Abstract
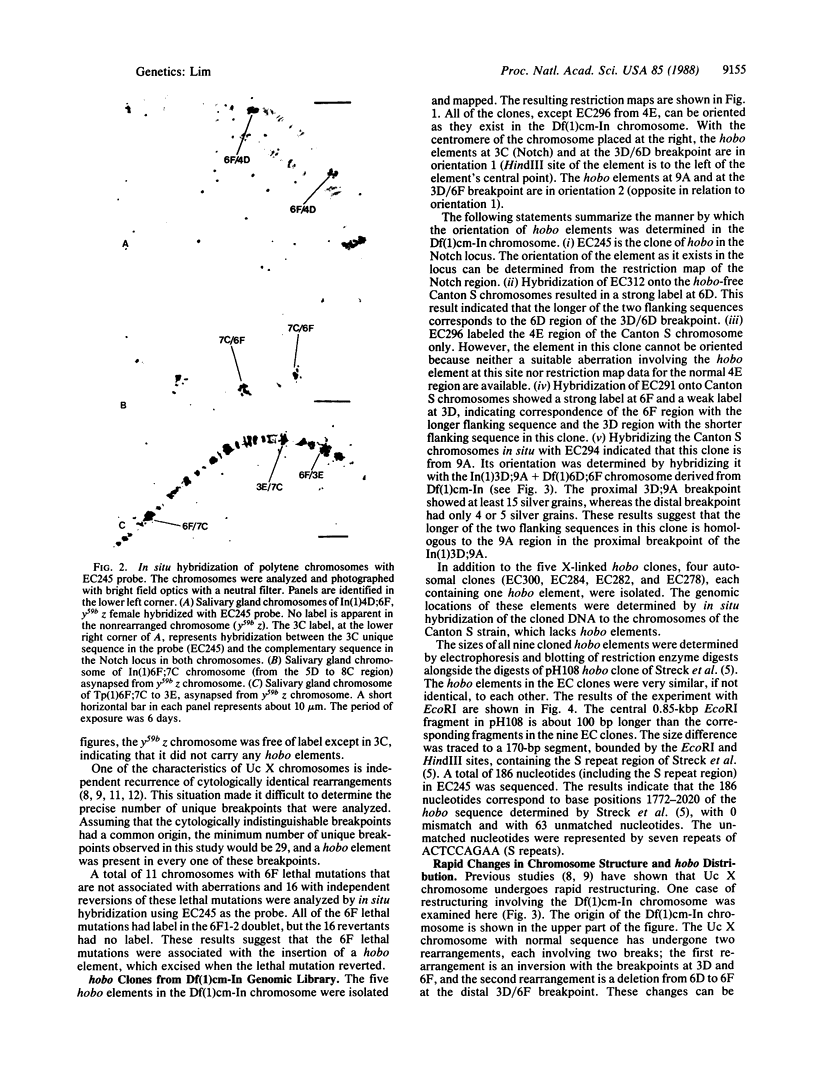
The recurring intrachromosomal rearrangements observed in an unstable X chromosome, designated Uc, of Drosophila melanogaster are shown to be mediated by hobo transposable elements. Each of 29 chromosome rearrangement breakpoints in 16 gross aberrations detected in the Uc-derived X chromosomes had a hobo element. In one particular unstable X chromosome line selected for detailed studies, a hobo element was found in each of the five hot spots for rearrangements. Furthermore, hobo elements at deletion hot spots were found to lie in the same orientation, whereas those hobo elements at inversion hot spots were in the opposite orientation. The restriction maps of two phage lambda clones containing rearrangement breakpoints indicated that a hobo element was inserted exactly at the breakpoints. Pairing of hobo elements in the same chromosome followed by recombination between the paired hobo elements is suggested as the explanation for the intrachromosomal aberrations observed in the Uc X chromosomes. A clear qualitative difference among the hobo elements in their ability to participate in rearrangement formation was noted. It was also found that each of the 11 recessive lethal mutations mapped in the 6F1-2 doublet had a hobo element in the doublet, whereas none of the 16 independent revertants of the mutation had a hobo element in the site. This observation indicates that hobo movement is responsible for production and subsequent instability of recessive lethal mutations in the 6F region of the Uc X chromosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender W., Spierer P., Hogness D. S. Chromosomal walking and jumping to isolate DNA from the Ace and rosy loci and the bithorax complex in Drosophila melanogaster. J Mol Biol. 1983 Jul 25;168(1):17–33. doi: 10.1016/s0022-2836(83)80320-9. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Blackman R. K., Grimaila R., Koehler M. M., Gelbart W. M. Mobilization of hobo elements residing within the decapentaplegic gene complex: suggestion of a new hybrid dysgenesis system in Drosophila melanogaster. Cell. 1987 May 22;49(4):497–505. doi: 10.1016/0092-8674(87)90452-1. [DOI] [PubMed] [Google Scholar]
- Davis P. S., Shen M. W., Judd B. H. Asymmetrical pairings of transposons in and proximal to the white locus of Drosophila account for four classes of regularly occurring exchange products. Proc Natl Acad Sci U S A. 1987 Jan;84(1):174–178. doi: 10.1073/pnas.84.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Fawcett D. H. Transposable elements in Drosophila melanogaster. Oxf Surv Eukaryot Genes. 1986;3:1–62. [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Goldberg M. L., Sheen J. Y., Gehring W. J., Green M. M. Unequal crossing-over associated with asymmetrical synapsis between nomadic elements in the Drosophila melanogaster genome. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5017–5021. doi: 10.1073/pnas.80.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Schlitz D., Lim J. K. Cytogenetics of Notch mutations arising in the unstable X chromosome Uc of Drosophila melanogaster. Genetics. 1987 Apr;115(4):701–709. doi: 10.1093/genetics/115.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S., Lockett T. J., Young M. W. The Notch locus of Drosophila melanogaster. Cell. 1983 Sep;34(2):421–433. doi: 10.1016/0092-8674(83)90376-8. [DOI] [PubMed] [Google Scholar]
- Laverty T. R., Lim J. K. Site-specific instability in Drosophila melanogaster: evidence for transposition of destabilizing element. Genetics. 1982 Jul-Aug;101(3-4):461–476. doi: 10.1093/genetics/101.3-4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. K., Simmons M. J., Raymond J. D., Cox N. M., Doll R. F., Culbert T. P. Homologue destabilization by a putative transposable element in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6624–6627. doi: 10.1073/pnas.80.21.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. K. Site-specific instability in Drosophila melanogaster: the origin of the mutation and cytogenetic evidence for site specificity. Genetics. 1979 Nov;93(3):681–701. doi: 10.1093/genetics/93.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J. K. Site-specific intrachromosomal rearrangements in Drosophila melanogaster: cytogenetic evidence for transposable elements. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):553–560. doi: 10.1101/sqb.1981.045.01.071. [DOI] [PubMed] [Google Scholar]
- Lim J. K., Snyder L. A. The mutagenic effects of two monofunctional alkylating chemicals on mature spermatozoa of drosophila. Mutat Res. 1968 Jul-Aug;6(1):129–137. doi: 10.1016/0027-5107(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Mathog D., Hochstrasser M., Gruenbaum Y., Saumweber H., Sedat J. Characteristic folding pattern of polytene chromosomes in Drosophila salivary gland nuclei. 1984 Mar 29-Apr 4Nature. 308(5958):414–421. doi: 10.1038/308414a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Shermoen A. W., Beckendorf S. K. A transposable element inserted just 5' to a Drosophila glue protein gene alters gene expression and chromatin structure. Cell. 1983 Aug;34(1):75–84. doi: 10.1016/0092-8674(83)90137-x. [DOI] [PubMed] [Google Scholar]
- McMullen M. D., Hunter B., Phillips R. L., Rubenstein I. The structure of the maize ribosomal DNA spacer region. Nucleic Acids Res. 1986 Jun 25;14(12):4953–4968. doi: 10.1093/nar/14.12.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrimpton A. E., Montgomery E. A., Langley C. H. OM Mutations in DROSOPHILA ANANASSAE Are Linked to Insertions of a Transposable Element. Genetics. 1986 Sep;114(1):125–135. doi: 10.1093/genetics/114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streck R. D., Macgaffey J. E., Beckendorf S. K. The structure of hobo transposable elements and their insertion sites. EMBO J. 1986 Dec 20;5(13):3615–3623. doi: 10.1002/j.1460-2075.1986.tb04690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Yannopoulos G., Stamatis N., Monastirioti M., Hatzopoulos P., Louis C. hobo is responsible for the induction of hybrid dysgenesis by strains of Drosophila melanogaster bearing the male recombination factor 23.5MRF. Cell. 1987 May 22;49(4):487–495. doi: 10.1016/0092-8674(87)90451-x. [DOI] [PubMed] [Google Scholar]





