 11 | 43 | <0.001 |
Effects of out-of-phase and in-phase deletions on dystrophin structure
To further investigate the mechanism by which phasing affects dystrophin function, we modeled the effects of deletion mutations on the rod domain of dystrophin for Group 2 mutations. Each spectrin repeat is composed of a long α-helix 1 connected to a shorter α-helix 2 by a flexible linker sequence (Supplemental Figure 2A). Figure 3B illustrates how the α-helices of the spectrin repeats interlock with each other forming a stable yet flexible rod-shaped structure. In-phase mutations remove one or more interlocking units and simply result in a shortening of the rod-domain (Supplemental Figure 2B). By contrast, out-of-phase mutations join the helices 1 and 2 together, removing the intervening linker sequence (Supplemental Figure 2C). This is predicted to result in potentially dramatic changes to the rod-domain that would affect the overall dystrophin structure. Based on our modeling, most out-of-phase mutations are predicted to bend the rod domain and reverse the directionality of the entire carboxyl-terminal part of dystrophin (Figure 3C). One exception is deletion of exon 48, which is predicted to introduce a new hinge adjacent to hinge 3 (Figure 3D). Such major alterations to the overall protein structure are likely to affect the function of dystrophin.
Discussion
An association of genotype with DCM has long been suspected but has not been clearly established 2–4, 24. In this study, we demonstrated that genotype is a determinant of the age of DCM manifestations in BMD and XLDCM patients. The large sample size (78 patients) and stringent selection criteria including only patients with small deletions (11 exons) enabled us to capture informative dystrophin protein domains. This allowed patient grouping based on dystrophin mutations affecting a single rather than multiple adjacent functional protein domains, thus increasing the statistical power of the study. We also provided novel evidence of a strong association of cardiomyopathy with specific structural alterations of the dystrophin rod domain. Prior studies reported contradictory findings on the potential link of deletions including exons 48 and/or 49 with severity and occurrence of DCM 2–4, 24. Analysis of our 56 patients in Group 2 showed that DCM in this region is more sensitive to altered phasing of the spectrin repeats rather than absence or presence of any one individual exon. Our analysis of the effects of specific deletions on the three-dimensional structure of dystrophin and their correlation with cardiac phenotype highlights the importance of integrating protein structure information in genotype-phenotype studies.
Our results also indicate that the rod domain of dystrophin may not be as permissive to alterations as previously thought. This study suggests that preservation of phasing delays the onset of DCM by about a decade. This is likely not due to a difference in expression of cardiac dystrophin protein since Arbustini et al. 20 reported similar amount and distribution of dystrophin in cardiac biopsy samples from BMD patients with either in-phase or out-of-phase mutations. Rather, our modeling suggests that the alterations caused by out-of-phase mutations extend beyond the spectrin repeat unit and may lead to a severely altered configuration of the rod domain, ultimately affecting the entire dystrophin protein. This major structural change is likely a main determinant of early onset DCM. Interestingly, most Group 2 BMD patients have late-onset skeletal muscle symptoms and mild disease progression, irrespective of the effects of their mutation on phasing. This is in agreement with studies in dystrophin-null mdx mice expressing a mini-dystrophin construct that lacks the exon 45 to 49 region but has an intact hinge 3 domain. In these mice only a partial restoration of cardiac function was achieved in spite of a complete rescue of the skeletal muscle pathology 25. Thus cardiac dystrophin may be particularly sensitive to structural disruptions of the exon 45 to 49 region compared to skeletal muscle dystrophin. The reasons for this disparity are currently unknown but highlight the importance of mapping domains of dystrophin essential for cardiac function to improve upon current treatment approaches relying on exon skipping or gene replacement with mini-/micro-dystrophin constructs.
This study provides expected median ages of onset of DCM associated with three distinct regions of the dystrophin protein and with specific re-arrangements of its rod-domain. The deletion mutations studied here are among the most frequent, rendering our findings relevant to most BMD and XLDCM patients. Of interest, within groups, XLDCM patients did not have an earlier age of cardiomyopathy compared to BMD patients. Instead, the earliest age of DCM is associated with mutations affecting the amino-terminal domain of the protein (early 20’s), and out-of-phase mutations in the exon 45 to 49 region of the dystrophin rod domain (mid 20’s). While cardiac expression of dystrophin has been confirmed in several out-of-phase Group 2 patients, such information is not available for Group 1 patients. The best studied mutations affecting the 5′ region of the dystrophin gene (including the muscle promoter, exon 1, or intronic regions that alter exon splicing 26–28) lead to a selective lack of cardiac dystrophin. While none of our Group 1 patients had mutations affecting non-coding regions or exon 1, we cannot exclude the possibility that the early DCM onset in Group 1 patients reflects a selective absence of cardiac dystrophin. Of note, the median age of XLDCM patients with lack of cardiac dystrophin (24 years for 6 independent families, Supplemental Table 1) is very similar to that of Group 1patients (23 years). Further studies are needed to determine the mechanism(s) by which deletion mutations in the amino-terminal region lead to earlier onset of cardiomyopathy compared to mutations affecting other regions. A greater awareness of the value of cardiac tissue sampling at the time of cardiac transplantation and the design of transgenic mdx mice mimicking human mutations could yield important information on cardiac-specific mechanisms regulating this region of dystrophin at a transcriptional and protein level.
Significantly later DCM onset is associated Group 2 in-phase (mid 30’s) and Group 3 mutations (mid 40’s). The cardio-protective effect of hinge 3 deletion seen in Group 3 patients mirrors findings reported for skeletal muscle in both mice and humans 6, 17. However, while loss of hinge 3 delays onset of cardiomyopathy, it correlated with slower disease progression in skeletal muscle but had no effect on age of onset 6. Due to the small number of Group 3 patients with identical deletions, we could not determine whether this partially protective effect is associated with a specific structural alteration of the dystrophin protein backbone. Further studies are needed to explain the significant cardio-protective effect conferred by the loss of hinge 3.
The median age of cardiac involvement for each patient group reported here is currently the best approximation available for this patient population. This information is valuable because cardiac involvement in BMD patients is often asymptomatic in its initial stages and can therefore be underestimated. Since genotyping has become a more common practice, the median ages reported here may prove valuable for individualized risk assessment, and for timely cardiac evaluation and intervention. An important next step is to conduct a large-scale longitudinal study to further refine the age of DCM onset associated with the dystrophin domains identified here. This information underscores the importance of genotype information in the cardiac care of BMD patients and bears relevance to the design of therapies aimed at the myocardium in BMD, XLDCM and DMD patients.
Supplementary Material
Acknowledgments
The authors acknowledge the input of the UDP Consortium including the following individuals: Brenda Wong at Cincinnati Children’s Hospital Medical Center, Richard Finkel, Carsten Bonnemann, and Livje Medne at Children’s Hospital of Philadelphia, Julaine Florence and Anne Connolly, Washington University, Katherine Mathews, University of Iowa, Jacinda Sampson, Mark Bromberg, and Kathryn J. Swoboda, University of Utah, and John W. Day, University of Minnesota. The authors thank Dr. Xiomara Rosales for her diagrams of the alignment of dystrophin exons with protein domains, and Brent Yetter for assistance in the identification of patients seen at MDA clinics who were suitable for this study. We are grateful for editing assistance from Drs. Carlos Miranda, Jennifer Thomas-Ahner and Christopher Pierson, and for mentorship and support to author R.W.K. from Dr. Donna McCarthy, Professor of Nursing at The Ohio State University. We are indebted to Dr. Christopher Holloman from the College of Mathematical and Physical Sciences at The Ohio State University for assistance with statistical analysis.
Funding sources
Rita Wen Kaspar was supported by a grant from the NIH Roadmap Training Program in Clinical Research (T32-RR023260-03). The UDP project is supported by grants from the National Institute of Neurologic Diseases and Stroke (R01 NS043264) and the National Center for Research Resources (M01-RR00064, to the University of Utah, Dr. L. Betz, P.I.).
Footnotes
Disclosures
None.
References
- 1.Bushby K, Muntoni F, Bourke JP. Neuromuscul Disord; 107th ENMC international workshop: the management of cardiac involvement in muscular dystrophy and myotonic dystrophy; 7th–9th June 2002; Naarden, the Netherlands. 2003. pp. 166–172. [DOI] [PubMed] [Google Scholar]
- 2.Nigro G, Politano L, Nigro V, Petretta VR, Comi LI. Mutation of dystrophin gene and cardiomyopathy. Neuromuscul Disord. 1994;4:371–379. doi: 10.1016/0960-8966(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 3.Melacini P, Fanin M, Danieli GA, Villanova C, Martinello F, Miorin M, Freda MP, Miorelli M, Mostacciuolo ML, Fasoli G, Angelini C, Dalla Volta S. Myocardial involvement is very frequent among patients affected with subclinical Becker’s muscular dystrophy. Circulation. 1996;94:3168–3175. doi: 10.1161/01.cir.94.12.3168. [DOI] [PubMed] [Google Scholar]
- 4.Melacini P, Fanin M, Danieli GA, Fasoli G, Villanova C, Angelini C, Vitiello L, Miorelli M, Buja GF, Mostacciuolo ML, Pegoraro E, Della Volta S. Cardiac involvement in Becker muscular dystrophy. J Am Coll Cardiol. 1993;22:1927–1934. doi: 10.1016/0735-1097(93)90781-u. [DOI] [PubMed] [Google Scholar]
- 5.Jefferies JL, Eidem BW, Belmont JW, Craigen WJ, Ware SM, Fernbach SD, Neish SR, Smith EOB, Towbin JA. Genetic Predictors and Remodeling of Dilated Cardiomyopathy in Muscular Dystrophy. Circulation. 2005;112:2799–2804. doi: 10.1161/CIRCULATIONAHA.104.528281. [DOI] [PubMed] [Google Scholar]
- 6.Carsana A, Frisso G, Tremolaterra MR, Lanzillo R, Vitale DF, Santoro L, Salvatore F. Analysis of dystrophin gene deletions indicates that the hinge III region of the protein correlates with disease severity. Ann Hum Genet. 2005;69:253–259. doi: 10.1046/j.1529-8817.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- 7.Beggs AH, Hoffman EP, Snyder JR, Arahata K, Specht L, Shapiro F, Angelini C, Sugita H, Kunkel LM. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am J Hum Genet. 1991;49:54–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Comi GP, Prelle A, Bresolin N, Moggio M, Bardoni A, Gallanti A, Vita G, Toscano A, Ferro MT, Bordoni A, Fortunato F, Ciscato P, Felisari G, Tedeschi S, Castelli E, Garghentino R, Turconi A, Fraschini P, Marchi E, Negretto GG, Adobbati L, Meola G, Tonin P, Papadimitriou D, Scarlato G. Clinical variability in Becker muscular dystrophy. Genetic, biochemical and immunohistochemical correlates. Brain. 1994;117 (Pt 1):1–14. doi: 10.1093/brain/117.1.1-a. [DOI] [PubMed] [Google Scholar]
- 9.Yazaki M, Yoshida K, Nakamura A, Koyama J, Nanba T, Ohori N, Ikeda S. Clinical characteristics of aged Becker muscular dystrophy patients with onset after 30 years. Eur Neurol. 1999;42:145–149. doi: 10.1159/000008089. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura A, Yoshida K, Fukushima K, Ueda H, Urasawa N, Koyama J, Yazaki Y, Yazaki M, Sakai T, Haruta S, Takeda S, Ikeda S. Follow-up of three patients with a large in-frame deletion of exons 45–55 in the Duchenne muscular dystrophy (DMD) gene. J Clin Neurosci. 2008;15:757–763. doi: 10.1016/j.jocn.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, Province MA. Clinical investigation in Duchenne dystrophy: 2. Determination of the “power” of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 12.Duboc D, Meune C, Lerebours G, Devaux JY, Vaksmann G, Becane HM. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J Am Coll Cardiol. 2005;45:855–857. doi: 10.1016/j.jacc.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 13.Thrush PT, Allen HD, Viollet L, Mendell JR. Re-examination of the electrocardiogram in boys with Duchenne muscular dystrophy and correlation with its dilated cardiomyopathy. Am J Cardiol. 2009;103:262–265. doi: 10.1016/j.amjcard.2008.08.064. [DOI] [PubMed] [Google Scholar]
- 14.Kusunoki H, Minasov G, Macdonald RI, Mondragon A. Independent movement, dimerization and stability of tandem repeats of chicken brain alpha-spectrin. J Mol Biol. 2004;344:495–511. doi: 10.1016/j.jmb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Worton RG, Thompson MW. Genetics of Duchenne muscular dystrophy. Annu Rev Genet. 1988;22:601–629. doi: 10.1146/annurev.ge.22.120188.003125. [DOI] [PubMed] [Google Scholar]
- 16.Worton RG, Molnar MJ, Brais B, Karpati G. The muscular Dystrophies. In: Scriver CR, Beaudet A, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. McGraw Hill; 2001. pp. 5493–5523. [Google Scholar]
- 17.Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, Chamberlain JS. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 18.Cross RA, Stewart M, Kendrick-Jones J. Structural predictions for the central domain of dystrophin. FEBS Lett. 1990;262:87–92. doi: 10.1016/0014-5793(90)80160-k. [DOI] [PubMed] [Google Scholar]
- 19.Maeda M, Nakao S, Miyazato H, Setoguchi M, Arima S, Higuchi I, Osame M, Taira A, Nomoto K, Toda H, Tahara M, Atsuchi Y, Tanaka H. Cardiac dystrophin abnormalities in Becker muscular dystrophy assessed by endomyocardial biopsy. Am Heart J. 1995;129:702–707. doi: 10.1016/0002-8703(95)90319-4. [DOI] [PubMed] [Google Scholar]
- 20.Arbustini E, Diegoli M, Morbini P, Dal Bello B, Banchieri N, Pilotto A, Magani F, Grasso M, Narula J, Gavazzi A, Vigano M, Tavazzi L. Prevalence and characteristics of dystrophin defects in adult male patients with dilated cardiomyopathy. J Am Coll Cardiol. 2000;35:1760–1768. doi: 10.1016/s0735-1097(00)00650-1. [DOI] [PubMed] [Google Scholar]
- 21.Politano L, Passamano L, Petretta VR, Nigro V, Papparella S, Nigro G, Santangelo L, Esposito MG, Come LI, Nigro G. Familial Dilated Cardiomyopathy Associated with the Typical Dystrophin BMD Mutation: Report on Two Additional Cases. Acta Myol. 1999:3329–3336. [Google Scholar]
- 22.Muntoni F, Di Lenarda A, Porcu M, Sinagra G, Mateddu A, Marrosu G, Ferlini A, Cau M, Milasin J, Melis MA, Marrosu MG, Cianchetti C, Sanna A, Falaschi A, Camerini F, Giacca M, Mestroni L. Dystrophin gene abnormalities in two patients with idiopathic dilated cardiomyopathy. Heart. 1997;78:608–612. doi: 10.1136/hrt.78.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fanin M, Melacini P, Angelini C, Danieli GA. Could utrophin rescue the myocardium of patients with dystrophin gene mutations? J Mol Cell Cardiol. 1999;31:1501–1508. doi: 10.1006/jmcc.1999.0987. [DOI] [PubMed] [Google Scholar]
- 24.Politano L, SC-R, Esposito MG, Nigro V, Comi LI, Passamano L, Nigro G. Genotype-phenotype correlation in patients with deletions of Duchenne/Becker gene. Acta Cardiomyologica. 1991;3:239–244. [Google Scholar]
- 25.Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, Duan D. Cardiac Expression of a Mini-dystrophin That Normalizes Skeletal Muscle Force Only Partially Restores Heart Function in Aged Mdx Mice. Mol Ther. 2008 doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milasin J, Muntoni F, Severini GM, Bartoloni L, Vatta M, Krajinovic M, Mateddu A, Angelini C, Camerini F, Falaschi A, Mestroni L, Giacca M. A point mutation in the 5′ splice site of the dystrophin gene first intron responsible for X-linked dilated cardiomyopathy. Hum Mol Genet. 1996;5:73–79. doi: 10.1093/hmg/5.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Muntoni F, Cau M, Ganau A, Congiu R, Arvedi G, Mateddu A, Marrosu MG, Cianchetti C, Realdi G, Cao A, Melis MA. Brief report: deletion of the dystrophin muscle-promoter region associated with X-linked dilated cardiomyopathy. N Engl J Med. 1993;329:921–925. doi: 10.1056/NEJM199309233291304. [DOI] [PubMed] [Google Scholar]
- 28.Muntoni F, Wilson L, Marrosu G, Marrosu MG, Cianchetti C, Mestroni L, Ganau A, Dubowitz V, Sewry C. A mutation in the dystrophin gene selectively affecting dystrophin expression in the heart. J Clin Invest. 1995;96:693–699. doi: 10.1172/JCI118112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.