Abstract
Highly attenuated rabies virus (RV) vaccine vectors were evaluated for their ability to protect against highly pathogenic SIVmac251 challenge. Mamu-A*01 negative rhesus macaques were immunized in groups of four with either: RV expressing SIVmac239-GagPol, a combination of RV expressing SIVmac239-Env and RV expressing SIVmac239-GagPol, or with empty RV vectors. Eight weeks later animals received a booster immunization with a heterologous RV expressing the same antigens. At twelve weeks post-boost, all animals were challenged intravenously with 100 TCID50 of pathogenic SIVmac251-CX. Immunized macaques in both vaccine groups had 1.3–1.6-log fold decrease in viral set point compared to control animals. The GagPol/Env immunized animals also had a significantly lower peak viral load. When compared to control animals following challenge, vaccinated macaques had a more rapid induction of SIVmac251 neutralizing antibodies and of CD8+ T cell responses to various SIV epitopes. Moreover, vaccinated macaques better-maintained peripheral memory CD4+ T cells and were able to mount a poly-functional CD8+ T cell response in the mucosa. These findings indicate promise for RV-based vectors and have important implications for the development of an efficacious HIV vaccine.
Keywords: rabies virus, HIV-1, vaccine, SIVmac251, rhabdovirus
INTRODUCTION
The HIV-1 pandemic has persisted for over two decades and little headway has been made in developing an effective HIV-1 vaccine. A variety of approaches to develop an HIV-1 vaccine have been attempted (for review see [1]), although the use of recombinant adenovirus serotype 5 (Ad5)-based vectors expressing HIV-1 genes have been the most common. Whereas vaccine studies with replication deficient Ad5 vectors and simian-human immunodeficiency virus (SHIV) looked promising in rhesus macaque models, a similar Ad5-based vaccine failed to induce protective immune responses in a large clinical study in humans [2, 3]. Despite this discouraging outcome and based on the finding that attenuated SIV can protect animals from a lethal challenge, a live viral vaccine seems to be the most promising candidate for protection against HIV-1 [4].
Rabies virus (RV) is an enveloped non-segmented negative strand RNA virus. In its attenuated form, RV-based vaccine vectors have been proven to be safe and effective [5–8]. RV has a relatively simple genome organization encoding five structural proteins: nucleoprotein, phosphoprotein, matrix protein, glycoprotein (G), and an RNA-dependent RNA polymerase. The RV lifecycle is exclusively cytoplasmic, thus abolishing concerns that the virus’ genetic material will integrate into the host cell genome.
We previously showed that a combination of RV-based vaccines expressing either SIVmac239 Gag or SHIV89.6P Env can protect against SHIV89.6P challenge [5]. Although the SHIV89.6P challenge model in rhesus macaques provides insight into the validity of the vaccine strategy, SIVmac251 infection of rhesus macaques induces a progressive disease and pathology more similar to human infection with HIV-1 [9].
In the present study, we investigate the efficacy of two vaccine strategies: immunization with either a recombinant RV expressing SIVmac239 GagPol or a combination of RV expressing SIVmac239 GagPol and RV expressing SIVmac239 Env. We see decreased viral set points in vaccinated animals as compared to the controls. Additionally, we observe RV-based vaccines induce neutralizing antibody production, CD8+ T cell responses, and increased protection in both vaccine cohorts.
METHODS
Recombinant Vaccine Vectors
The RV vaccine strains SPBN-333 and SPBN-IG, have been described previously [5, 7]. SIVmac239GagPol or SIVmac239Env were amplified by polymerase chain reaction (Vent, Biolabs Inc.) from p239SpSp5’ [10]. The SIVmac239Env cytoplasmic domain (CD) was replaced with that of RV G. SIVmac239GagPol or SIVmac239Env-RVG-CD where then cloned into pSPBN-333 or pSPBN-IG utilizing the BsiWI and NheI restriction sites (Figure 1A). Sequences were confirmed by DNA sequencing. Infectious RVs were recovered by standard methods [7] and designated RV-333-GagPol, RV-333-Env, RV-IG-GagPol, or RV-IG-Env (Fig. 1).
Figure 1. Rabies virus vectors and immunization schedule.
(A) Timeline of experimental design: Rhesus macaques were primed i.m. at week 0 and boosted i.m. at week 8. All animals were challenged i.v. at week 20 with SIVmac251. (B) Schematics of the recombinant RVs used for the prime and boost immunizations.
Animals and Vaccination
A total of 12 rhesus macaques (Macaca mulatta; 10 male, 2 female) between 2–8 years old were used in this study. All animals were housed at the Tulane National Primate Research Center in accordance with the regulations of the American Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and all experiments were reviewed and approved by the Tulane Institutional Animal Care and Use Committee. Monkeys were screened for the presence of the Mamu-A*01, Mamu-A*02, Mamu-A*08, Mamu-A*11, Mamu-B*01, Mamu-B*03, Mamu-B*04, Mamu-B*08, Mamu-B*17, and DR2011 alleles using a PCR-based technique as previously described [11].
Animals were immunized in three groups of four macaques. On day 0 of the study, animals were immunized intramuscularly with: (1) 108 foci forming units (ffu) RV-333-GagPol, (2) 108 ffu RV-333-GagPol and 108 ffu RV-333-Env, or (3) 108 ffu RV-333. On week 8 of the study, animals were intramuscularly boosted with heterologous viruses: (1) 108 ffu RV-IG-GagPol, (2) 108 ffu RV-IG-GagPol and 108 ffu RV-IG-Env, or (3) 108 ffu RV-IG. On week 20 of the study animals were challenged intravenously with 100 TCID50 of SIVmac251 i.v. [12].
Tissue Collection
Peripheral blood and intestinal lymphocytes were collected at various time points throughout the course of the study. PBMC samples were obtained from heparinized and EDTA anticoagulated blood samples at each time point (−4, 2, 6, 8, 10, 14, 20, 22, 24, 26, 28, 32, 36, 40, 44, 48, 52, and 56 weeks). Intestinal lamina propria lymphocytes (LPL) were obtained from jejunal pinch biopsies collected by endoscopy at study weeks −4, 6, 20, 22, 32, 44, and 52 [13, 14].
Flow cytometry
Intracellular cytokine staining was performed as described previously [15, 16]. Briefly, mononuclear cells were collected from peripheral blood or jejunum LPL, and 1×106 cells were stimulated with peptides (15-mer with 11 amino acid overlap from the NIH AIDS Research & Reference Reagent Program) derived from SIV-Gag (Cat# 6204), SIV-Env (Cat# 6883) or SIV-Pol (Cat# 6443) in the presence of 0.5 µg/ml of -CD28 and α-CD49d. Stimulation was done at 37° C for 1 hour prior to adding 10 µg/ml Brefeldin A (Sigma) and then for an additional 5 hours. Positive and negative control cells were stimulated with PMA/ Ionomycin (Sigma) and media, respectively. Following stimulation, the cells were stained with fluorescently labeled α-CD3, α-CD4 and α-CD8, α-CD28, α-CD95, α-CD45RA and α-CCR5 at 25° C for 25 min and then fixed and permeabilized with Fixation/Permeabilization solution (BD Biosciences). After permeabilization, cells were stained with fluorescently labeled α-IFN-γ·, α-TNF-α, α-IL-2 and α-MIP1-β at 25°C for 25 min. Cells were suspended in 300 µl of 1X Stabilizing Fixative buffer (BD Biosciences) and analyzed with a BD LSRII System.
Quantitation of plasma viral RNA
Viral RNA in plasma was quantified by a commercial bDNA signal amplification assay specific for SIV [17].
Vector neutralizing antibodies
Rabies virus: Neutralizing antibody titers were determined with a CVS-11 reference strain and transformed into international units using the World Health Organization’s anti-rabies virus antibody standard as described previously [5]. Vesicular stomatitis virus: The neutralizing antibody titers were determined with the SPBN-IG reference strain and reported as the serum dilution that achieved 50% reduction in foci-forming units of input virus as described previously [5]. Simian immunodeficiency virus (SIVmac251): Neutralization of a T cell line adapted stock of SIVmac251 (TCLA-SIVmac251) was measured by using 5.25.EGFP.Luc.M7 (M7-Luc) cells (kindly provided by Dr. Nathaniel R. Landau) as previously described [18]. The M7-Luc cell line is a CEMx174 cell clone that was produced by retroviral vector transduction to express CCR5 (CD4 and CXCR4 are expressed naturally) and transfection to contain Tat-responsive luciferase (Luc) and green fluorescence protein (GFP) reporter genes [19]. The assay stock of TCLA-SIVmac251 was produced in H9 cells and titrated in M7-Luc cells. Briefly, a 500 tissue culture infectious dose 50 (TCID50) of virus was incubated with serial dilutions of serum samples in triplicate for 1 hr at 37°C. Then, 5×104 cells M7-Luc cells were added to each well. One set of control wells received cells and virus (virus control) and another set received cells only (background control). The plates were incubated until approximately 10% of cells in virus control wells were positive for GFP expression by fluorescence microscopy (approximately 3 days). Alternatively, neutralization of an SIVmac239 Env-pseudotyped virus (clone 23) was measured as a reduction in luciferase reporter gene expression after a single round of infection in TZM-bl cells (NIH AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu) as previously described [18, 20]. The assay stock of SIVmac239.18 was prepared by transfection in 293T cells and was titrated in TZM-bl cells as previously described [18, 20]. Briefly, 200 TCID50 of virus was incubated with serial 3-fold dilutions of serum sample in duplicate for 1 hr at 37° C. Then, 10,000 freshly trypsinized cells were added to each well. One set of control wells received cells and virus (virus control) and another set received cells only (background control). The plates were incubated for 48 hours. Following incubation with either TCLA-SIVmac251 or SIVmac239.18, luminescence was measured using the Britelite Luminescence Reporter Gene Assay System (PerkinElmer Life Sciences). Neutralization titers are the dilution at which relative luminescence units (RLU) were reduced by 50% compared to virus control wells after subtraction of background RLUs.
Statistics
Viral load trajectories: Log (base 10) transformed viral load trajectories were analyzed in the framework of NLME [21]. Animal-specific trajectories were modeled as the sum of a linear function with intercept (A), slope (B) (representing long-term behavior) and hyperbolic function (C) over Day (representing sharp decline in the viral load after 2 weeks post-challenge). The common NLME model (based on data from all animals) included random effects of animal incorporated into parameters A and B of animal-specific trajectories. The differences between the groups of trajectories were incorporated into the model as fixed effects. The interest was focused on the long-term behavior, and the group average slopes of the long-term linear trend were compared between the groups using the estimates from the fitted NLME model. CD4+ T cell count trajectories: Log (base 10) transformed CD4+ T cell counts were analyzed by fitting a LME model [22]. The linear time trends in log transformed CD4+ T cell trajectories were modeled with the slopes and intercepts varying between the groups, incorporating random effects of animal, and adjusting for the baseline CD4+ T cell counts computed as the average log transformed CD4+ T cell count before the challenge. Long-term SIVmac251 antibody trajectories: Log (base 10) transformed antibodies measures at 28 weeks or later were analyzed by fitting a LME model [22], similar to the CD4+ T cell counts, except for the baseline adjustment. In addition, the model adjusted for the difference between the two experiments (blocking factor).
In addition, the overall comparison of viral loads in three groups at 2 and 16 weeks after challenge were performed using the Kruscall-Wallis test. Wilcoxon two-sample test was used for the corresponding paired comparisons. Due to the small sample size for these analyses (12 animals for the overall and 8 animals for the paired group comparison), the exact versions of the Kruscall-Wallis and Wilcoxon test were used. Also, for weeks 6 to 22, separate overall comparison of SIVmac251 NAb in three groups were performed using the exact Kruscall-Wallis test and p-values were a djusted for multiple testing using the Hommel's closed testing procedure [23]. Exact Wilcoxon two-sample test was used for the corresponding paired comparisons. The data at −4 and 2 weeks were not analyzed because 20 of 24 values were below the detection limit.
RESULTS
Vaccination regimen of rhesus macaques
Twelve Indian-origin rhesus macaques were immunized in two independent experiments. All animals were MHC typed [11], and all were determined to be Mamu-A*01 negative. However, one control animal, CJ15, was positive for the MHC class I allele Mamu-B*17. It is well documented that the presence of the Mamu-B*17 allele reduces plasma viremia and allows elite control of virus [24, 25]. Despite this, CJ15 data was included in all statistical analyses unless otherwise indicated.
Each animal was given two immunizations (Fig. 1A). The first was an attenuated replication-competent RV vector and the second, eight weeks later, was a heterologous RV containing vesicular stomatitis virus (VSV) G instead of RV G (Fig. 1B). The animals were challenged i.v. with 100 tissue culture infectious dose (TCID50) of pathogenic SIVmac251 twenty weeks after the initial immunization (Fig. 1A).
To determine whether or not immunization with multiple SIV genes would improve the efficacy of RV-based vaccine vectors, we used two different vaccine regimens. The first group of animals (n=4) was primed and boosted with vectors that expressed SIVmac239GagPol (GagPol). The second group (n=4) was primed and boosted with two vectors, one that expressed SIVmac239 GagPol and one that expressed SIVmac239 Env (GagPol/Env). The control group (n=4) was immunized and boosted with empty vectors.
Target cell population frequency and level of viremia following challenge with SIVmac251
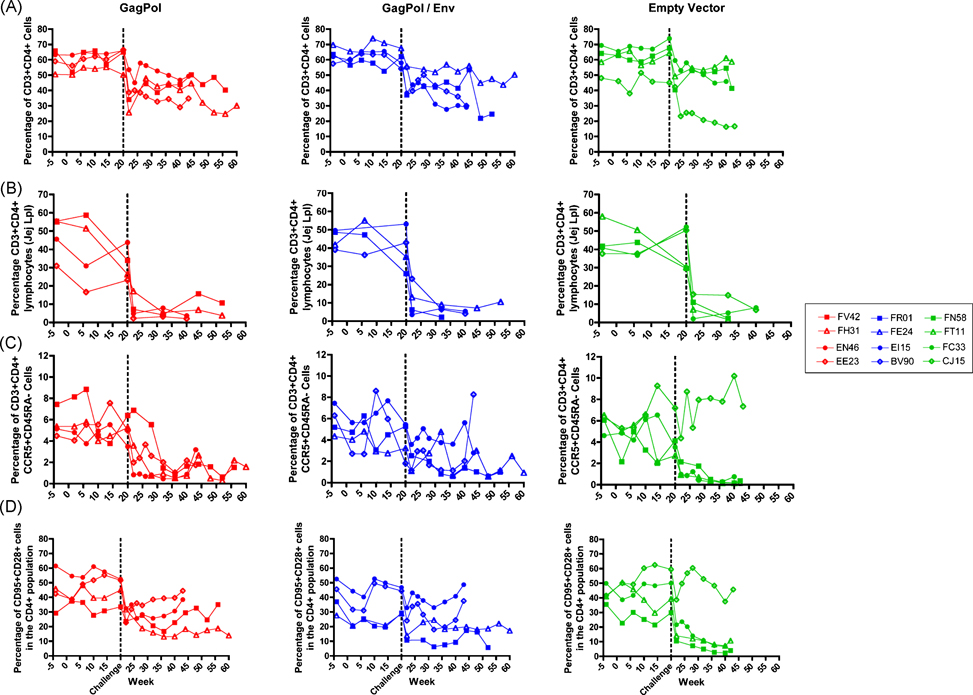
Twelve weeks after the second immunization with RV-based vaccines, the animals were challenged with a pathogenic SIV strain. In order to monitor disease progression, the percentage of CD4+ T cells in peripheral blood mononuclear cells (PBMCs) and intestinal biopsies was analyzed over time. In PBMCs, we detected a rapid loss of CD4+ T cells in all animals at 2 weeks post challenge. However, the population stabilized by 6 weeks post challenge (Fig. 2A). In order to determine significant trends in this data, group average estimates from a linear mixed effects (LME) model for CD4+ T cell counts were performed [22]. No significant differences in the slopes or intercepts of the CD4 trajectories were found between the groups. When observing the percentage of CD3+CD4+ cells in the small intestinal lymphocyte population, we saw a rapid and profound loss of CD4+ T cells after challenge in all groups (Fig. 2B). This data indicates that vaccination was not able to protect against the initial loss of CD4+ cells.
Figure 2. CD4+ T lymphocyte counts in the three immunization groups over time.
The change in percentage of CD4+ T cells was monitored in the CD3+ PBMC (A) or in the CD3+ jejunal lymph node (B) populations. The population of CCR5+CD45RA- memory cells (C) and central memory CD95+CD28+ cells (D) in the CD4+CD3+ PBMC population was also monitored.
It is known that HIV predominantly infects memory CD4+ T cells [26] and the maintenance of CD4+ memory cells is associated with a better disease outcome [27]. Therefore, we monitored the loss of CD4+ CD45RA- (memory) cells. As shown in Figure 2C, following challenge, there was a decrease in the percentage of CD45RA-CCR5+ target cells in blood in all animals except the control animal CJ15. In control animals, the percentage of CD45RA-CCR5+ cells continuously decreased after challenge, while vaccinees had more variability in the level of target cell depletion (Fig. 2C). The preservation of memory cells in vaccinated macaques was also seen when looking at the CD4+ central memory cells (CD95+CD28+); after an initial drop in the percentage of cells, there is a slight restoration (Fig. 2D). This fluctuation in target cells following challenge indicates that RV-based vaccines can contribute to the maintenance and/or restoration of memory CD4+ T cells.
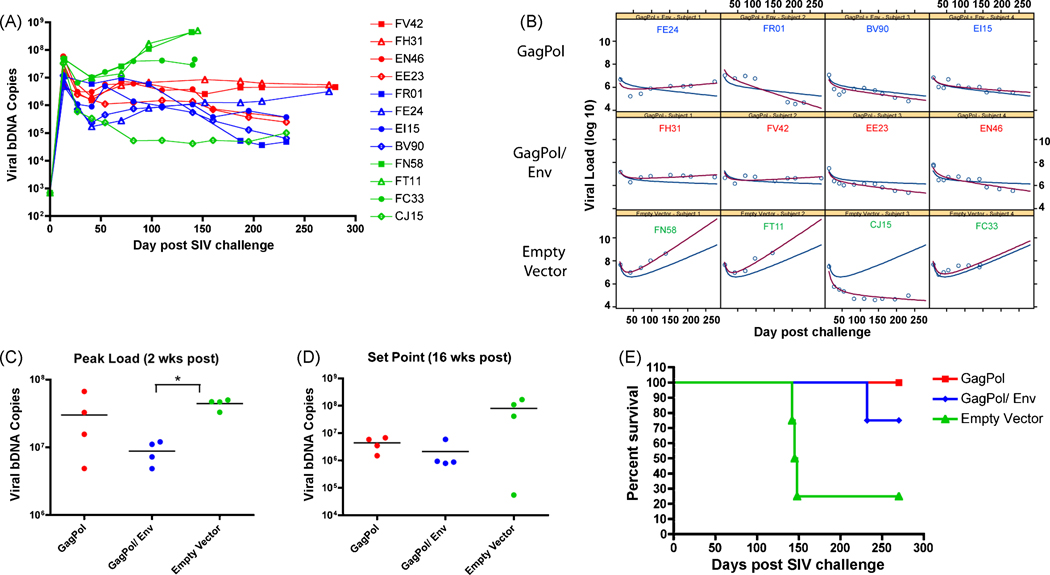
We also monitored challenge virus replication as a measure of vaccine-induced efficacy. As indicated by the drop in CD4+ cells, all animals became infected following challenge (Fig. 3A). In order to determine whether the overall trend of viral loads were different among vaccination regimens, we modeled the average parameter estimates for viral load intercept and slope with a non-linear mixed effects (NLME, Tab. 1). Notably, the empty vector group had positive long-term slope, while both GagPol and GagPol/Env groups had small negative slopes. The average long-term slope was significantly different between empty vector and GagPol (p=0.015) and between empty vector and GagPol/Env (p=0.007), Figure 3B.
Figure 3. SIV viral loads and survival of animals.
(A) Viral loads were sampled various days after challenge and viral bDNA copies are plotted over time. (B) A viral load trajectory was estimated from the NLME model for each group (blue line). The animal-specific viral load trajectories are also shown (red line). (C) Two weeks post SIVmac251 challenge, peak viral loads were compared for each immunization group. (D) SIV viral set point at 16 weeks post challenge was compared for each immunization group. (E) Percent survival of monkeys was also monitored in days post challenge. Comparison among groups was done by a two-sided Wilcoxon’s rank-sum test and (*) indicates a p-value of less than 0.05.
Table 1.
Parameter estimates for viral load and antibody models.
| Parameter | Group | Estimate | Standard Error |
Lower Confidence Limita |
Upper Confidence Limita |
|---|---|---|---|---|---|
| Interceptb | Empty Vector | 5.36 | 0.31 | 4.66 | 6.05 |
| GagPol | 6.36 | 0.26 | 5.79 | 6.93 | |
| GagPol/ Env | 6.07 | 0.26 | 5.49 | 6.65 | |
| Slopeb | Empty Vector | 0.0139 | 0.0038 | 0.0055 | 0.0223 |
| GagPol | −0.0009 | 0.0034 | −0.0084 | 0.0066 | |
| GagPol/ Env | −0.0031 | 0.0034 | −0.0106 | 0.0044 | |
| Hypreb weightb | Empty Vector | 28.05 | 4.22 | 18.66 | 37.45 |
| GagPol | 11.95 | 3.33 | 4.52 | 19.37 | |
| GagPol/ Env | 10.45 | 3.61 | 2.42 | 18.49 | |
| Interceptc | Empty Vector | 2.68 | 0.5 | 1.58 | 3.79 |
| GagPol | 4.78 | 0.49 | 3.68 | 5.88 | |
| GagPol/ Env | 4.95 | 0.49 | 3.85 | 6.05 | |
| Slopec | Empty Vector | −0.0082 | 0.0018 | −0.0121 | −0.0043 |
| GagPol | −0.001 | 0.0015 | −0.0046 | 0.0025 | |
| GagPol/ Env | −0.0006 | 0.0015 | −0.0041 | 0.003 |
95% confidence interval
Parameter description for NLME model of log (base 10) of transfored viral load data
Parameter description for LME model of log (base 10) of transformed SIVmac251-TCLA NAb titer (after week 28) data
The overall difference in peak levels of SIVmac251 titers (2 weeks post challenge) among the three groups was significant (p=0.037, Fig. 3C). Pair-wise comparisons showed that only the difference between empty vector and GagPol/Env animals was significant (p=0.029). When observing the viral set points (16 weeks post challenge), the GagPol and GagPol/Env immunized animals had 1.26-log or 1.58-log lower viral titers, respectively, than empty vector controls (Fig. 3D). However, no overall or pair-wise significant differences were found among viral loads at this time point. When the non-parametric analysis of viral set point values was repeated without Mamu-B*17 (+) CJ15, the overall difference among 3 groups is significant (p=0.011).
Lastly, when evaluating the overall survival of animals at 270 days post challenge, we detected a statistically significant increased rate of survival (p=0.0395) in GagPol and GagPol/Env immunized animals when compared to the empty vector immunized controls (Fig. 3E). Necropsy data indicated that the cause of death in all animals was AIDS-defining illnesses, namely encephalomyelitis, glomerulosclerosis, thrombosis, and pneumonia.
RV vaccine induced humoral immune responses in macaques
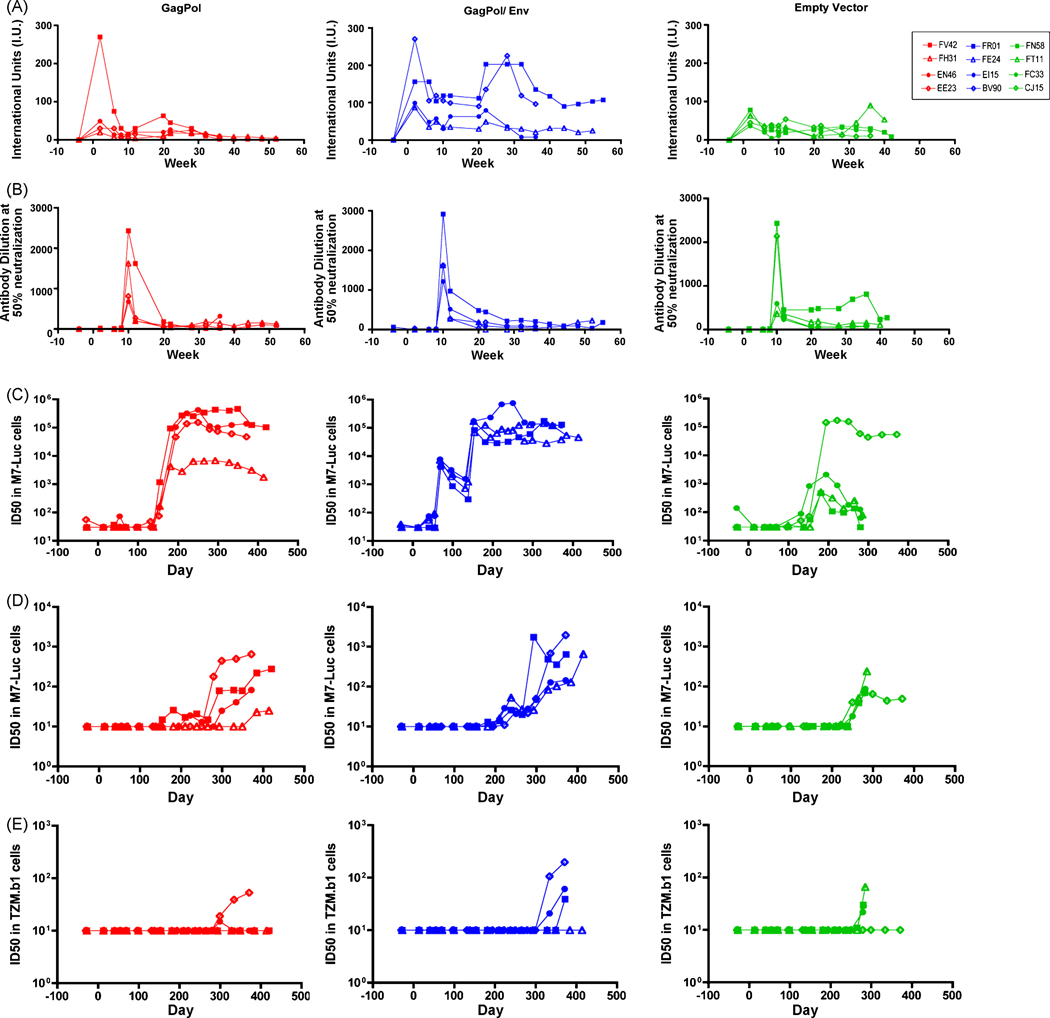
We monitored the humoral immune response of the immunized monkeys for both vector-specific (Fig. 4A–B) and SIV-specific antibodies (Fig. 4C–E). All immunization regimens induced strong neutralizing antibody (NAb) responses against RV two weeks following the initial immunization (Fig. 4A). Two weeks following the booster immunization with the chimeric RV-VSV vectors, we also detected high levels of anti-VSV Nab (Fig. 4B). The level of neutralizing antibodies against both vectors decreased over time; however, they were maintained at levels considered to prevent re-infection with the same vector.
Figure 4. Neutralization antibody titers for vectors and SIVmac251.
Serum from rhesus macaques was tested for the presence of neutralizing antibodies throughout the course of this study. Neutralizing antibody titers for RV (A) and VSV-G (B) are shown here for each animal. Neutralization of TCLA SIVmac251 (C) or SIVmac239-CX (D) was measured using 5.25.EGFP.Luc.M7 cells. Alternatively, neutralization of SIVmac239 (clone 23) was measured on TZM-bl cells (E). Titers are indicated by the dilution at which a 50% reduction was seen as compared to the control virus.
We also quantified the NAb titer against SIVmac251-TCLA, SIVmac251-CX (challenge virus) and SIVmac239. Prior to SIVmac251 challenge, we detected no NAb against SIVmac251-CX or SIVmac239 (Fig. 4D–E). However, the vaccine regimen that included the RV-expressing SIVmac239 Env did induce NAb response against SIVmac251-TCLA two weeks after boost, reaching titers as high as 7.7 × 103 (Fig. 4C). A three-group comparison by Kruskal-Wallis exact nonparametric test showed that the NAb levels were significantly different between immunization groups at weeks 10, 12, and 20 (p=0.018, p=0.018, and p=0.012, respectively). Pair-wise comparisons of GagPol/Env with empty vector at weeks 10 and 20 show that the level of NAb in the GagPol/Env group was significantly higher (p=0.029, p=0.029, respectively).
Following challenge, the NAb titers against SIVmac251 increased. After eight weeks, the GagPol/Env and the GagPol immunized animals generated high NAb titers against SIVmac251-TCLA (Fig. 4C). Furthermore, the GagPol/Env immunized animals had a significantly faster NAb response compared to GagPol immunized and control animals 2 weeks post challenge (p=0.029 and p=0.029, respectively). To distinguish the long-term trends in the SIVmac251-TCLA NAb titers, LME modeling was performed [22](Tab. 1). The difference in group average slope was significantly different between GagPol and empty vector immunized animals (p=0.012) and between GagPol/Env and empty vector animals (p=0.009). Although titers against SIVmac251-CX were lower than those seen for SIVmac251-TCLA, NAb titers began to increase as early as 12 weeks post challenge in vaccinated animals (Fig. 4D).
RV vaccine induced potent and poly-functional cellular immune responses in vaccinated macaques
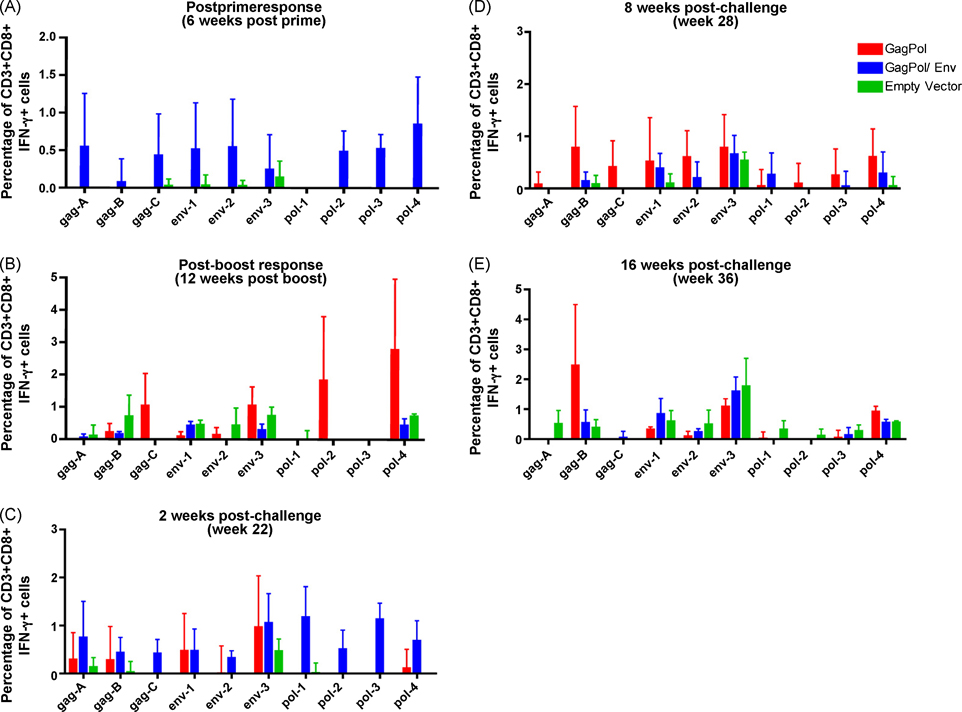
Antigen specific CD8+ T cell responses were determined by intracellular cytokine staining. PBMCs were stimulated ex vivo with various peptides pools from SIVmac251 Gag, Pol, or Env. Six weeks after th e first immunization with RV-vectors, we noted a greater percentage of antigen specific IFN-γ producing CD8+ T cells in the GagPol/Env immunized animals when compared to GagPol or empty vector immunized macaques. Additionally, the IFN-γ production was induced in response to Gag, Env, and Pol stimulation in the GagPol/Env animals (Fig. 5A). Twelve weeks following the booster immunization, we reexamined antigen specific CD8+ T cell responses. Interestingly, when comparing the GagPol immunized to the control immunized macaques, we saw an increased response to Gag and Pol peptide pools in some animals (Fig. 5B). This data indicates that RV-based vaccines did induce a broad range of antigen-specific CD8+ T cells.
Figure 5. CD8+ T cell response to vaccine antigens following SIV challenge.
PBMC were isolated from rhesus macaques at experimental week 6, 20, 22, 28, or 36 (A–E, respectively). Cells were stimulated with overlapping Gag, Pol, or Env peptide pools ex vivo and then stained for the presence of IFN-γ. The average percentage of CD3+CD8+ IFN-γ secreting cells in each group is shown here.
Likewise, two weeks following SIVmac251 challenge we detected a greater number of peripheral blood CD8+ T cells producing IFN-γ in the GagPol/Env and GagPol immunized animal cohorts as compared to control animals (Fig. 5C). Interestingly, the response to Pol epitopes was principally observed in the GagPol/Env immunized animals, and only very low levels of IFN-γ-secreting cells were seen in the GagPol immunized animals following stimulation with Pol peptides. On the other hand, the response to Env peptide pools was equal between the GagPol/Env and GagPol immunization groups 2 weeks post challenge. This data indicate that both RV-based immunization regimens induce a broad CD8+ T cell response by 2 weeks post SIV challenge while the empty vector immunization does not. The CD8+ IFN-γ+ T cell response was maintained in the GagPol/Env and GagPol macaques at 8 weeks post challenge (Fig. 5D). The controls animals did not generate an IFN-γ response comparable to vaccinated animals until 16 weeks post SIVmac251 challenge (Fig. 5E). However, this response was inadequate, or too late, to control viral replication in control macaques.
It has been suggested that the induction of poly-functional T cells is an important parameter for successful HIV vaccine [28, 29]. Thus, in addition to IFN-γ we included TNF-α , Mip1-β (CCL4), and IL-2 in the intracellular cytokine-staining panel. We saw that all three of the immunization regimens induced poly-functional CD8+ T cells in peripheral blood and that the Env peptide stimulus induced the greatest number of poly-functional cells (Fig. 6A–C). However, there was no difference between GagPol/Env or GagPol immunization when compared to empty vector controls following SIVmac251 challenge.
Figure 6. Poly-functional CD8+ T cell response to vaccine antigens.
PBMC were isolated from rhesus macaques 2, 8, 12 and 16 weeks post-challenge with SIVmac251. Cells were stimulated ex vivo with overlapping Gag peptide pools (A), Env peptide pools (B), or Pol peptide pools (C). Alternatively, jejunal lymphocytes were isolated from rhesus macaques pre-challenge (week 0) or 2 and 12 weeks post-challenge with SIVmac251 and then stimulated with overlapping Gag peptide pools ex vivo (D). Following stimulation, cells were then stained for the presence of IFN-γ, IL-2, Mip1-b and TNF-α. Each segment in the pie chart indicates the proportion of CD3+CD8+ cells secreting multiple cytokines.
We also isolated intestinal lymphocytes, because the majority of SIV replication initially occurs in the gastrointestinal tract [30, 31]. At the time of challenge (week 0), the GagPol/Env and GagPol immunized animals had more cells expressing a combination of two or three cytokines in response to Gag stimulation than the empty vector controls. Furthermore, at two weeks post-challenge, the GagPol/Env immunized animals had an appreciable population of cells that were positive for all four cytokines (Fig. 6D). At twelve weeks post-challenge, both the GagPol/Env and the GagPol immunized animals had a greater population of cells simultaneously expressing IFN-γ , IL-2, Mip1-β , and TNF-α than the control immunized animals (Fig. 6D). This data indicates that peripheral immunization with RV-based vaccines is a ble to efficiently induce high-quality CD8+ T cells in the mucosa.
DISCUSSION
The holy grail of the HIV field for the last 25 years has been the development of an effective vaccine. It is important that candidate vaccines be tested against simian viruses with different pathogenic properties in order to fully understand their protective potential. Like other potential HIV-1 vaccines, RV-based vaccine vectors have been seen to be efficacious against SHIV89.6P challenge [5]. However, oftentimes a vaccine effective against SHIV89.6P challenge fails to protect macaques from pathogenic SIVmac251 [32]. In this study we see increased survival of vaccinated animals after SIVmac251 challenge.
Here we examine two different RV-based vaccines, Gag and Gag/Env, in order to determine if the inclusion of Env in the vaccine design significantly increases the immune response. There is up to 30% amino acid diversity in HIV-1 Env [33], thus using Env as a vaccine antigen may not induce protection following a natural HIV infection. We see here that although the Gag/Env vaccinees had a lower peak viral load and more rapid antibody induction, there was no difference between vaccine cohorts in the ability to maintain a low viral set point and to prevent disease. Thus, it appears that while Env may help immediately following infection, the long-term benefits are minimal. Of note, SIVmac239 Env (which was used in our vaccine) and SIVmac251 Env (which is expressed by the challenge virus) have strong sequence similarity. Therefore, it will also be necessary to test the RV-based vaccine strategy after a pathogenic challenge with a heterologous virus such as SIVmacE660.
The correlate of protection for HIV/SIV infection is, as of yet, unknown. However, evidence suggests that both antibodies and CD8+ T cell responses are important. Passive transfer of a variety of anti-HIV1-Env antibodies to macaques induces complete or partial protection following vaginal SHIV challenge [34]. Additionally, the decrease of HIV levels in the blood has been associated with high levels of HIV-specific CD8+ T cell activity [35, 36] and CD8+ T cell depletion in SIV-infected rhesus macaques causes an increase in plasma viremia [37, 38]. Of note, following SIV infection of a natural host there is a general absence of chronic immune activation and this may need to be emulated by candidate vaccine vectors in order to generate appropriate immunity [39].
Using the RV-based vaccine vectors expressing GagPol or Env, SIVmac251-TCLA-specific NAb titers were detected as early as 2 weeks after boost in the GagPol/ Env immunized cohort and 2 weeks post infection in all vaccinees. Furthermore, vaccinees generated more NAb against SIVmac251-CX and SIVmac239. It is unclear if these responses were due to the vaccination regimen or the challenge virus, however, the vaccinees clearly showed a greater humoral immune response than the controls.
As noted above, the CD8+ T cell response also plays a central role in the control of HIV infection. We saw that peripherally IFN-γ secreting CD8+ T cells are more rapidly induced in the vaccinees as compared to the controls. Additionally, although the overall profile of poly-functional cells in the blood was similar among all immunization groups, we did detect a larger numbers of poly-functional cells in the CD8+ intestinal lymphocyte population for the vaccinated animals. This may be significant for our protection because the mucosa is known to be the primary site of viral replication following infection [30, 31].
One critical marker for HIV vaccines is the ability to reduce viral load in vaccinated individuals. Similar to other vaccine approaches, we saw that there was a significant decrease in peak viral load in the GagPol/Env immunization group when compared with the controls. Although similar decreases have been induced by DNA prime/ Ad5 boost vaccine strategies following SIVmac239 challenge, viral loads in vaccinated animals began to increase 10 weeks post challenge [40]. Following a RV-based vaccine, however, vaccinated animals maintain viral loads 1.3 to 1.6 logs lower than control animals 29 weeks post challenge. Another Ad vaccine strategy, in which serologically distinct Ad was tested, resulted in similar levels of SIVmac251 viral load reduction as we see in our study [41]. However, it appears that currently available vaccine technologies are not efficient to combat HIV infection [2, 3] and thus, the use of other viral vector vaccines needs to be revisited.
In addition to lowering viral loads, our results indicate that the RV vaccine protects memory T cells. It has been reported that HIV-infected individuals that maintain their CD4+ memory cells do not progress to AIDS [27]. Likewise, restoration following initial destruction of the CD4+CCR5+ cell compartment in the gut of SIVmac239 infected macaques is associated with long term non-progression of an AIDS-like disease [42]. The RV-based vaccines had some preservation of CD4+ cells expressing CCR5+CD45RA− or CD28+CD95+ with what appears to be repeated cycles of partial restoration and loss of this population. Although the vaccine did not completely protect against loss of intestinal target cells, it is important to note that the primary loss of intestinal CD4+ T cells also occurs in non-progressive infections [43–45]. Furthermore, it is the restoration of these target cells after acute infection that is important in disease outcome.
The mechanism of protection against challenge in vaccine recipients is not clear for the RV vectors, however it may be due to the collaborative activity of NAb and CD8+ T cells. The high level of NAb present in the GagPol/Env vaccinees at the time of challenge may be responsible for the significant reduction in peak viral load observed in these animals. However, the initial decrease observed in this vaccine cohort may also have been caused by Env specific CD8+ T cells, which were present in GagPol/Env vaccinated animals at 6 weeks post-prime. To delineate the importance of NAb in RV-vaccine induced protection, a further challenge experiment using the highly neutralization resistant SIVmac239 strain may be used.
To further evaluate the RV-based vaccine it may be important to consider similarities and differences it has with other ongoing vaccine approaches. A related rhabdovirus, VSV, was seen to be efficacious against SHIV89.6P [46] but it has not yet been shown to protect against a highly pathogenic SIV strain. It will be interesting to see if there are differences between the highly cytotoxic VSV and the non-cytotoxic RV. Of note, for both VSV and RV there is no preexisting immunity in the human population. This may prove to be important, as one factor contributing to the failure of the Ad5-based STEP vaccine trial was pre-existing vector immunity [2, 3]. Additionally, the simplicity of the RV vector immunization schedule (two inoculations) should be highlighted. Furthermore, it is not unlikely that the combination of RV vectors with other vectors may increase the observed immune responses further. This study indicated RV-vaccines induce strong humoral immune responses, and thus we suspect that RV-vectors will be well suited to express novel designed Env antigens, when they become available.
In summary, the results presented here indicate that both of the RV-based vaccines induced potent cellular and humoral immune responses in macaques and an increase in immunogenicity against SIV. The anti-SIV immune responses induced by RV- vaccines can be translated into increased protection from an AIDS-like disease for the challenged animal.
Acknowledgement
This study was supported by NIH/ NIAID R01 AI049153 to M.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial issues.
REFERENCES
- 1.Duerr A, Wasserheit JN, Corey L. HIV vaccines: new frontiers in vaccine development. Clin Infect Dis. 2006 Aug 15;43(4):500–511. doi: 10.1086/505979. [DOI] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008 Nov 29;372(9653):1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, et al. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006 Jan;7(1):19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 5.McKenna PM, Koser ML, Carlson KR, Montefiori DC, Letvin NL, Papaneri AB, et al. Highly attenuated rabies virus-based vaccine vectors expressing simian-human immunodeficiency virus89.6P Env and simian immunodeficiency virusmac239 Gag are safe in rhesus macaques and protect from an AIDS-like disease. J Infect Dis. 2007 Apr 1;195(7):980–988. doi: 10.1086/512243. [DOI] [PubMed] [Google Scholar]
- 6.Faber M, Lamirande EW, Roberts A, Rice AB, Koprowski H, Dietzschold B, et al. A single immunization with a rhabdovirus-based vector expressing severe acute respiratory syndrome coronavirus (SARS-CoV) S protein results in the production of high levels of SARS-CoV-neutralizing antibodies. J Gen Virol. 2005 May;86(Pt 5):1435–1440. doi: 10.1099/vir.0.80844-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGettigan JP, Pomerantz RJ, Siler CA, McKenna PM, Foley HD, Dietzschold B, et al. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J Virol. 2003 Jan;77(1):237–244. doi: 10.1128/JVI.77.1.237-244.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siler CA, McGettigan JP, Dietzschold B, Herrine SK, Dubuisson J, Pomerantz RJ, et al. Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology. 2002 Jan 5;292(1):24–34. doi: 10.1006/viro.2001.1212. [DOI] [PubMed] [Google Scholar]
- 9.Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T. HIV/AIDS: in search of an animal model. Trends Biotechnol. 2007 Aug;25(8):333–337. doi: 10.1016/j.tibtech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, et al. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 11.Knapp LA, Lehmann E, Piekarczyk MS, Urvater JA, Watkins DI. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens. 1997 Dec;50(6):657–661. doi: 10.1111/j.1399-0039.1997.tb02927.x. [DOI] [PubMed] [Google Scholar]
- 12.Smith SM, Mefford M, Sodora D, Klase Z, Singh M, Alexander N, et al. Topical estrogen protects against SIV vaginal transmission without evidence of systemic effect. AIDS. 2004 Aug 20;18(12):1637–1643. doi: 10.1097/01.aids.0000131393.76221.cc. [DOI] [PubMed] [Google Scholar]
- 13.Veazey RS, Mansfield KG, Tham IC, Carville AC, Shvetz DE, Forand AE, et al. Dynamics of CCR5 expression by CD4(+) T cells in lymphoid tissues during simian immunodeficiency virus infection. J Virol. 2000 Dec;74(23):11001–11007. doi: 10.1128/jvi.74.23.11001-11007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veazey RS, Tham IC, Mansfield KG, DeMaria M, Forand AE, Shvetz DE, et al. Identifying the target cell in primary simian immunodeficiency virus (SIV) infection: highly activated memory CD4(+) T cells are rapidly eliminated in early SIV infection in vivo. J Virol. 2000 Jan;74(1):57–64. doi: 10.1128/jvi.74.1.57-64.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pahar B, Li J, Rourke T, Miller CJ, McChesney MB. Detection of antigen-specific T cell interferon gamma expression by ELISPOT and cytokine flow cytometry assays in rhesus macaques. J Immunol Methods. 2003 Nov;282(1–2):103–115. doi: 10.1016/j.jim.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Pahar B, Wang X, Dufour J, Lackner AA, Veazey RS. Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3) Virology. 2007 Jun 20;363(1):36–47. doi: 10.1016/j.virol.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veazey RS, Klasse PJ, Ketas TJ, Reeves JD, Piatak M, Jr., Kunstman K, et al. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency virus infection. J Exp Med. 2003 Nov 17;198(10):1551–1562. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EMW, Strober MW, Coico R, editors. Current Protocols in Immunology. 2004. [DOI] [PubMed] [Google Scholar]
- 19.Brandt SM, Mariani R, Holland AU, Hope TJ, Landau NR. Association of chemokine-mediated block to HIV entry with coreceptor internalization. J Biol Chem. 2002 May 10;277(19):17291–17299. doi: 10.1074/jbc.M108232200. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005 Aug;79(16):10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vonesh EF, Chinchilli VM. Linear and Nonlinear Models for the Analysis of Repeated Measurements. New York, NY: Marcel Dekker; 1997. [Google Scholar]
- 22.Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000. [Google Scholar]
- 23.Hommel G. A Comparison of Two Modified Bonferroni Procedures. Biometrika. 1988;75:383–386. [Google Scholar]
- 24.Maness NJ, Yant LJ, Chung C, Loffredo JT, Friedrich TC, Piaskowski SM, et al. Comprehensive immunological evaluation reveals surprisingly few differences between elite controller and progressor Mamu-B*17-positive Simian immunodeficiency virus-infected rhesus macaques. J Virol. 2008 Jun;82(11):5245–5254. doi: 10.1128/JVI.00292-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, et al. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol. 2006 May;80(10):5074–5077. doi: 10.1128/JVI.80.10.5074-5077.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnittman SM, Lane HC, Greenhouse J, Justement JS, Baseler M, Fauci AS. Preferential infection of CD4+ memory T cells by human immunodeficiency virus type 1: evidence for a role in the selective T-cell functional defects observed in infected individuals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6058–6062. doi: 10.1073/pnas.87.16.6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakraborty R, Morel AS, Sutton JK, Appay V, Ripley RM, Dong T, et al. Correlates of delayed disease progression in HIV-1-infected Kenyan children. J Immunol. 2005 Jun 15;174(12):8191–8199. doi: 10.4049/jimmunol.174.12.8191. [DOI] [PubMed] [Google Scholar]
- 28.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006 Jun 15;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007 Jul;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 30.Stahl-Hennig C, Steinman RM, Tenner-Racz K, Pope M, Stolte N, Matz-Rensing K, et al. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science. 1999 Aug 20;285(5431):1261–1265. doi: 10.1126/science.285.5431.1261. [DOI] [PubMed] [Google Scholar]
- 31.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998 Apr 17;280(5362):427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 32.Demberg T, Boyer JD, Malkevich N, Patterson LJ, Venzon D, Summers EL, et al. Sequential priming with simian immunodeficiency virus (SIV) DNA vaccines, with or without encoded cytokines, and a replicating adenovirus-SIV recombinant followed by protein boosting does not control a pathogenic SIVmac251 mucosal challenge. J Virol. 2008 Nov;82(21):10911–10921. doi: 10.1128/JVI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaschen B, Taylor J, Yusim K, Foley B, Gao F, Lang D, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002 Jun 28;296(5577):2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 34.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000 Feb;6(2):207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 35.Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994 Sep;68(9):6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koup RA, Safrit JT, Cao Y, Andrews CA, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994 Jul;68(7):4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999 Mar 15;189(6):991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999 Feb 5;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 39.Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009 Aug;15(8):861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, Shipley T, et al. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol. 2002 Jul;76(14):7187–7202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, O'Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009 Jan 1;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling B, Veazey RS, Hart M, Lackner AA, Kuroda M, Pahar B, et al. Early restoration of mucosal CD4 memory CCR5 T cells in the gut of SIV-infected rhesus predicts long term non-progression. AIDS. 2007 Nov 30;21(18):2377–2385. doi: 10.1097/QAD.0b013e3282f08b32. [DOI] [PubMed] [Google Scholar]
- 43.Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007 Sep 1;179(5):3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol. 2007 Sep 1;179(5):3026–3034. doi: 10.4049/jimmunol.179.5.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007 Nov;117(11):3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schell J, Rose NF, Fazo N, Marx PA, Hunter M, Ramsburg E, et al. Long-term vaccine protection from AIDS and clearance of viral DNA following SHIV89.6P challenge. Vaccine. 2009 Feb 11;27(7):979–986. doi: 10.1016/j.vaccine.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]